Early Defoliation Techniques Enhance Yield Components, Grape and Wine Composition of cv. Trnjak (Vitis vinifera L.) in Dalmatian Hinterland Wine Region
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Vineyard Site
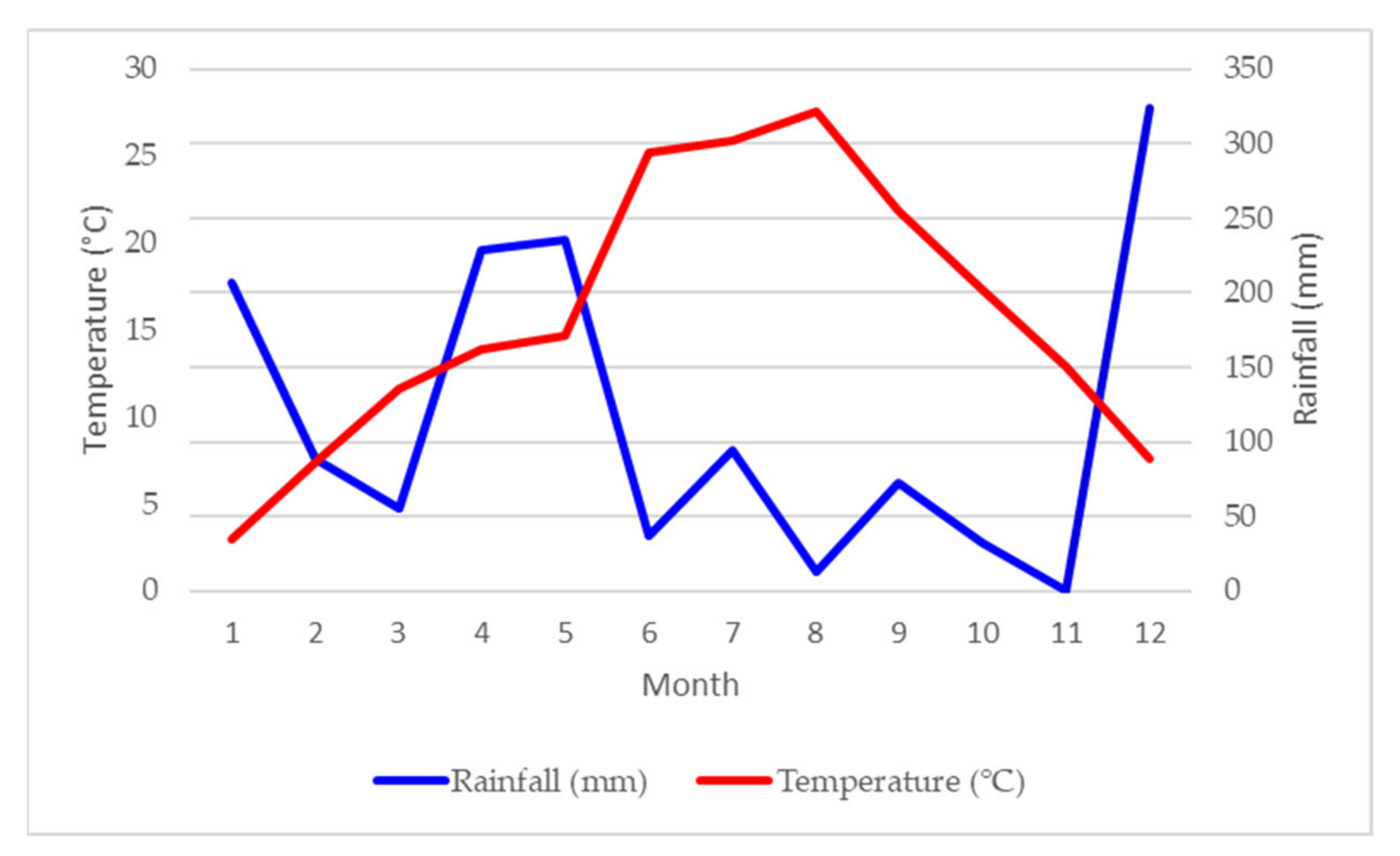
Climate Conditions at the Experimental Site
4.3. Experimental Set-Up
4.4. Yield Components
4.5. Clusters Characterization
4.6. Analysis of Physiochemical Components of Fresh Juice
4.7. Wines
4.8. Analysis of Standard Components of Wine
4.9. Analysis of (Non)Flavonoid Compounds by HPLC
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pastore, C.; Zenoni, S.; Fasoli, M.; Pezzotti, M.; Tornielli, G.B.; Filippetti, I. Selective defoliation affects plant growth, fruit transcriptional ripening program and flavonoid metabolism in grapevine. BMC Plant Biol. 2013, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Verdenal, T.; Zufferey, V.; Dienes-Nagy, A.; Bourdin, G.; Gindro, K.; Viret, O.; Spring, J.-L. Timing and Intensity of Grapevine Defoliation: An Extensive Overview on Five Cultivars in Switzerland. Am. J. Enol. Vitic. 2019, 70, 427–434. [Google Scholar] [CrossRef]
- Zenoni, S.; Santo, S.D.; Tornielli, G.B.; d’ Incà, E.; Filippetti, I.; Pastore, C.; Allegro, G.; Silvestroni, O.; Lanari, V.; Pisciotta, A.; et al. Transcriptional Responses to Pre-flowering Leaf Defoliation in Grapevine Berry from Different Growing Sites, Years, and Genotypes. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Poni, S.; Casalini, L.; Bernizzoni, F.; Civardi, S.; Intrieri, C. Effects of early defoliation on shoot photosynthesis, yield components, and grape composition. Am. J. Enol. Vitic. 2006, 57, 397–407. [Google Scholar]
- Drenjančević, M.; Jukić, V.; Zmaić, K.; Kujundžić, T.; Rastija, V. Effects of early leaf removal on grape yield, chemical characteristics, and antioxidant activity of grape variety Cabernet Sauvignon and wine from eastern Croatia. Acta Agric. Scand. Sect. B Plant Soil Sci. 2017, 67, 1–7. [Google Scholar] [CrossRef]
- Carbonell-Bejerano, P.; Diago, M.-P.; Martínez-Abaigar, J.; Martínez-Zapater, J.M.; Tardáguila, J.; Núñez-Olivera, E. Solar ultraviolet radiation is necessary to enhance grapevine fruit ripening transcriptional and phenolic responses. BMC Plant Biol. 2014, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, J.; Dokoozlian, N.; Ebisuda, N. Sunlight exposure and temperature effects on berry growth and composition of Cabernet Sauvignon and Grenache in the Central San Joaquin Valley of California. Am. J. Enol. Vitic. 2001, 52, 1–7. [Google Scholar]
- Kliewer, W.M. Effect of high temperatures during the bloom-set period on fruit-set, ovule fertility, and berry growth of several grape cultivars. Am. J. Enol. Vitic. 1997, 28, 215–222. [Google Scholar]
