Allelopathic Effect of Serphidium kaschgaricum (Krasch.) Poljak. Volatiles on Selected Species
Abstract
1. Introduction
2. Results
2.1. Allelopathic Potential of VOCs
2.2. Composition of the Volatile Oils
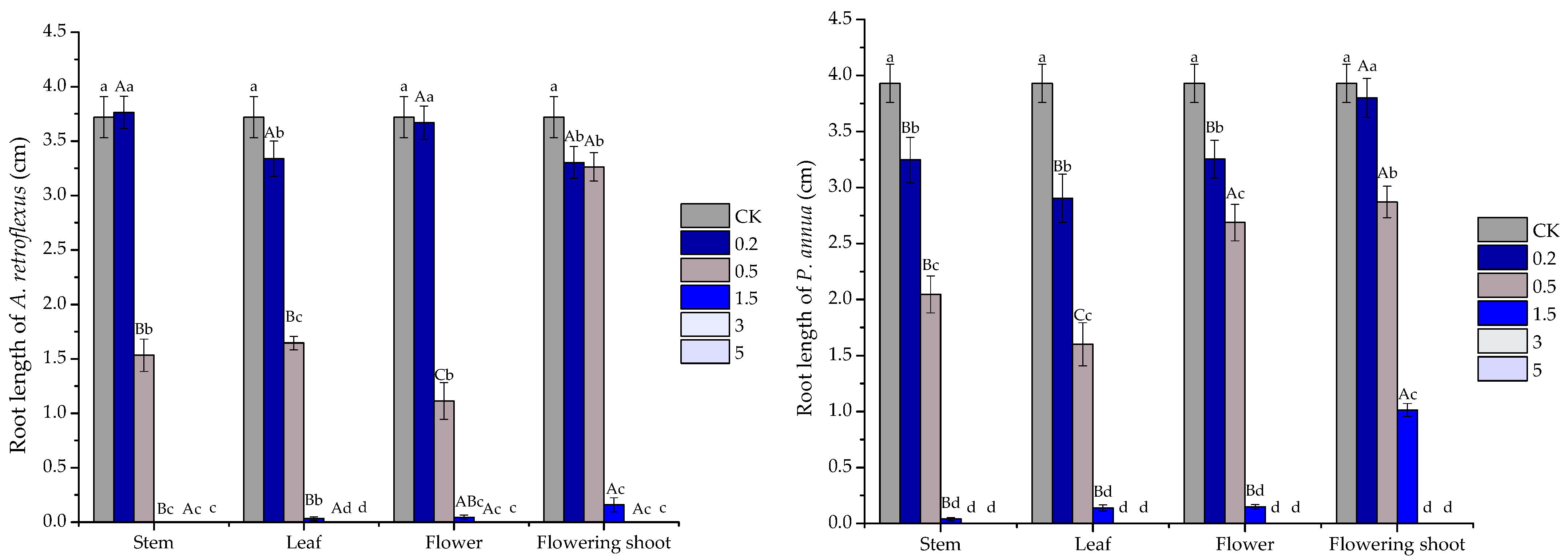
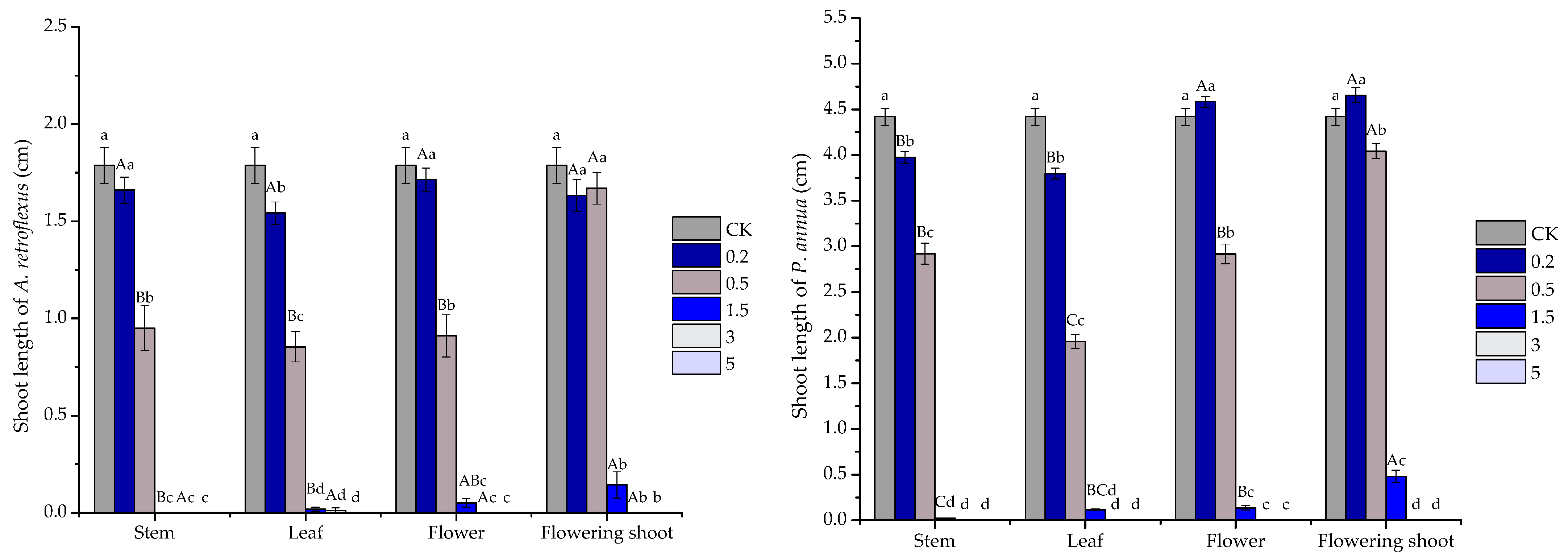
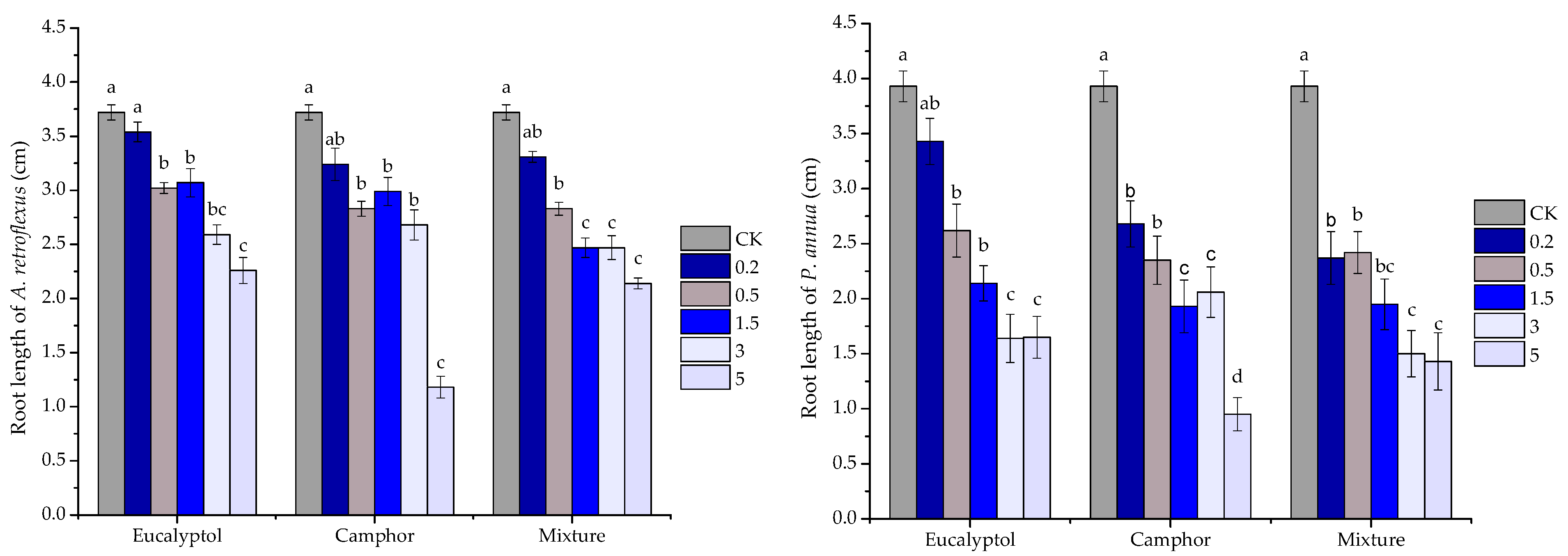
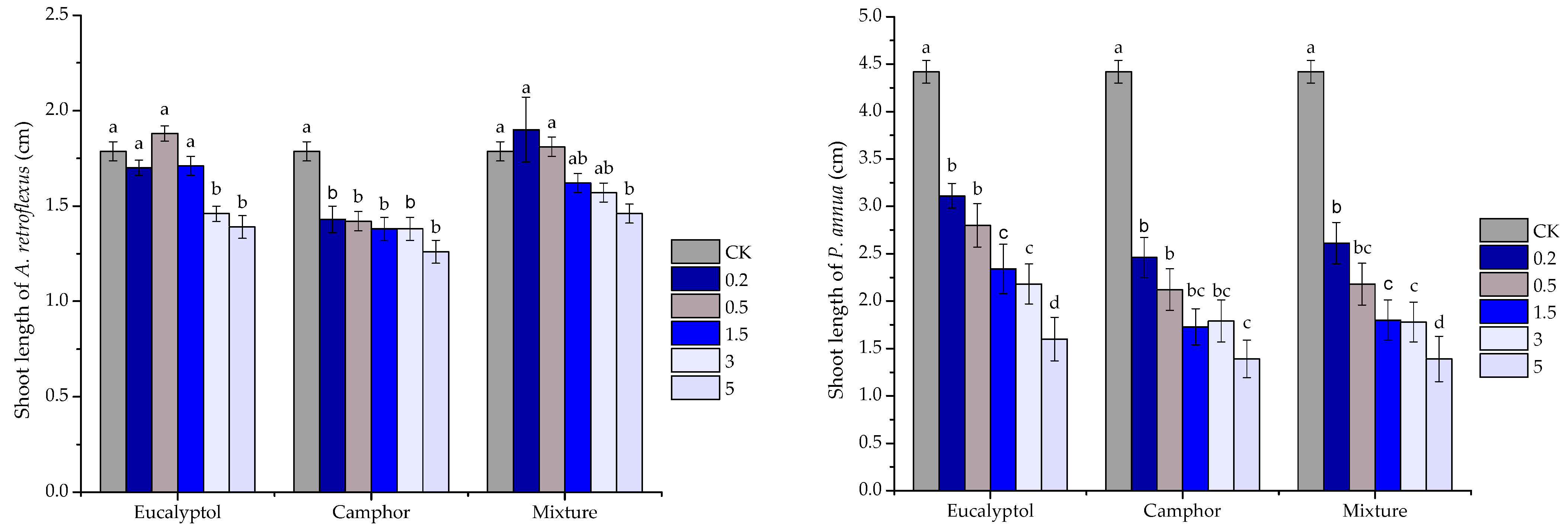
2.3. Phytotoxic Activity of the Volatile Oils and Their Major Constituents
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Potential Allelopathic Effect of VOCs
4.3. Isolation of the Volatile Oil
4.4. Gas Chromatography-Mass Spectrometry Analysis
4.5. Phytotoxic Effect of the Volatile Oils
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.J.; He, X.L. Chromosome numbers and karyotype of five species from Seriphidium (Asteraceae). Plant. Syst. Evol. 2005, 290, 109–113. [Google Scholar] [CrossRef]
- Malik, S.; Vitales, D.; Hayat, M.Q.; Korobkov, A.A.; Joan, V. Phylogeny and biogeography of Artemisia subgenus Seriphidium (asteraceae: Anthemideae). Taxon 2017, 66, 934–952. [Google Scholar] [CrossRef]
- Editorial Committee of Flora of China, Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1991; Volume 76, p. 257. [Google Scholar]
- Boriky, D.; Berrada, M.; Talbi, M.; Keravis, G.; Rouessac, F. Eudesmanolides from Artemisia herba-alba. Phytochemistry 1996, 43, 309–311. [Google Scholar] [CrossRef]
