QTL Mapping for Gummy Stem Blight Resistance in Watermelon (Citrullus spp.)
Abstract
1. Introduction
2. Results
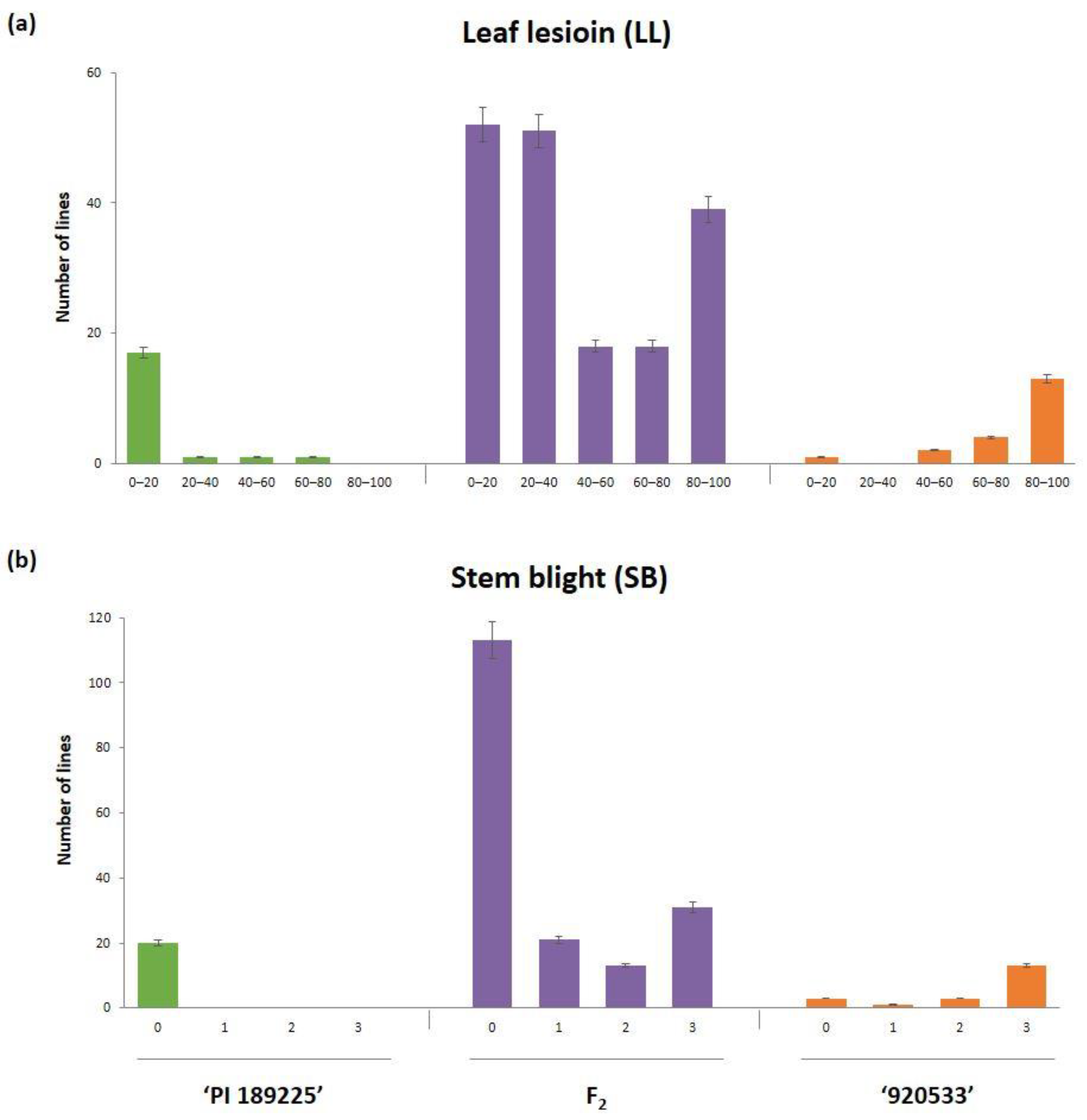
2.1. Resistance to GSB in Parents and an F2 Population
2.2. SNP Genotyping Using Fluidigm® SNP TypeTM Assays
2.3. Identification of SNPs Using NGS
2.4. Development of HRM Markers Using Identified SNPs
2.5. Construction of a Watermelon Linkage Map
2.6. Identification of QTLs Conferring Resistance to GSB in Watermelon
2.7. Validation of Flanking Markers Using Watermelon Accessions and Cultivars
2.8. Identification of Candidate Genes for GSB Resistance
3. Discussion
4. Materials and Methods
4.1. Plant Materials and DNA Extraction
4.2. Pathogen Inoculation and Disease Assessment
4.3. SNP Genotyping Using Fluidigm® SNP TypeTM Assays
4.4. Next-Generation Re-Sequencing and SNP Detection
4.5. HRM Primer Design and Genotyping
4.6. Genotyping Using Previously Reported KASPTM Markers
4.7. Construction of Linkage Map and QTL Analysis
4.8. Validation of Flanking SNP Markers and Identification of Candidate Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BFB | Bacterial Fruit Blotch |
| BLASTN | Basic Local Alignment Search Tool Nucleotide |
| BSA | Bulked Segregant Analysis |
| CAPS | Cleaved Amplified Polymorphic Sequence |
| CIM | Composite interval mapping |
| DAI | Days After Inoculation |
| dCAPS | derived Cleaved Amplified Polymorphic Sequence |
| DI | Disease Index |
| GBS | Genotyping-by-Sequencing |
| GO | Gene Ontology |
| GSB | Gummy Stem Blight |
| HRM | High Resolution Melting |
| HT | High-throughput |
| KASP | Kompetitive Allele Specific PCR |
| LG | Linkage Group |
| LL | Leaf Lesion |
| LOD | Logarithm of Odds |
| LRR | Leucine-Rich Repeat |
| MAS | Marker-assisted Selection |
| NGS | Next Generation Sequencing |
| PRSV-W | Papaya Ringspot Virus-Watermelon (Strain) |
| QTL | Quantitative trait locus |
| RAPD | Random Amplified Polymorphic DNA |
| RIL | Recombinant Inbred Line |
| RLK | Receptor-Like Kinase |
| SB | Stem Blight |
| SCAR | Sequence Characterized Amplified Region |
| SNP | Single Nucleotide Polymorphism |
| SSR | Simple Sequence Repeat |
References
- Food and Agriculture Organization. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/ (accessed on 10 October 2018).
- Soteriou, G.; Kyriacou, M.; Siomos, A.; Gerasopoulos, D. Evolution of watermelon fruit physicochemical and phytochemical composition during ripening as affected by grafting. Food Chem. 2014, 165, 282–289. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, S.H.; Hwang, S.J.; Kim, G.H.; Eun, J.-B. Physicochemical characteristics and functional com-ponents of Mudeungsan watermelon and the other cultivars from Korea. Korean J. Food Sci. Technol. 2013, 45, 345–349. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, K.S.; Choi, Y.H.; Kim, J.-C.; Choi, G.J. Development of an efficient screening system for re-sistance of watermelon plants to Didymella bryoniae. Res. Plant. Dis. 2016, 22, 72–112. [Google Scholar] [CrossRef]