- Verdenal, T.; Zufferey, V.; Dienes-Nagy, A.; Belcher, S.; Lorenzini, F.; Rösti, J.; Koestel, C.; Gindro, K.; Spring, J.-L. Intensity and timing of defoliation on white cultivar Chasselas under the temperate climate of Switzerland. Oeno One 2018, 52, 93–104. [Google Scholar] [CrossRef]
- Tardaguila, J.; de Toda, F.M.; Poni, S.; Diago, M.P. Impact of early leaf removal on yield and fruit and wine composition of Vitis Vinifera L. Graciano and Carignan. Am. J. Enol. Vitic. 2010, 61, 372–381. [Google Scholar]
- Bubola, M.; Rusjan, D.; Lukić, I. Crop level vs. leaf removal: Effects on Istrian Malvasia wine aroma and phenolic acids composition. Food Chem. 2020, 312, 126046. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, V.; Murisier, F.; Schultz, H.R. A Model of Vitis Vinifera L. cvs Riesling and Chasselas leaves in the field: I. Interaction of age, light and temperature. Vitis 2000, 39, 19–26. [Google Scholar] [CrossRef]
- Xi, X.; Zha, Q.; He, Y.; Tian, Y.; Jiang, A. Influence of cluster thinning and girdling on aroma composition in ‘Jumeigui’ table grape. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Sivilotti, P.; Falchi, R.; Vanderweide, J.; Sabbatini, P.; Bubola, M.; Vanzo, A.; Lisjak, K.; Peterlunger, E.; Herrera, J.C. Yield reduction through cluster or selective berry thinning similarly modulates anthocyanins and proanthocyanidins composition in Refosco dal peduncolo rosso (Vitis vinifera L.) grapes. Sci. Hortic. 2020, 264, 109166. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Schlosser, J.; Sorokowsky, D.; Roberts, R.; Willwerth, J.; de Savigny, C. Magnitude of viticultural and enological effects. II. Relative impacts of cluster thinning and yeast strain on composition and sensory attributes of Chardonnay Musqué. Am. J. Enol. Vitic. 2007, 58, 25–41. [Google Scholar]
- Schelezki, O.J.; Antalick, G.; Šuklje, K.; Jeffery, D.W. Pre-fermentation approaches to producing lower alcohol wines from Cabernet Sauvignon and Shiraz: Implications for wine quality based on chemical and sensory analysis. Food Chem. 2020, 309, 125698. [Google Scholar] [CrossRef]
- Zdunić, G.; Preece, J.E.; Dangl, G.S.; Koehmstedt, A.; Mucalo, A.; Maletić, E.; Pejić, I. Genetic Characterization of Grapevine Cultivars Collected throughout the Dalmatian Region. Am. J. Enol. Vitic. 2013, 64, 285–290. [Google Scholar] [CrossRef]
- Katalinic, V.; Maleš, P.; Konja, G. Low molecular weight flavans in wine. J. Wine Res. 1997, 8, 19–27. [Google Scholar] [CrossRef]
- Poni, S.; Bernizzoni, F.; Civardi, S. The effect of early leaf removal on whole-canopy gas exchange and vine performance of Vitis vinifera L. ‘Sangiovese’. Vitis 2008, 47, 1–6. [Google Scholar]