- Wright, J.M.; Chambers, J.C. Restoring riparian meadows currently dominated by Artemisa using alternative state concepts—above-ground vegetation response. Appl. Veg. Sci. 2002, 5, 237–246. [Google Scholar]
- Ferreira, J.F.S.; Luthria, D.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with srtemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [PubMed]
- Valles, J.; Garcia, S.; Hidalgo, O.; Martin, J.; Pellicer, J.; Sanz, M.; Garnatje, T. Biology, genome evolution, biotechnological issues and research including applied perspectives in Artemisia (Asteraceae). Adv. Bot. Res. 2011, 60, 349–419. [Google Scholar]
- Shafiq, N.; Shafiq, S.; Rafiq, N.; Parveen, S.; Javed, I.; Majeed, H.N. Review: Phytochemicals of the Seriphidium, economically and pharmaceutically important genus of Asteraceae family. Mini Rev. Org. Chem. 2020, 17, 158–168. [Google Scholar] [CrossRef]
- Shao, H.; Hu, Y.X.; Han, C.X.; Wei, C.X.; Zhou, S.X.; Zhang, C.P.; Zhang, C. Chemical composition and phytotoxic activity of Seriphidium terrae-albae (Asteraceae) essential oil. Chem. Biodivers. 2018, 15, e1800348. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984; p. 422. [Google Scholar]
- Yuan, Z.G.; Zheng, X.W.; Zhao, Y.; Liu, Y.; Zhou, S.X.; Wei, C.X.; Hu, Y.X.; Shao, H. Phytotoxic compounds isolated from leaves of the invasive weed Xanthium spinosum. Molecules 2018, 23, 2840. [Google Scholar] [CrossRef] [PubMed]