- Shim, C.K.; Seo, I.K.; Jee, H.J.; Kim, H.K. Genetic diversity of Didymella bryoniae for RAPD profiles substantiated by SCAR marker in Korea. Plant. Pathol. J. 2006, 22, 36–45. [Google Scholar] [CrossRef][Green Version]
- Skarshaug, A.J. Centrum development in Didymella bryoniae. Am. J. Bot. 1981, 68, 1096–1103. [Google Scholar] [CrossRef]
- Maynard, D.N.; Hopkins, D.L. Watermelon Fruit Disorders. HortTechnology 1999, 9, 155–161. [Google Scholar] [CrossRef]
- Kwon, M.K.; Hong, H.J.; Sung, K.Y.; Cho, B.H.; Kim, K.L. Standardization of a mass-production technique for pycnidiospores of Didymella bryoniae, gummy stem blight fungus of Cucurbits. Korea J. Plant. Pathol. 1997, 13, 105–112. [Google Scholar]
- Dos Santos, G.R.; Sousa, S.C.R.; Juliatti, F.C.; Rodrigues, A.C.; Dalcin, M.S.; Bonifácio, A. Control of gummy stem blight in watermelon through different management systems. Biosci. J. 2016, 32, 371–377. [Google Scholar] [CrossRef]
- Wolukau, J.N.; Zhou, X.-H.; Li, Y.; Zhang, Y.-B.; Chen, J.-F. Resistance to gummy stem blight in melon (Cucumis melo L.) germplasm and inheritance of resistance from plant introductions 157076, 420145, and 323498. HortScience 2007, 42, 215–221. [Google Scholar] [CrossRef]
- Lou, L.; Wang, H.; Qian, C.; Liu, J.; Bai, Y.; Chen, J. Genetic mapping of gummy stem blight (Didymella bryoniae) resistance genes in Cucumis sativus-hystrix introgression lines. Euphytica 2013, 192, 359–369. [Google Scholar] [CrossRef]
- Norton, J.D.; Boyan, G.; Smith, D.A.; Abrahams, B.R. ‘AU-Sweet Scarlet’ watermelon. HortScience 1995, 30, 393–394. [Google Scholar] [CrossRef]
- Sowell, G. An additional source of resistance to gummy stem blight in watermelon. Plant. Dis. Rep. 1975, 59, 413–415. [Google Scholar]
- Sowell, G.; Pointer, G.R. Gummy stem blight resistance introduced watermelons. Plant. Dis. Rep. 1962, 46, 883–885. [Google Scholar]
- Gusmini, G.; Song, R.; Wehner, T.C. New sources of resistance to gummy stem blight in watermelon. Crop. Sci. 2005, 45, 582–588. [Google Scholar] [CrossRef]
- Norton, J.D. Inheritance of resistance to gummy stem blight caused by Didymella bryoniae in watermelon. HortScience 1979, 14, 630–632. [Google Scholar]
- Gusmini, G.; Rivera-Burgos, L.A.; Wehner, T.C. Inheritance of resistance to gummy stem blight in watermelon. HortScience 2017, 52, 1477–1482. [Google Scholar] [CrossRef]
- Branham, S.E.; Levi, A.; Katawczik, M.L.; Wechter, W.P. QTL mapping of resistance to bacterial fruit blotch in Citrullus amarus. Theor. Appl. Genet. 2019, 132, 1463–1471. [Google Scholar] [CrossRef]
