Phytodepuration of Nitrate Contaminated Water Using Four Different Tree Species
Abstract
:1. Introduction
2. Results
2.1. Leaf Net Photosynthesis (Pn) and Chlorophyll Content
2.2. Plant Growth and Evapotranspirated Water
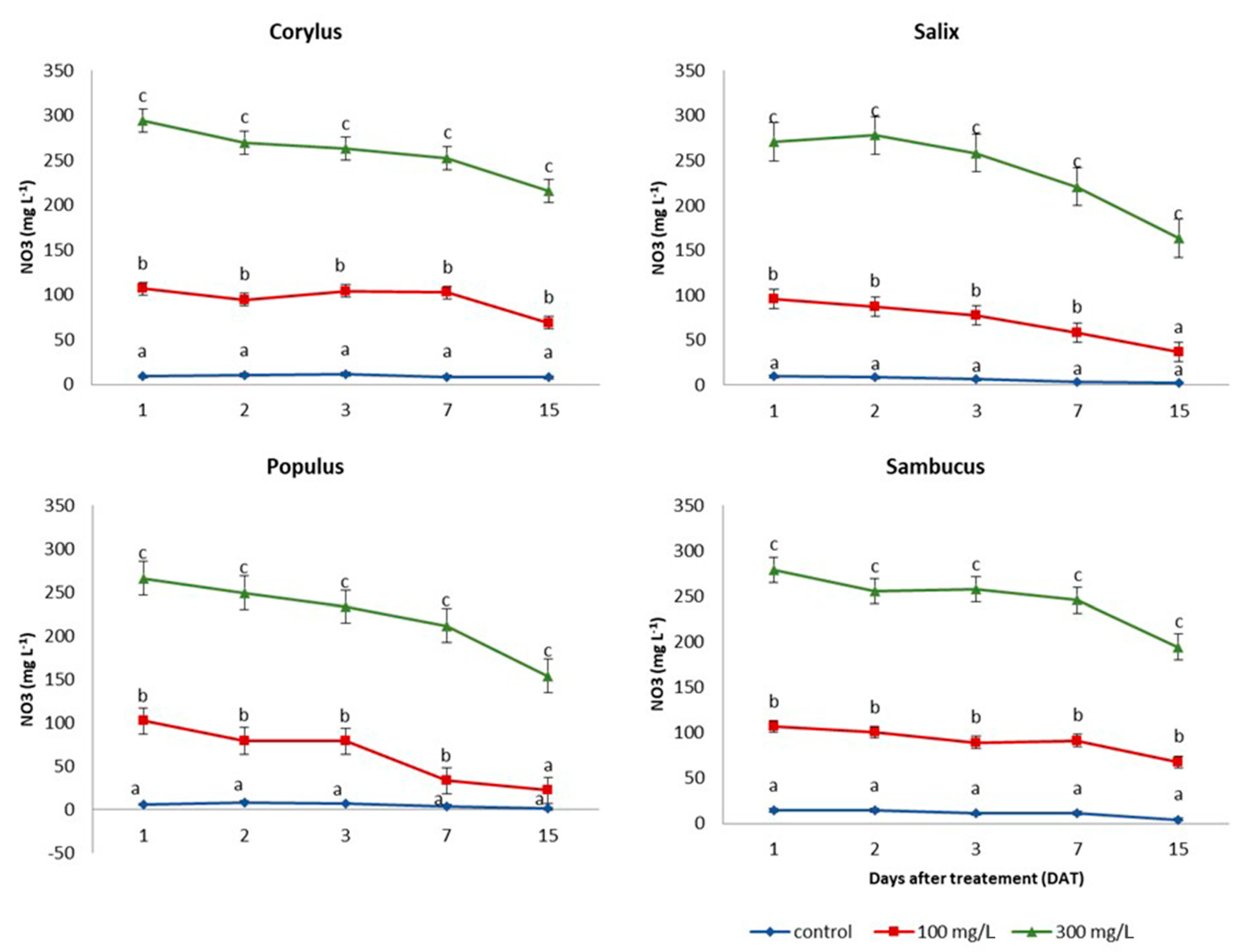
2.3. Nitrate Absorption
2.4. Bioconcentration Factor (BCF)
3. Discussion
4. Materials and Methods
4.1. Plant Material, Hydroponic System, and Nitrate Treatment
4.2. Leaf Net Photosynthesis (Pn) and Chlorophyll Content
4.3. Evapotranspiration and Plant Growth
4.4. Nitrate Absorption
4.5. Bioconcentration Factor (BCF)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartucca, M.L.; Celletti, S.; Mimmo, T.; Cesco, S.; Astolfi, S.; Del Buono, D. Terbuthylazine Interferes with Iron Nutrition in Maize (Zea Mays) Plants. Acta Physiol. Plant. 2017, 39, 235. [Google Scholar] [CrossRef]
- Del Buono, D.; Terzano, R.; Panfili, I.; Bartucca, M.L. Phytoremediation and Detoxification of Xenobiotics in Plants: Herbicide-Safeners as a Tool to Improve Plant Efficiency in the Remediation of Polluted Environments. A Mini-Review. Int. J. Phytoremediat. 2020, 22, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tang, X.; Li, Q.; Yang, W.; Jin, F.; Tang, M.; Scholz, M. Review of Ecological Engineering Solutions for Rural Non-Point Source Water Pollution Control in Hubei Province, China. Water Air Soil Pollut. 2013, 224. [Google Scholar] [CrossRef]
- Gomez Isaza, D.F.; Cramp, R.L.; Franklin, C.E. Living in Polluted Waters: A Meta-Analysis of the Effects of Nitrate and Interactions with Other Environmental Stressors on Freshwater Taxa. Environ. Pollut. 2020, 261, 114091. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, D. Can Biostimulants Be Used to Mitigate the Effect of Anthropogenic Climate Change on Agriculture? It Is Time to Respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Regni, L.; Proietti, P. Effects of Nitrogen Foliar Fertilization on the Vegetative and Productive Performance of the Olive Tree and on Oil Quality. Agriculture 2019, 9, 252. [Google Scholar] [CrossRef] [Green Version]
- Tei, F.; De Neve, S.; De Haan, J.; Kristensen, H.L. Nitrogen management of vegetable crops. Agric. Water Manag. 2020, 241, 106316. [Google Scholar] [CrossRef]
- Dodds, W.K.; Smith, V.H. Nitrogen, Phosphorus, and Eutrophication in Streams. Inland Waters 2016, 6, 155–164. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Mimmo, T.; Cesco, S.; Del Buono, D. Nitrate Removal from Polluted Water by Using a Vegetated Floating System. Sci. Total Environ. 2016, 542, 803–808. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, M.; Jones, R.; Brender, J.; de Kok, T.; Weyer, P.; Nolan, B.; Villanueva, C.; van Breda, S. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [Green Version]