- Bogicevic, M.; Maraš, V.; Mugoša, M.; Kodžulović, V.; Raičević, J.; Radonjić, S.; Failla, O. The effects of early leaf removal and cluster thinning treatments on berry growth and grape composition in cultivars Vranac and Cabernet Sauvignon. Chem. Biol. Technol. Agric. 2015, 2, 13. [Google Scholar] [CrossRef]
- Zdunić, G.; Mucalo, A.; Budić, L.I.; Humar, I.; Pejić, I.; Maletić, E. Cluster architecture of old, neglected Croatian grapevine varieties (Vitis vinifera L.). Vitis 2015, 54, 177–180. [Google Scholar] [CrossRef]
- De Bolt, S.; Ristic, R.; Iland, P.G.; Ford, C.M. Altered Light Interception Reduces Grape Berry Weight and Modulates Organic Acid Biosynthesis During Development. HortScience 2008, 43, 957–961. [Google Scholar] [CrossRef]
- Cholet, C.; Claverol, S.; Claisse, O.; Rabot, A.; Osowsky, A.; Dumot, V.; Ferrari, G.; Gény, L. Tartaric acid pathways in Vitis vinifera L. (cv. Ugni blanc): A comparative study of two vintages with contrasted climatic conditions. BMC Plant Biol. 2016, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sadras, V.; Morán, M. Elevated temperature decouples anthocyanins and sugars in berries of Shiraz and Cabernet Franc. Aust. J. Grape Wine Res. 2012, 18, 115–122. [Google Scholar] [CrossRef]
- Osrečak, M.; Karoglan, M.; Kozina, B. Influence of leaf removal and reflective mulch on phenolic composition and antioxidant activity of Merlot, Teran and Plavac mali wines (Vitis vinifera L.). Sci. Hortic. 2016, 209, 261–269. [Google Scholar] [CrossRef]
- Duchêne, É.; Dumas, V.; Butterlin, G.; Jaegli, N.; Rustenholz, C.; Chauveau, A.; Bérard, A.; le Paslier, M.C.; Gaillard, I.; Merdinoglu, D. Genetic variations of acidity in grape berries are controlled by the interplay between organic acids and potassium. Theor. Appl. Genet. 2020, 133, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Rogiers, S.Y.; Sadras, V.O.; Tyerman, S.D. Hypoxia in grape berries: The role of seed respiration and lenticels on the berry pedicel and the possible link to cell death. J. Exp. Bot. 2018, 69, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Toda, F.; Balda, P. Reducing the pH of wine by increasing grape sunlight exposure: A method to mitigate the effects of climate warming. Vitis J. Grapevine Res. 2014, 53, 17–20. [Google Scholar]
- Sun, R.-Z.; Cheng, G.; Li, Q.; Zhu, Y.-R.; Zhang, X.; Wang, Y.; He, Y.-N.; Li, S.-Y.; He, L.; Chen, W.; et al. Comparative physiological, metabolomic, and transcriptomic analyses reveal developmental stage-dependent effects of cluster bagging on phenolic metabolism in Cabernet Sauvignon grape berries. BMC Plant Biol. 2019, 19, 1–21. [Google Scholar] [CrossRef]