- Jang, Y.J.; Seo, M.; Hersh, C.P.; Rhee, S.-J.; Kim, Y.; Lee, G.P. An evolutionarily conserved non-synonymous SNP in a leucine-rich repeat domain determines anthracnose resistance in watermelon. Theor. Appl. Genet. 2018, 132, 473–488. [Google Scholar] [CrossRef]
- Fall, L.A.; Clevenger, J.; McGregor, C. Assay development and marker validation for marker assisted selection of Fusarium oxysporum f. sp. niveum race 1 in watermelon. Mol. Breed. 2018, 38, 130. [Google Scholar] [CrossRef]
- Branham, S.E.; Wechter, W.P.; Lambel, S.; Massey, L.; Ma, M.; Fauve, J.; Farnham, M.W.; Levi, A. QTL-seq and marker development for resistance to Fusarium oxysporum f. sp. niveum race 1 in cultivated watermelon. Mol. Breed. 2018, 38, 139. [Google Scholar] [CrossRef]
- Branham, S.E.; Wechter, W.P.; Ling, K.-S.; Chanda, B.; Massey, L.; Zhao, G.; Guner, N.; Bello, M.; Kabelka, E.; Fei, Z.; et al. QTL mapping of resistance to Fusarium oxysporum f. sp. niveum race 2 and Papaya ringspot virus in Citrullus amarus. Theor. Appl. Genet. 2020, 133, 677–687. [Google Scholar] [CrossRef]
- Branham, S.E.; Levi, A.; Farnham, M.W.; Wechter, W.P. A GBS-SNP-based linkage map and quantitative trait loci (QTL) associated with resistance to Fusarium oxysporum f. sp. niveum race 2 identified in Citrullus lanatus var. citroides. Theor. Appl. Genet. 2017, 130, 319–330. [Google Scholar] [CrossRef]
- Liu, S.; Shi, Y.; Miao, H.; Wang, M.; Li, B.; Gu, X.; Zhang, S. Genetic analysis and QTL mapping of resistance to gummy stem blight in Cucumis sativus seedling stage. Plant. Dis. 2017, 101, 1145–1152. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.; Miao, H.; Shi, Y.; Wang, M.; Wang, Y.; Li, B.; Gu, X. Inheritance and QTL mapping of resistance to gummy stem blight in cucumber stem. Mol. Breed. 2017, 37, 49. [Google Scholar] [CrossRef]
- Hu, Z.; Deng, G.; Mou, H.; Xu, Y.; Chen, L.; Yang, J.; Zhang, M. A re-sequencing-based ultra-dense genetic map reveals a gummy stem blight resistance-associated gene in Cucumis melo. DNA Res. 2018, 25, 1–10. [Google Scholar] [CrossRef]
- Hassan, Z.; Rahim, A.; Natarajan, S.; Robin, A.H.K.; Kim, H.-T.; Park, J.-I.; Nou, I.-S. Gummy stem blight resistance in melon: Inheritance pattern and development of molecular markers. Int. J. Mol. Sci. 2018, 19, 2914. [Google Scholar] [CrossRef]
- Ren, R.; Xu, J.; Zhang, M.; Liu, G.; Yao, X.; Zhu, L.; Hou, Q. Identification and molecular mapping of a gummy stem blight resistance gene in wild watermelon (Citrullus amarus) Germplasm PI 189225. Plant. Dis. 2020, 104, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Gimode, W.; Bao, K.; Fei, Z.; McGregor, C. QTL associated with gummy stem blight resistance in watermelon. Theor. Appl. Genet. 2021, 134, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Hwang, J.-H.; Han, D.-Y.; Park, M.; Kim, S.; Choi, D.; Kim, Y.; Lee, G.P.; Kim, S.-T.; Park, Y.-H. Major quantitative trait loci and putative candidate genes for powdery mildew resistance and fruit-related traits revealed by an intraspecific genetic map for watermelon (Citrullus lanatus var. lanatus). PLoS ONE 2015, 10, e0145665. [Google Scholar] [CrossRef] [PubMed]