- Taneja, P.; Labhasetwar, P.; Nagarnaik, P.; Ensink, J.H.J. The Risk of Cancer as a Result of Elevated Levels of Nitrate in Drinking Water and Vegetables in Central India. J. Water Health 2017, 15, 602–614. [Google Scholar] [CrossRef] [Green Version]
- Weinthal, E.; Vengosh, A.; Marei, A.; Gutierrez, A.; Kloppmann, W. The Water Crisis in the Gaza Strip: Prospects for Resolution. Groundwater 2005, 43, 653–660. [Google Scholar] [CrossRef]
- Usharani, K.; Keerthi, K.V. Nitrate Bioremoval by Phytotechnology Using Utricularia Aurea Collected from Eutrophic Lake of Theerthamkara, Kerala, India. Pollution 2020, 6. [Google Scholar] [CrossRef]
- Pannacci, E.; Del Buono, D.; Bartucca, M.L.; Nasini, L.; Proietti, P.; Tei, F. Herbicide Uptake and Regrowth Ability of Tall Fescue and Orchardgrass in S-Metolachlor-Contaminated Leachates from Sand Pot Experiment. Agriculture 2020, 10, 487. [Google Scholar] [CrossRef]
- Gholipour, M.; Mehrabanjoubani, P.; Abdolzadeh, A.; Raghimi, M.; Seyedkhademi, S.; Karimi, E.; Sadeghipour, H.R. Facilitated Decrease of Anions and Cations in Influent and Effluent of Sewage Treatment Plant by Vetiver Grass (Chrysopogon Zizanioides): The Uptake of Nitrate, Nitrite, Ammonium, and Phosphate. Environ. Sci. Pollut. Res. 2020, 27, 21506–21516. [Google Scholar] [CrossRef]
- Almeida, A.; Ribeiro, C.; Carvalho, F.; Durao, A.; Bugajski, P.; Kurek, K.; Pochwatka, P.; Jóźwiakowski, K. Phytoremediation Potential of Vetiveria Zizanioides and Oryza Sativa to Nitrate and Organic Substance Removal in Vertical Flow Constructed Wetland Systems. Ecol. Eng. 2019, 138, 19–27. [Google Scholar] [CrossRef]
- Shyamala, S.; Arul Manikandan, N.; Pakshirajan, K.; Tang, V.T.; Rene, E.R.; Park, H.-S.; Behera, S.K. Phytoremediation of Nitrate Contaminated Water Using Ornamental Plants. J. Water Supply Res. Technol. AQUA 2019, 68, 731–743. [Google Scholar] [CrossRef]
- Asrari, E.; Avatefinezhad, G. Study of Nitrate Removal from the Water by Using Eichhornia Crassipes. Asian J. Water Environ. Pollut. 2017, 14, 69–74. [Google Scholar] [CrossRef]