- Bubola, M.; Peršurić, Đ.; Kovačević, G.K.; Karoglan, M.; Kozina, B. Effects of fruit zone leaf removal on the concentrations of phenolic and organic acids in Istrian Malvasia grape juice and wine. Food Technol. Biotechnol. 2012, 50, 159–166. [Google Scholar]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.C.; Gascueña, J.M.; Romero, E.G. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef]
- Wang, X.-J.; Li, Y.-K.; Song, H.-C.; Tao, Y.-S.; Russo, N. Phenolic matrix effect on aroma formation of terpenes during simulated wine fermentation—Part I: Phenolic acids. Food Chem. 2021, 341, 128288. [Google Scholar] [CrossRef]
- Cheynier, V.; Souquet, J.-M.; Kontek, A.; Moutounet, M. Anthocyanin degradation in oxidising grape musts. J. Sci. Food Agric. 1994, 66, 283–288. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Rustioni, L.; Fracassetti, D.; Prinsi, B.; Geuna, F.; Ancelotti, A.; Fauda, V.; Tirelli, A.; Espen, L.; Failla, O. Oxidations in white grape (Vitis vinifera L.) skins: Comparison between ripening process and photooxidative sunburn symptoms. Plant Physiol. Biochem. 2020, 150, 270–278. [Google Scholar] [CrossRef]
- Bindon, K.; Holt, H.; Williamson, P.O.; Varela, C.; Herderich, M.; Francis, I.L. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon 2. Wine sensory properties and consumer preference. Food Chem. 2014, 154, 90–101. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. The effect of bunch shading on berry development and flavonoid accumulation in Shiraz grapes. Aust. J. Grape Wine Res. 2008, 10, 55–73. [Google Scholar] [CrossRef]
- Yu, R.; Cook, M.G.; Yacco, R.S.; Watrelot, A.A.; Gambetta, G.; Kennedy, J.A.; Kurtural, S.K. Effects of Leaf Removal and Applied Water on Flavonoid Accumulation in Grapevine (Vitis vinifera L. cv. Merlot) Berry in a Hot Climate. J. Agric. Food Chem. 2016, 64, 8118–8127. [Google Scholar] [CrossRef] [PubMed]
- Talaverano, M.I.; Moreno, D.; Rodríguez-Pulido, F.J.; Valdés, M.E.; Gamero, E.; Jara-Palacios, M.J.; Heredia, F.J. Effect of early leaf removal on Vitis Vinifera L. cv. Tempranillo seeds during ripening based on chemical and image analysis. Sci. Hortic. 2016, 209, 148–155. [Google Scholar] [CrossRef]
- Kennedy, A.J. Grape and wine phenolics: Observations and recent findings. Cienc. Investig. Agrar. 2008, 35, 107–120. [Google Scholar] [CrossRef]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, A.J.M.; Zamora, F. Influence of Ethanol Concentration on the Extraction of Color and Phenolic Compounds from the Skin and Seeds of Tempranillo Grapes at Different Stages of Ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef]