- Han, B.K.; Rhee, S.J.; Jang, Y.J.; Sim, T.Y.; Kim, Y.J.; Park, T.S.; Lee, G.P. Identification of a causal pathogen of watermelon powdery mildew in Korea and development of a genetic linkage marker for resistance in water-melon (Citrullus lanatus). Korean J. Hortic. Sci. 2016, 34, 912–923. [Google Scholar]
- Ling, K.-S.; Harris, K.R.; Meyer, J.D.F.; Levi, A.; Guner, N.; Wehner, T.C.; Bendahmane, A.; Havey, M.J. Non-synonymous single nucleotide polymorphisms in the watermelon eIF4E gene are closely associated with resistance to Zucchini yellow mosaic virus. Theor. Appl. Genet. 2009, 120, 191–200. [Google Scholar] [CrossRef]
- Wittwer, C.T.; Reed, G.H.; Gundry, C.N.; Vandersteen, J.G.; Pryor, R.J. High-resolution genotyping by amplicon melting analysis using LCGreen. Clin. Chem. 2003, 49, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-W.; Kim, K.-T.; Kang, S.-C.; Yang, H.-B. Rapid and practical molecular marker development for rind traits in watermelon. Hortic. Environ. Biotechnol. 2016, 57, 385–391. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, J.; Hong, J.P.; Kim, D.-S.; Kim, M.; Huh, Y.-C.; Back, C.-G.; Lee, J.; Lee, H.-E. Development of HRM markers based on SNPs identified from next generation resequencing of susceptible and resistant parents to gummy stem blight in watermelon. Korean J. Breed. Sci. 2018, 50, 424–433. [Google Scholar] [CrossRef]
- Lee, H.-E.; Hong, J.P.; Suh, H.Y.; Huh, Y.-C.; Ahn, Y.-K.; Kim, J.; Kim, D.-S. Survey of SNP markers based on genome related to gummy stem blight resistance in watermelon. J. Agric. Sci. Chungbuk Natl. Univ. 2015, 31, 107–113. [Google Scholar]
- Hassan, Z.; Rahim, A.; Jung, H.-J.; Park, J.-I.; Kim, H.-T.; Nou, I.-S. Genome-wide characterization of NBS-encoding genes in watermelon and their potential association with gummy stem blight resistance. Int. J. Mol. Sci. 2019, 20, 902. [Google Scholar] [CrossRef]
- Zuniga, T.L.; Jantz, J.P.; Zitter, T.A.; Jahn, M.K. Monogenic dominant resistance to gummy stem blight in two melon (Cucumis melo) accessions. Plant. Dis. 1999, 83, 1105–1107. [Google Scholar] [CrossRef]
- Cheng, Y.; Luan, F.; Wang, X.; Gao, P.; Zhu, Z.; Liu, S.; Baloch, A.M.; Zhang, Y. Construction of a genetic linkage map of watermelon (Citrullus lanatus) using CAPS and SSR markers and QTL analysis for fruit quality traits. Sci. Hortic. 2016, 202, 25–31. [Google Scholar] [CrossRef]