- Moore, M.T.; Locke, M.A.; Kröger, R. Using Aquatic Vegetation to Remediate Nitrate, Ammonium, and Soluble Reactive Phosphorus in Simulated Runoff. Chemosphere 2016, 160, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Takayanagi, S.; Takagi, Y.; Hasegawa, H. The Shoot of Ranunculus Nipponicus Var. Submersus, a Submerged Vascular Plant, Can Actively Take up Nitrate from Cool Water. Plant Biotechnol. 2015, 32, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Sundaralingam, T.; Gnanavelrajah, N. Phytoremediation Potential of Selected Plants for Nitrate and Phosphorus from Ground Water. Int. J. Phytoremediat. 2014, 16, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of Contaminated Soils by Heavy Metals and PAHs. A Brief Review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Muro-González, D.A.; Mussali-Galante, P.; Valencia-Cuevas, L.; Flores-Trujillo, K.; Tovar-Sánchez, E. Morphological, Physiological, and Genotoxic Effects of Heavy Metal Bioaccumulation in Prosopis Laevigata Reveal Its Potential for Phytoremediation. Environ. Sci. Pollut. Res. 2020, 27, 40187–40204. [Google Scholar] [CrossRef]
- Covre, W.P.; Pereira, W.V.D.S.; Gonçalves, D.A.M.; Teixeira, O.M.M.; Amarante, C.B.D.; Fernandes, A.R. Phytoremediation Potential of Khaya Ivorensis and Cedrela Fissilis in Copper Contaminated Soil. J. Environ. Manag. 2020, 268. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Liu, L.; Yan, D.; He, Y. Physiological Stress Responses, Mineral Element Uptake and Phytoremediation Potential of Morus Alba L. in Cadmium-Contaminated Soil. Ecotoxicol. Environ. Saf. 2020, 189. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodríguez, V.; García-Gutiérrez, A.; Canales, J.; Cañas, R.A.; Kirby, E.G.; Avila, C.; Cánovas, F.M. Poplar Trees for Phytoremediation of High Levels of Nitrate and Applications in Bioenergy. Plant Biotechnol. J. 2016, 14, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Warsaw, A.L.; Thomas Fernandez, R.; Kort, D.R.; Cregg, B.M.; Rowe, B.; Vandervoort, C. Remediation of Metalaxyl, Trifluralin, and Nitrate from Nursery Runoff Using Container-Grown Woody Ornamentals and Phytoremediation Areas. Ecol. Eng. 2012, 47, 254–263. [Google Scholar] [CrossRef]
- Groffman, P.M.; Gold, A.J.; Addy, K. Nitrous Oxide Production in Riparian Zones and Its Importance to National Emission Inventories. Chemosphere Glob. Chang. Sci. 2000, 2, 291–299. [Google Scholar] [CrossRef]
- Van Den Heuvel, R.N.; Bakker, S.E.; Jetten, M.S.M.; Hefting, M.M. Decreased N2O Reduction by Low Soil PH Causes High N2O Emissions in a Riparian Ecosystem. Geobiology 2011, 9, 294–300. [Google Scholar] [CrossRef]