- Mucalo, A.; Maletić, E.; Zdunić, G. Extended Harvest Date Alter Flavonoid Composition and Chromatic Characteristics of Plavac Mali (Vitis vinifera L.) Grape Berries. Foods 2020, 9, 1155. [Google Scholar] [CrossRef] [PubMed]
- Casassa, L.F.; Harbertson, J.F. Extraction, Evolution, and Sensory Impact of Phenolic Compounds During Red Wine Maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Goto-Yamamoto, N.; Hashizume, K. Influence of Maceration Temperature in Red Wine Vinification on Extraction of Phenolics from Berry Skins and Seeds of Grape (Vitis vinifera). Biosci. Biotechnol. Biochem. 2007, 71, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Bois, B.; Joly, D.; Quénol, H.; Pieri, P.; Gaudillère, J.-P.; Guyon, D.; Saur, E.; van Leeuwen, C. Temperature-based zoning of the Bordeaux wine region. Oeno One 2018, 52. [Google Scholar] [CrossRef]
- Falcão, L.D.; Brighenti, E.; Rosier, J.-P.; Bordignon-Luiz, M.T.; Burin, V.M.; Chaves, E.S.; Vieira, H.J. Vineyard altitude and mesoclimate influences on the phenology and maturation of Cabernet-Sauvignon grapes from Santa Catarina State. Oeno One 2010, 44, 135. [Google Scholar] [CrossRef]
- Hall, A.; Jones, G. Spatial analysis of climate in winegrape-growing regions in Australia. Aust. J. Grape Wine Res. 2010, 16, 389–404. [Google Scholar] [CrossRef]
- Tello, J.; Cubero, S.; Blasco, J.; Tardáguila, J.; Aleixos, N.; Ibáñez, J. Application of 2D and 3D image technologies to characterize morphological attributes of grapevine clusters. J. Sci. Food Agric. 2016, 96, 4575–4583. [Google Scholar] [CrossRef]
- Office International de la Vigne et du Vin. Compendium of International Methods of Wine and Must Analysis—Vol I.; Organisation Internationale de la Vigne et du Vin (OIV): Paris, France, 2015. [Google Scholar]
- Tomaz, I.; Maslov, L. Simultaneous Determination of Phenolic Compounds in Different Matrices using Phenyl-Hexyl Stationary Phase. Food Anal. Methods 2016, 9, 401–410. [Google Scholar] [CrossRef]
- Berente, B.; García, D.D.L.C.; Reichenbächer, M.; Danzer, K. Method development for the determination of anthocyanins in red wines by high-performance liquid chromatography and classification of German red wines by means of multivariate statistical methods. J. Chromatogr. A 2000, 871, 95–103. [Google Scholar] [CrossRef]
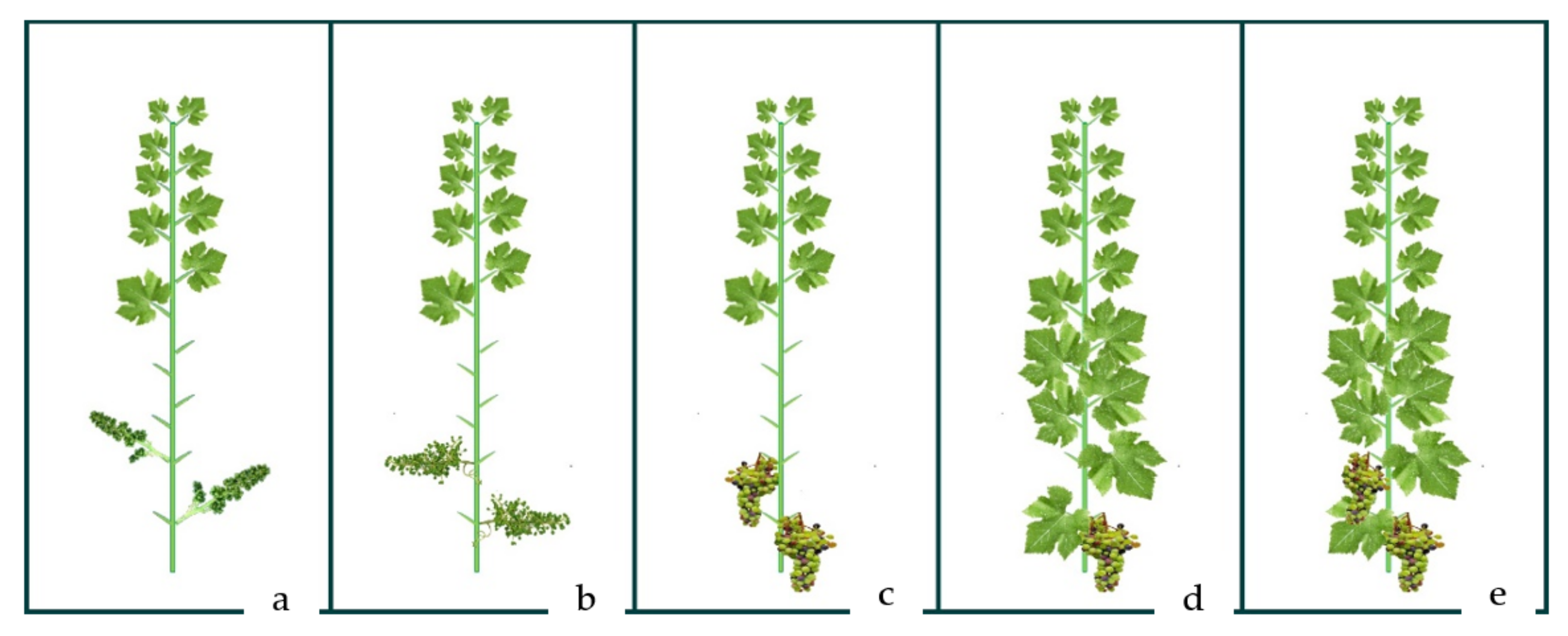
| T1 | T2 | T3 | T4 | C | |
|---|---|---|---|---|---|
| Agronomic parameters | |||||
| Cluster number/vine | 11.78 ± 3.67 a | 10.36 ± 3.12 b | 10.16 ± 2.95 b | 7.2 ± 2.46 c | 10.67 ± 3.13 ab |
| Yield/vine (kg) | 1.54 ± 0.63 b | 1.33 ± 0.52 bc | 1.15 ± 0.45 c | 1.24 ± 0.51 c | 1.83 ± 0.71 a |
| Cluster architecture | |||||
| Cluster weight (g) | 244.54 ± 34.17 b | 251.73 ± 64.22 b | 227.47 ± 57.83 b | 325.07 ± 54.30 a | 360.74 ± 58.93 a |