- Sandlin, K.; Prothro, J.; Heesacker, A.; Khalilian, N.; Okashah, R.; Xiang, W.; Bachlava, E.; Caldwell, D.G.; Taylor, C.A.; Seymour, D.K.; et al. Comparative mapping in watermelon [Citrullus lanatus (Thunb.) Matsum. et Nakai]. Theor. Appl. Genet. 2012, 125, 1603–1618. [Google Scholar] [CrossRef]
- Esteras, C.; Gomez, P.; Monforte, A.J.; Blanca, J.; Vicente-Dolera, N.; Roig, C.; Nuez, F.; Pico, B. High-throughput SNP genotyping in Cucurbita pepo for map construction and quantitative trait loci mapping. BMC Genom. 2012, 13, 80. [Google Scholar] [CrossRef]
- Deleu, W.; Esteras, C.; Roig, C.; González-To, M.; Fernandez-Silva, I.; Gonzalez-Ibeas, D.; Blanca, J.; Aranda, M.A.; Arús, P.; Nuez, F.; et al. A set of EST-SNPs for map saturation and cultivar identification in melon. BMC Plant. Biol. 2009, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Guo, S.; Zhang, J.; Sun, H.; Salse, J.; Lucas, W.J.; Zhang, H.; Zheng, Y.; Mao, L.; Ren, Y.; Wang, Z.; et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 2013, 45, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- He, C.; Holme, J.; Anthony, J. SNP genotyping: The KASP Assay. In Crop Breeding; Fleury, D., Whitford, R., Eds.; Human Press: New York, NY, USA, 2014; pp. 75–86. [Google Scholar]
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1943, 12, 172–175. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef]
- Ooijen, J.; Kyazma, B. MapQTL 6; Software for the Mapping of Quantitative Trait Loci in Experimental Populations of Dihaploid Species; Kyazma: Wageningen, The Netherlands, 2009. [Google Scholar]
- de Mendiburu, F. Package ‘Agricolae’. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 7 September 2020).



| Linkage Group | Number of Markers | Map Length (cM) | Marker Density (cM/Marker) | Marker Types | |||
|---|---|---|---|---|---|---|---|
| Fluidigm® Markers | Previously Reported HRM Markers | Newly Designed HRM Markers | Previously Reported KASP™ Markers | ||||
| Chr.1a | 6 | 59.1 | 9.85 | 1 | 1 | 4 | - |
| Chr.1b | 4 | 10.0 | 2.5 | 3 | - | 1 | - |
| Chr.1c | 7 | 39.2 | 5.6 | 3 | 1 | 3 | - |
| Chr.2a | 11 | 57.8 | 5.25 | 10 | - | 1 | - |
| Chr.2b | 7 | 34.8 | 4.97 | 6 | - | 1 | - |
| Chr.3a | 5 | 21.5 | 4.3 | 3 | 1 | 1 | - |
| Chr.3b | 5 | 30.2 | 6.04 | 4 | - | 1 | - |
| Chr.4 | 10 | 81.4 | 8.14 | 4 | - | 6 | - |
| Chr.5a | 5 | 44.0 | 8.8 | 4 | - | 1 | - |
| Chr.5b | 6 | 44.7 | 7.45 | 6 | - | - | - |
| Chr.6 | 22 | 97.0 | 4.41 | 5 | - | 17 | - |
| Chr.7 | 20 | 98.8 | 4.94 | 12 | - | 8 | - |
| Chr.8 | 38 | 131.7 | 3.47 | 10 | - | 21 | 7 |
| Chr.9 | 18 | 134.2 | 7.46 | 15 | - | 3 | - |
| Chr.10 | 14 | 89.5 | 6.39 | 11 | 1 | 2 | - |
| Chr.11 | 10 | 96.3 | 9.63 | 7 | - | 3 | - |
| Unmapped | 23 | - | - | 9 | - | 11 | 3 |
| Total | 211 | 1070.2 | 5.69 | 113 | 4 | 84 | 10 |