- Audet, J.; Hoffmann, C.C.; Andersen, P.M.; Baattrup-Pedersen, A.; Johansen, J.R.; Larsen, S.E.; Kjaergaard, C.; Elsgaard, L. Nitrous Oxide Fluxes in Undisturbed Riparian Wetlands Located in Agricultural Catchments: Emission, Uptake and Controlling Factors. Soil Biol. Biochem. 2014, 68, 291–299. [Google Scholar] [CrossRef]
- Wang, S.; Pi, Y.; Jiang, Y.; Pan, H.; Wang, X.; Wang, X.; Zhou, J.; Zhu, G. Nitrate Reduction in the Reed Rhizosphere of a Riparian Zone: From Functional Genes to Activity and Contribution. Environ. Res. 2020, 180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.P.; Ma, B.L.; Hu, Y.G.; Liu, J.H. Leaf Photosynthesis, Biomass Production and Water and Nitrogen Use Efficiencies of Two Contrasting Naked vs. Hulled Oat Genotypes Subjected to Water and Nitrogen Stresses. J. Plant Nutr. 2011, 34, 2139–2157. [Google Scholar] [CrossRef]
- Liu, G.; Du, Q.; Li, J. Interactive Effects of Nitrate-Ammonium Ratios and Temperatures on Growth, Photosynthesis, and Nitrogen Metabolism of Tomato Seedlings. Sci. Hortic. 2017, 214, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Piwpuan, N.; Zhai, X.; Brix, H. Nitrogen Nutrition of Cyperus Laevigatus and Phormium Tenax: Effects of Ammonium versus Nitrate on Growth, Nitrate Reductase Activity and N Uptake Kinetics. Aquat. Bot. 2013, 106, 42–51. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, R.; Wang, L.; Wu, W.; Chen, Y. Removal of Nitrogen from Wastewater with Perennial Ryegrass/Artificial Aquatic Mats Biofilm Combined System. J. Environ. Sci. 2013, 25, 670–676. [Google Scholar] [CrossRef]
- Bravo, D.; Hill, A.R. The Effect of Chronic High Groundwater Nitrate Loading on Riparian Forest Growth and Plant-Soil Processes. Water Air Soil Pollut. 2012, 223, 73–84. [Google Scholar] [CrossRef]
- Cui, Y.; Ning, S.; Jin, J.; Jiang, S.; Zhou, Y.; Wu, C. Quantitative Lasting Effects of Drought Stress at a Growth Stage on Soybean Evapotranspiration and Aboveground BIOMASS. Water 2020, 13, 18. [Google Scholar] [CrossRef]
- Mousavi, S.; Regni, L.; Bocchini, M.; Mariotti, R.; Cultrera, N.G.M.; Mancuso, S.; Googlani, J.; Chakerolhosseini, M.R.; Guerrero, C.; Albertini, E.; et al. Physiological, epigenetic and genetic regulation in some olive cultivars under salt stress. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Tsubo, M.; Walker, S. A Model of Radiation Interception and Use by a Maize-Bean Intercrop Canopy. Agric. For. Meteorol. 2002, 110, 203–215. [Google Scholar] [CrossRef]
- Chen, R.; Kang, S.; Hao, X.; Li, F.; Du, T.; Qiu, R.; Chen, J. Variations in Tomato Yield and Quality in Relation to Soil Properties and Evapotranspiration under Greenhouse Condition. Sci. Hortic. 2015, 197, 318–328. [Google Scholar] [CrossRef]