| Cluster length (cm) | 14.57 ± 2.01 b | 15.04 ± 1.04 ab | 14.61 ± 1.87 b | 15.29 ± 1.28 ab | 16.36 ± 1.41 a |
| Cluster width (cm) | 10.49 ± 1.27 ab | 10.61 ± 1.26 ab | 10.36 ± 2.40 b | 12.12 ± 2.19 a | 11.77 ± 2.10 ab |
| Berries/cluster | 120 ± 23.63 c | 131 ± 33.38 bc | 129 ± 39.55 bc | 151 ± 28.34 b | 183 ± 28.83 a |
| Rachis weight (g) | 6.81 ± 1.14 b | 7.43 ± 1.94 b | 7.49 ± 2.53 b | 10.58 ± 2.69 a | 11.53 ± 3.59 a |
| Total berry weight (g) | 237.73 ± 33.37 b | 244.30 ± 62.57 b | 219.99 ± 55.79 b | 314.49 ± 52.41 a | 349.21 ± 56.10 a |
| Mean mass (1 berry) | 2.07 ± 0.25 a | 1.94 ± 0.29 ab | 1.81 ± 0.32 b | 2.17 ± 0.25 a | 1.98 ± 0.17 ab |
| Compactness | 1.2 ± 0.35 ab | 1.11 ± 0.23 b | 1.07 ± 0.22 b | 1.41 ± 0.32 a | 1.36 ± 0.23 a |
| Cluster elongation | 1.43 ± 0.37 a | 1.44 ± 0.20 a | 1.46 ± 0.29 a | 1.29 ± 0.22 a | 1.43 ± 0.27 a |
| Ripening parameters | |||||
| Weight 100 berries | 227.83 ± 3.04 ab | 218.84 ± 4.50 cd | 214.88 ± 4.89 d | 233.85 ± 1.88 a | 224.71 ± 4.55 bc |
| Sugar (°Brix) | 19.47 ± 0.15 d | 20.37 ± 0.06 b | 22.30 ± 0.00 a | 20.37 ± 0.12 b | 20.10 ± 0.20 c |
| pH | 3.54 ± 0.01 d | 3.63 ± 0.01 c | 3.75 ± 0.01 a | 3.67 ± 0.01 b | 3.62 ± 0.02 c |
| Total acidity (g/L) | 6.12 ± 0.05 a | 5.30 ± 0.04 d | 5.01 ± 0.06 e | 5.56 ± 0.09 c | 5.87 ± 0.08 b |
| T1 | T2 | T3 | T4 | C | |
|---|---|---|---|---|---|
| Alcohol (% v/v) | 11.9 ± 0.0 c | 12.5 ± 0.1 b | 13.9 ± 0.0 a | 12.47 ± 0.4 b | 12.1 ± 0.0 c |
| Total dry extract (g/L) | 28.6 ± 0.66 b | 28.57 ± 0.29 b | 33.67 ± 0.4 a | 29 ± 0.17 b | 27.8 ± 0.17 c |
| Reducing sugars (g/L) | 1.67 ± 0.06 c | 2.2 ± 0.1 b | 2.53 ± 0.15 a | 1.7 ± 0.2 c | 1.87 ± 0.06 c |
| pH | 3.90 ± 0.06 a | 3.87 ± 0.02 a | 3.89 ± 0.00 a | 3.86 ± 0.03 a | 3.85 ± 0.02 a |
| Total acidity (g/L) (as tartaric acid) | 5.5 ± 0.44 c | 5.97 ± 0.06 ab | 6.3 ± 0.00 a | 5.93 ± 0.23 ab | 5.63 ± 0.06 bc |
| Volatile acidity (g/L) (as acetic acid) | 0.43 ± 0.06 b | 0.5 ± 0.0 ab | 0.47 ± 0.06 ab | 0.47 ± 0.06 ab | 0.53 ± 0.06 a |
| Nonflavonoid Compounds | T1 | T2 | T3 | T4 | C |
|---|---|---|---|---|---|
| Caftaric acid | 46.91 ± 1.5 a | 43.02 ± 0.84 b | 29.71 ± 2.10 d | 43.64 ± 0.91 b | 40.29 ± 1.12 c |
| Caffeic acid | 2.78 ± 0.18 c | 3.57 ± 0.08 b | 8.11 ± 0.27 a | 3.32 ± 0.37 b | 2.55 ± 0.28 c |
| p-coumaric acid | n.d. | n.d. | 1.60 ± 0.08 a | n.d. | n.d. |
| Total Hydroxycinnamic acids | 49.70 ± 1.39 a | 46.59 ± 0.92 b | 39.42 ± 1.99 d | 46.96 ± 1.26 b | 42.84 ± 0.90 c |
| Gallic acid | 13.08 ± 0.34 bc | 11.47 ± 0.98 c | 24.38 ± 1.93 a | 13.70 ± 0.3 b | 12.09 ± 0.27 bc |
| Protocatechic acid | 2.82 ± 0.19 c | 2.87 ± 0.95 c | 8.07 ± 0.20 a | 4.32 ± 0.20 b | 3.66 ± 0.48 bc |
| Vanillic acid | 3.32 ± 0.39 c | 3.46 ± 0.21 c | 7.00 ± 0.42 a | 4.05 ± 0.26 b | 4.03 ± 0.16 b |