| Trait | QTL | Chr. | Position 1 (cM) | Marker Interval | Location (bp) | LOD 2 | R2 (%) 3 | Gene Effect | |
|---|---|---|---|---|---|---|---|---|---|
| Additive Effect | Dominance Effect | ||||||||
| Leaf lesion | qLL8.1 | 8 | 85.376 | chr8_WGRS240–chr8_WGRS(3)185 | 20,663,001–21,535,005 | 4.28 | 10.5 | 9.21 | −15.04 |
| Stem blight | qSB6.1 | 6 | 57.139 | chr6_WGRS(3)089–chr6_WGRS(3)092 | 7,533,583–7,625,669 | 3.96 | 9.7 | 0.07 | −0.72 |
| qSB8.1 | 8 | 85.263 | chr8_WGRS240–chr8_WGRS(3)185 | 20,663,001–21,535,005 | 4.02 | 10.0 | 0.28 | −0.64 | |
| Marker Name | Chromosome | Location (bp) | Allele | Fluorescent Primer | Sequence (5′-3′) |
|---|---|---|---|---|---|
| KASP_WGRS(3)089 | 6 | 7,533,583 | T | Allele-specific 1 (FAM) | GAATTCAAACTGACATCCAGCACCA |
| C | Allele-specific 2 (HEX) | AATTCAAACTGACATCCAGCACCG | |||
| - | Common | GTAACGACGGTCAATCTGTAACGACAA | |||
| KASP_WGRS(3)092 | 6 | 7,625,669 | A | Allele-specific 1 (FAM) | GAGGCAACAGAAGAAGAAGGCAT |
| G | Allele-specific 2 (HEX) | GAGGCAACAGAAGAAGAAGGCAC | |||
| - | Common | GAGGCTTATCTTACGTTTCTAGTTCGTTT | |||
| KASP_WGRS240 | 8 | 20,663,001 | A | Allele-specific 1 (FAM) | TGATGAGTAAGAAAAAGAGATTAAAAGCAAAA |
| G | Allele-specific 2 (HEX) | GATGAGTAAGAAAAAGAGATTAAAAGCAAAG | |||
| - | Common | GACTCATTTCAAAAGATTTTCTCTGAGGTA | |||
| KASP_WGRS(3)185 | 8 | 21,535,005 | C | Allele-specific 1 (FAM) | ATATGATTCATCTTGGCGGAAACAATG |
| A | Allele-specific 2 (HEX) | AAAATATGATTCATCTTGGCGGAAACAATT | |||
| - | Common | TCCAAACCATCATCATCGCTATGACTTA |
| Watermelon Accessions/Commercial Cultivars | Phenotype | Genotype | |||||
|---|---|---|---|---|---|---|---|
| Common Name | Origin | Scientific Name 1 | Disease Index (DI) 2 | KASP_WGRS240 (Chr.8) | KASP_WGRS(3)185 (Chr.8) | KASP_WGRS(3)089 (Chr.6) | KASP_WGRS(3)092 (Chr.6) |
| PI 189225 | Congo | CA | R | R | R | R | R |
| ‘920533’ | South Korea | CL | S | S | S | S | S |
| PI 500335 | Zambia | CA | R | R | R | R | R |
| PI 482283 | Zimbabwe | CA | R | R | R | R | R |
| PI 164248 | Liberia | CM | R | S | S | R | - |
| PI 500334 | Zambia | CA | R | R | R | R | R |
| PI 244019 | South Africa | CA | R | R | R | R | R |
| PI 482315 | Zimbabwe | CA | R | R | R | R | R |
| PI 379243 | North Macedonia | CA | R | R | R | R | R |
| PI 279461 | Japan | CL | S | S | S | R | R |
| PI 505590 | Zambia | CL | S | S | S | S | S |
| ‘Fair Fax’ | USA | CL | S | S | S | R | R |
| ‘Au-Jubilant’ | USA | CL | S | S | S | S | S |
| ‘Au-Producer’ | USA | CL | S | S | S | S | S |
| ‘Crimson Sweet’ | USA | CL | S | S | S | R | R |
| ‘Seupidpeulleoskkul‘ | South Korea | CL | S | S | S | H | H |
| ‘Heugho‘ | South Korea | CL | S | S | S | - | S |
| ‘Norangmanidara‘ | South Korea | CL | S | S | S | S | S |
| ‘Dalgona‘ | South Korea | CL | S | S | S | H | H |