- Hill, A.R. Groundwater Nitrate Removal in Riparian Buffer Zones: A Review of Research Progress in the Past 20 Years. Biogeochemistry 2019, 143, 347–369. [Google Scholar] [CrossRef]
- Søvik, A.K.; Mørkved, P.T. Use of Stable Nitrogen Isotope Fractionation to Estimate Denitrification in Small Constructed Wetlands Treating Agricultural Runoff. Sci. Total Environ. 2008, 392, 157–165. [Google Scholar] [CrossRef]
- Clément, J.-C.; Pinay, G.; Marmonier, P. Seasonal Dynamics of Denitrification along Topohydrosequences in Three Different Riparian Wetlands. J. Environ. Qual. 2002, 31, 1025–1037. [Google Scholar] [CrossRef]
- Sabater, S.; Butturini, A.; Clement, J.-C.; Burt, T.; Dowrick, D.; Hefting, M.; Maître, V.; Pinay, G.; Postolache, C.; Rzepecki, M.; et al. Nitrogen Removal by Riparian Buffers along a European Climatic Gradient: Patterns and Factors of Variation. Ecosystems 2003, 6, 20–30. [Google Scholar] [CrossRef]
- Mayer, P.M.; Reynolds, S.K., Jr.; McCutchen, M.D.; Canfield, T.J. Meta-Analysis of Nitrogen Removal in Riparian Buffers. J. Environ. Qual. 2007, 36, 1172–1180. [Google Scholar] [CrossRef]
- King, S.E.; Osmond, D.L.; Smith, J.; Burchell, M.R.; Dukes, M.; Evans, R.O.; Knies, S.; Kunickis, S. Effects of Riparian Buffer Vegetation and Width: A 12-Year Longitudinal Study. J. Environ. Qual. 2016, 45, 1243–1251. [Google Scholar] [CrossRef] [Green Version]
- Valkama, E.; Usva, K.; Saarinen, M.; Uusi-Kämppä, J. A Meta-Analysis on Nitrogen Retention by Buffer Zones. J. Environ. Qual. 2019, 48, 270–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clément, J.-C.; Holmes, R.M.; Peterson, B.J.; Pinay, G. Isotopic Investigation of Denitrification in a Riparian Ecosystem in Western France. J. Appl. Ecol. 2003, 40, 1035–1048. [Google Scholar] [CrossRef]
- Dhondt, K.; Boeckx, P.; Van Cleemput, O.; Hofman, G. Quantifying Nitrate Retention Processes in a Riparian Buffer Zone Using the Natural Abundance of 15N in NO3-. Rapid Commun. Mass Spectrom. 2003, 17, 2597–2604. [Google Scholar] [CrossRef]
- Borin, M.; Bigon, E. Abatement of NO3-N Concentration in Agricultural Waters by Narrow Buffer Strips. Environ. Pollut. 2002, 117, 165–168. [Google Scholar] [CrossRef]