| Syringic acid | 2.31 ± 0.23 c | 3.12 ± 0.12 b | 7.13 ± 0.58 a | 3.70 ± 0.38 b | 3.57 ± 0.29 b |
| Total Hydroxybenzoic acids | 21.53 ± 0.09 c | 20.92 ± 0.92 c | 46.58 ± 3.10 a | 25.78 ± 1.08 b | 23.35 ± 0.27 bc |
| Viniferin | 0.21 ± 0.02 a | 0.21 ± 0.04 a | 0.20 ± 0.05 a | 0.22 ± 0.01 a | 0.19 ± 0.02 a |
| Resveratrol-3-O-glucoside | 2.74 ± 0.09 ab | 2.61 ± 0.26 ab | 1.78 ± 0.14 c | 2.78 ± 0.08 a | 2.50 ± 0.06 b |
| Resveratrol | 0.07 ± 0.02 b | 0.10 ± 0.03 b | 0.16 ± 0.03 a | 0.10 ± 0.02 b | 0.08 ± 0.01 b |
| Total stilbenes | 3.02 ± 0.11 ab | 2.92 ± 0.27 ab | 2.14 ± 0.10 c | 3.10 ± 0.07 a | 2.77 ± 0.08 b |
| Flavonoid Compounds | T1 | T2 | T3 | T4 | K |
|---|---|---|---|---|---|
| Delphinidin-3-glucoside | 0.72 ± 0.37 a | 0.74 ± 0.14 a | 0.20 ± 0.17 b | 0.68 ± 0.09 a | 0.14 ± 0.03 b |
| Petunidin-3-glucoside | 4.01 ± 0.38 a | 3.6 ± 0.68 ab | 1.42 ± 0.20 d | 2.87 ± 0.75 bc | 2.34 ± 0.36 cd |
| Peonidin-3-glucoside | 1.09 ± 0.32 a | 1.37 ± 0.16 a | 0.31 ± 0.04 c | 1.33 ± 0.11 a | 0.68 ± 0.20 b |
| Malvidin-3-glucoside | 110.27 ± 6.25 a | 113.63 ± 2.98 a | 61.36 ± 21.45 c | 86.59 ± 3.67 b | 95.84 ± 6.56 ab |
| Cyanidin-3-(6″acetyl) glucoside | n.d. | n.d. | 2.81 ± 0.3 a | 1.95 ± 0.18 b | n.d. |
| Delphinidin-3-(6″caffeoyl) glucoside | 0.36 ± 0.04 a | 0.32 ± 0.04 a | 0.12 ± 0.03 b | 0.10 ± 0.08 b | 0.27 ± 0.08 a |
| Peonidin-3-(6″acetyl) glucoside | 1.8 ± 0.12 c | 2.74 ± 0.12 a | 1.03 ± 0.23 d | 2.37 ± 0.20 b | 2.1 ± 0.13 b |
| Malvidin-3-(6″acetyl) glucoside | 30.79 ± 6.10 a | 32.21 ± 2.82 a | 12.97 ± 2.25 c | 23.48 ± 2.53 b | 28.33 ± 2.31 ab |
| Peonidin-3-(6″-p-coumaroyl) glucoside | 1.28 ± 0.10 a | 1.19 ± 0.12 a | 0.47 ± 0.11 c | 0.74 ± 0.13 b | 0.80 ± 0.15 b |
| Malvidin-3-(6″-p-coumaroyl) glucoside | 6.74 ± 1.93 a | 6.40 ± 2.7 a | 2.0 ± 0.8 b | 6.93 ± 1.31 a | 6.79 ± 0.68 a |
| Total anthocyanins | 157.05 ± 14.22 a | 162.20 ± 8.55 a | 82.69 ± 23.31 c | 127.04 ± 4.83 b | 137.29 ± 10.06 ab |
| Myricetin-3-O-glucuronide | 7.09 ± 0.47 a | 4.37 ± 0.52 b | 0.35 ± 0.15 e | 2.12 ± 0.73 d | 3.00 ± 0.25 c |
| Myricetin-3-O-glucoside | 1.41 ± 0.02 ab | 1.28 ± 0.36 b | 0.70 ± 0.25 c | 1.77 ± 0.10 a | 1.62 ± 0.16 ab |
| Quercetin-3-O-glucoside | 13.68 ± 0.67 a | 11.82 ± 0.17 b | 4.82 ± 0.75 c | 5.47 ± 0.50 c | 5.43 ± 0.02 c |
| Quercetin-3-O-galactoside | 1.49 ± 0.10 a | 1.16 ± 0.04 b | 0.35 ± 0.18 c | 0.45 ± 0.10 c | 0.38 ± 0.09 c |
| Kaempferol-3-glucuronide | 0.32 ± 0.02 ab | 0.30 ± 0.04 ab | 0.21 ± 0.07 b | 0.35 ± 0.13 a | 0.25 ± 0.06 ab |
| Kaempferol-3-glucoside | 2.06 ± 0.05 a | 2.03 ± 0.05 a | 1.90 ± 0.01 b | 1.89 ± 0.01 b | 1.86 ± 0.04 b |
| Isorhamnetin-3-glucoside | 0.14 ± 0.02 a | 0.16 ± 0.06 a | 0.08 ± 0.01 a | 0.12 ± 0.03 a | 0.15 ± 0.07 a |