| ‘Urikkul‘ | South Korea | CL | S | S | S | S | S |
| ‘Orenjiking‘ | South Korea | CL | S | S | S | H | H |
| ‘Santakkul‘ | South Korea | CL | S | S | S | H | H |
| ‘Charleston Gray’ | USA | CL | S | S | S | R | R |
| ‘Seotaeja‘ | South Korea | CL | S | S | S | S | S |
| Gene ID | Location | Annotation | SNP (‘920533’/‘PI 189225’) | ||
|---|---|---|---|---|---|
| Chr. | Position (bp, Start–End) | Nucleotide | Position (bp) | ||
| Cla022133 | 8 | 20,710,211–20,711,725 | Receptor-like protein kinase | A/G | 20,710,460 |
| A/C | 20,710,552 | ||||
| A/G | 20,710,776 | ||||
| C/A | 20,710,810 | ||||
| A/G | 20,710,904 | ||||
| G/A | 20,711,132 | ||||
| Cla022184 | 8 | 21,315,878–21,318,792 | Receptor kinase | G/T | 21,316,051 |
| G/A | 21,316,141 | ||||
| T/C | 21,316,345 | ||||
| T/C | 21,316,486 | ||||
| G/A | 21,317,089 | ||||
| C/T | 21,317,371 | ||||
| C/T | 21,318,065 | ||||
| C/T | 21,318,362 | ||||
| T/C | 21,318,714 | ||||
| Cla022195 | 8 | 21,421,183–21,423,645 | Receptor kinase | G/T | 21,421,391 |
| G/A | 21,421,694 | ||||
| G/T | 21,421,865 | ||||
| A/G | 21,421,905 | ||||
| T/C | 21,422,026 | ||||
| C/T | 21,422,027 | ||||
| A/G | 21,422,704 | ||||
| T/C | 21,422,920 | ||||
| G/T | 21,423,010 | ||||
| G/A | 21,423,031 | ||||
| C/T | 21,423,285 | ||||
| G/A | 21,423,367 | ||||
| A/G | 21,423,644 | ||||
| Cla022196 | 8 | 21,423,671–21,424,567 | Leucine-rich repeat receptor-like protein kinase | T/C | 21,423,751 |
| T/C | 21,423,821 | ||||
| C/T | 21,423,899 | ||||
| C/T | 21,423,995 | ||||
| C/T | 21,424,071 | ||||
| A/C | 21,424,242 | ||||
| A/G | 21,424,352 | ||||
| G/A | 21,424,355 | ||||
| G/T | 21,424,503 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.S.; Kim, D.-S.; Kim, S.G.; Huh, Y.-C.; Back, C.-G.; Lee, Y.-R.; Siddique, M.I.; Han, K.; Lee, H.-E.; Lee, J. QTL Mapping for Gummy Stem Blight Resistance in Watermelon (Citrullus spp.). Plants 2021, 10, 500. https://doi.org/10.3390/plants10030500
Lee ES, Kim D-S, Kim SG, Huh Y-C, Back C-G, Lee Y-R, Siddique MI, Han K, Lee H-E, Lee J. QTL Mapping for Gummy Stem Blight Resistance in Watermelon (Citrullus spp.). Plants. 2021; 10(3):500. https://doi.org/10.3390/plants10030500
Chicago/Turabian StyleLee, Eun Su, Do-Sun Kim, Sang Gyu Kim, Yun-Chan Huh, Chang-Gi Back, Ye-Rin Lee, Muhammad Irfan Siddique, Koeun Han, Hye-Eun Lee, and Jundae Lee. 2021. "QTL Mapping for Gummy Stem Blight Resistance in Watermelon (Citrullus spp.)" Plants 10, no. 3: 500. https://doi.org/10.3390/plants10030500
APA StyleLee, E. S., Kim, D.-S., Kim, S. G., Huh, Y.-C., Back, C.-G., Lee, Y.-R., Siddique, M. I., Han, K., Lee, H.-E., & Lee, J. (2021). QTL Mapping for Gummy Stem Blight Resistance in Watermelon (Citrullus spp.). Plants, 10(3), 500. https://doi.org/10.3390/plants10030500