- Glass, A.D.M.; Britto, D.T.; Kaiser, B.N.; Kinghorn, J.R.; Kronzucker, H.J.; Kumar, A.; Okamoto, M.; Rawat, S.; Siddiqi, M.Y.; Unkles, S.E.; et al. The Regulation of Nitrate and Ammonium Transport Systems in Plants. J. Exp. Bot. 2002, 53, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Dix, M.E.; Klopfenstein, N.B.; Zhang, J.W.; Workman, S.W.; Kim, M.S. Potential Use of Populus for Phytoremediation of Environmental Pollution in Riparian Zones. In Micropropagation, Genetic Engineering, and Molecular Biology of Populus; RM-GTR-297; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1997. [Google Scholar]
- Zhang, X.; Zhang, Y.; Shi, P.; Bi, Z.; Shan, Z.; Ren, L. The Deep Challenge of Nitrate Pollution in River Water of China. Sci. Total Environ. 2021, 770, 144674. [Google Scholar] [CrossRef]
| 1 DAT µmol CO2 m−2 s−1 | 7 DAT µmol CO2 m−2 s−1 | 15 DAT µmol CO2 m−2 s−1 | |
|---|---|---|---|
| Corylus-0 | 1.84 ± 0.23 a | 3.12 ± 0.41 a | 2.64 ± 0.34 a |
| Corylus-100 | 2.12 ± 0.19 a | 2.32 ± 0.27 a | 2.23 ± 0.45 a |
| Corylus-300 | 2.05 ± 0.25 a | 2.95 ± 0.34 a | 3.92 ± 0.56 b |
| Salix-0 | 2.61 ± 0.37 a | 2.12 ± 0.38 a | 2.63 ± 0.37 a |
| Salix-100 | 2.72 ± 0.29 a | 3.36 ± 0.54 a | 4.60 ± 0.43 b |
| Salix-300 | 2.36 ± 0.42 a | 2.34 ± 0.59 a | 4.67 ± 0.39 b |
| Sambucus-0 | 2.43 ± 0.41 a | 2.09 ± 0.37 a | 1.90 ± 0.42 a |
| Sambucus-100 | 1.95 ± 0.34 a | 2.48 ± 0.24 a | 2.25 ± 0.51 a |
| Sambucus-300 | 2.05 ± 0.45 a | 1.82 ± 0.21 a | 4.10 ± 0.63 b |
| Populus-0 | 2.45 ± 0.23 a | 2.79 ± 0.38 a | 2.63 ± 0.31 a |
| Populus-100 | 2.39 ± 0.31 a | 4.28 ± 0.57 a | 5.18 ± 0.89 b |
| Populus-300 | 3.30 ± 0.29 a | 3.50 ± 0.34 a | 5.33 ± 0.92 b |
| 1 DAT | 7 DAT | 15 DAT | |
|---|---|---|---|
| Corylus-0 | 25.63 ± 3.74 a | 27.26 ± 3.24 a | 25.78 ± 2.01 a |
| Corylus-100 | 27.14 ± 4.15 a | 26.54 ± 2.21 a | 24.13 ± 1.87 a |
| Corylus-300 | 25.14 ± 3.95 a | 25.64 ± 3.16 a | 29.36 ± 1.41 b |
| Salix-0 | 28.84 ± 2.75a | 31.12 ± 2.77 a | 30.72 ± 1.47 a |
| Salix-100 | 27.56 ± 4.32 a | 38.54 ± 3.24 b | 34.47 ± 2.13 b |
| Salix-300 | 29.25 ± 3.12 a | 37.47 ± 3.12 b | 36.07 ± 3.42 b |
| Sambucus-0 | 26.32 ± 3.13 a | 24.78 ± 4.13 a | 25.47 ± 4.32 a |
| Sambucus-100 | 27.12 ± 4.84 a | 25.63 ± 3.45 a | 26.34 ± 3.15 a |
| Sambucus-300 | 26.83 ± 2.98 a | 27.54 ± 2.23 a | 30.27 ± 2.76 b |
| Populus-0 | 27.74 ± 5.23 a | 26.15 ± 2.13 a | 28.86 ± 2.57 a |
| Populus-100 | 28.10 ± 3.21 a | 31.23 ± 2.21 b | 34.00 ± 1.94 b |
| Populus-300 | 26.65 ± 2.72 a | 32.11 ± 2.05 b | 36.07 ± 2.34 b |
| DW Roots | DW Stem + Lateral Shoots | DW Leaves | DW Total | |
|---|---|---|---|---|
| (g) | (g) | (g) | (g) | |
| Corylus-0 | 1.97 ± 0.43 a | 1.99 ± 0.43 a | 1.22 ± 0.17 a | 5.18 ± 0.54 a |
| Corylus-100 | 1.84 ± 0.59 a | 2.00 ± 0.51 a | 1.31 ± 0.18 a | 5.15 ± 0.61 a |
| Corylus-300 | 1.09 ± 0.31 a | 2.56 ± 0.32 a | 0.86 ± 0.12 a | 4.51 ± 0.53 a |