| Syringetin-3-glucoside | 8.85 ± 0.33 a | 8.88 ± 0.26 a | 7.66 ± 0.76 b | 7.02 ± 0.30 bc | 6.35 ± 0.13 c |
| Total favonols | 35.03 ± 1.57 a | 30 ± 0.59 b | 16.08 ± 1.46 d | 19.19 ± 1.21 c | 19.05 ± 0.03 c |
| Epigallocatechin-gallate (EGCG) | 3.62 ± 0.21 c | 6.12 ± 0.08 a | 4.58 ± 0.21 b | 5.52 ± 0.41 a | 3.87 ± 0.99 bc |
| Epicatechingallate (ECG) | 8.09 ± 0.67 a | 7.59 ± 0.96 a | 3.97 ± 0.25 c | 6.94 ± 0.85 ab | 6.31 ± 0.17 b |
| Gallocatechin | 102.59 ± 3.58 a | 110.84 ± 7.16 a | 85.95 ± 5.82 b | 73.79 ± 7.22 c | 66.04 ± 1.38 c |
| Epigallocatechin | 5.94 ± 0.30 a | 5.96 ± 0.82 a | n.d. | 5.61 ± 0.40 a | n.d. |
| Dimer B1 | 24.04 ± 1.49 a | 20.31 ± 1.89 b | 19.91 ± 3.01 b | 19.98 ± 0.65 b | 19.52 ± 0.66 b |
| Catechin | 19.08 ± 1.53 a | 16.18 ± 1.88 ab | 16.55 ± 2.70 ab | 16.85 ± 1.23 ab | 15.43 ± 0.47 b |
| Dimer B3 | 4.55 ± 0.20 a | 3.39 ± 0.34 c | 4.18 ± 0.24 ab | 3.87 ± 0.08 b | 3.39 ± 0.19 c |
| Dimer B4 | 2.92 ± 0.08 ab | 2.55 ± 0.06 cd | 3.03 ± 0.19 a | 2.71 ± 0.19 bc | 2.45 ± 0.05 d |
| Dimer B2 | 12.14 ± 1.14 ab | 9.30 ± 1.10 c | 13.91 ± 2.26 a | 11.49 ± 0.94 bc | 10.35 ± 0.35 bc |
| Epicatechin | 13.89 ± 1.42 a | 10.30 ± 1.48 c | 14.46 ± 2.11 a | 13.01 ± 0.53 ab | 11.38 ± 0.55 bc |
| Total (catechin+epicatechin) | 32.97 ± 2.92 a | 26.49 ± 3.35 b | 31.01 ± 4.80 ab | 29.86 ± 1.74 ab | 26.81 ± 1.00 b |
| Total dimers B | 43.66 ± 2.79 a | 35.55 ± 2.65 b | 41.04 ± 5.42 ab | 38.05 ± 1.11 b | 35.70 ± 1.11 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mucalo, A.; Budić-Leto, I.; Lukšić, K.; Maletić, E.; Zdunić, G. Early Defoliation Techniques Enhance Yield Components, Grape and Wine Composition of cv. Trnjak (Vitis vinifera L.) in Dalmatian Hinterland Wine Region. Plants 2021, 10, 551. https://doi.org/10.3390/plants10030551
Mucalo A, Budić-Leto I, Lukšić K, Maletić E, Zdunić G. Early Defoliation Techniques Enhance Yield Components, Grape and Wine Composition of cv. Trnjak (Vitis vinifera L.) in Dalmatian Hinterland Wine Region. Plants. 2021; 10(3):551. https://doi.org/10.3390/plants10030551
Chicago/Turabian StyleMucalo, Ana, Irena Budić-Leto, Katarina Lukšić, Edi Maletić, and Goran Zdunić. 2021. "Early Defoliation Techniques Enhance Yield Components, Grape and Wine Composition of cv. Trnjak (Vitis vinifera L.) in Dalmatian Hinterland Wine Region" Plants 10, no. 3: 551. https://doi.org/10.3390/plants10030551
APA StyleMucalo, A., Budić-Leto, I., Lukšić, K., Maletić, E., & Zdunić, G. (2021). Early Defoliation Techniques Enhance Yield Components, Grape and Wine Composition of cv. Trnjak (Vitis vinifera L.) in Dalmatian Hinterland Wine Region. Plants, 10(3), 551. https://doi.org/10.3390/plants10030551