| Salix-0 | 0.37 ± 0.15 a | 2.60 ± 0.32 a | 0.57 ± 0.11 a | 3.54 ± 0.38 a |
| Salix-100 | 0.41 ± 0.18 a | 2.80 ± 0.27 a | 0.79 ± 0.13 a | 4.00 ± 0.55 a |
| Salix-300 | 0.37 ± 0.13 a | 4.46 ± 0.72 b | 1.05 ± 0.24 a | 5.88 ± 0.43 b |
| Sambucus-0 | 0.43 ± 0.17 a | 2.12 ± 0.43 a | 0.53 ± 0.14 a | 3.08 ± 0.32 a |
| Sambucus-100 | 0.31 ± 0.12 a | 2.16 ± 0.37 a | 0.53 ± 0.17 a | 3.00 ± 0.27 a |
| Sambucus-300 | 0.42 ± 0.17 a | 1.43 ± 0.22 a | 0.44 ± 0.12 a | 2.29 ± 0.32 a |
| Populus-0 | 0.28 ± 0.14 a | 3.42 ± 0.31 a | 0.54 ± 0.10 a | 4.24 ± 0.45 a |
| Populus-100 | 0.47 ± 0.18 a | 3.23 ± 0.37 a | 1.72 ± 0.23 b | 5.42 ± 0.33 b |
| Populus-300 | 0.41 ± 0.15 a | 4.68 ± 0.43 b | 1.67 ± 0.31 b | 6.76 ± 0.41 b |
| Evapotranspirated H2O | |
|---|---|
| (mL) | |
| Corylus-0 | 2.550 ±545 a |
| Corylus-100 | 2.492 ± 314 a |
| Corylus -300 | 2.104 ± 274 a |
| Salix-0 | 3.296 ± 412 a |
| Salix-100 | 4.494 ± 719 b |
| Salix-300 | 4.730 ± 516 b |
| Sambucus-0 | 1.544 ± 187 a |
| Sambucus-100 | 1.930 ± 210 a |
| Sambucus-300 | 1.735 ± 197 a |
| Populus-0 | 2.471 ± 214 a |
| Populus-100 | 3.758 ± 423 b |
| Populus-300 | 3.984 ± 456 b |
| BCF | |
|---|---|
| Corylus-0 | 0.30 ± 0.13 |
| Corylus-100 | 0.43 ± 0.11 |
| Corylus-300 | 0.43 ± 0.12 |
| Salix-0 | 2.61 ± 1.31 |
| Salix-100 | 1.56 ± 0.18 |
| Salix-300 | 0.64 ± 0.14 |
| Sambucus-0 | 1.52 ± 0.57 |
| Sambucus-100 | 0.43 ± 0.15 |
| Sambucus-300 | 0.43 ± 0.16 |
| Populus-0 | 6.30 ± 2.45 |
| Populus-100 | 2.43 ± 0.31 |
| Populus-300 | 0.72 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regni, L.; Bartucca, M.L.; Pannacci, E.; Tei, F.; Del Buono, D.; Proietti, P. Phytodepuration of Nitrate Contaminated Water Using Four Different Tree Species. Plants 2021, 10, 515. https://doi.org/10.3390/plants10030515
Regni L, Bartucca ML, Pannacci E, Tei F, Del Buono D, Proietti P. Phytodepuration of Nitrate Contaminated Water Using Four Different Tree Species. Plants. 2021; 10(3):515. https://doi.org/10.3390/plants10030515
Chicago/Turabian StyleRegni, Luca, Maria Luce Bartucca, Euro Pannacci, Francesco Tei, Daniele Del Buono, and Primo Proietti. 2021. "Phytodepuration of Nitrate Contaminated Water Using Four Different Tree Species" Plants 10, no. 3: 515. https://doi.org/10.3390/plants10030515
APA StyleRegni, L., Bartucca, M. L., Pannacci, E., Tei, F., Del Buono, D., & Proietti, P. (2021). Phytodepuration of Nitrate Contaminated Water Using Four Different Tree Species. Plants, 10(3), 515. https://doi.org/10.3390/plants10030515