- Van der Putten, W.H. Impacts of soil microbial communities on exotic plant invasions. Trends Ecol. Evol. 2010, 25, 512–519. [Google Scholar]
- Shao, H.; Zeng, R.S.; Wang, R.L.; Zhang, B.C.; Zhang, C. Selective phytotoxicity of xanthinin and xanthatin from invasive weed Xanthium italicum Morretti on test plants. Allelopath. J. 2015, 35, 77–86. [Google Scholar]
- Vidotto, F.; Tesio, F.; Ferrero, A. Allelopathic effects of Ambrosia artemisiifolia L. in the invasive process. Crop. Prot. 2013, 54, 161–167. [Google Scholar] [CrossRef]
- Rawat, L.S.; Maikhuri, R.K.; Bahuguna, Y.M.; Maletha, A.; Phondani, P.C.; Jha, N.K. Interference of Eupatorium adenophorum (Spr.) and its allelopathic effect on growth and yield attributes of traditional food crops in Indian Himalayan region. Ecol. Res. 2019, 34, 587–599. [Google Scholar] [CrossRef]
- Kong, C.; Hu, F.; Xu, X. Allelopathic potential and chemical constituents of volatiles from Ageratum conyzoides under stress. J. Chem. Ecol. 2002, 28, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, A.A.; Weston, P.A.; Gurusinghe, S.; Latif, S.; Adkins, S.W.; Weston, L.A. Toxic potential and metabolic profiling of two australian biotypes of the invasive plant parthenium weed (Parthenium hysterophorus L.). Toxins 2020, 12, 447. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Yuan, L.; Weber, E.; Kleunen, M.V. Effect of allelopathy on plant performance: A meta-analysis. Ecol. Lett. 2020, 24, 348–362. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef]
- Dudai, N.; Poljakoff-Mayber, A.; Mayer, A.M.; Putievsky, E.; Lerner, H.R. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089. [Google Scholar] [CrossRef]
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefevre, F.; Pierman, B.; Lynch, J.H.; Alam, I.; Junqueira, B.; Benke, R.; Ray, S. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 2017, 356, 1386–1388. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant. Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, H.P.; Mittal, S.; Batish, D.R.; Kohli, R.K. Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as a bioherbicide. Ind. Crop. Prod. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, Y.; Nan, P.; Huang, X.; Zhang, C. Chemical composition and phytotoxic activity of the volatile oil of invasive Xanthium italicum Moretti from China. J. Arid Land 2013, 5, 324–330. [Google Scholar] [CrossRef]
- Puig, C.G.; Goncalves, R.F.; Valento, P.; Andrade, P.B.; Reigosa, M.J.; Nuria, P. The consistency between phytotoxic effects and the dynamics of allelochemicals release from Eucalyptus globulus leaves used as bioherbicide green manure. J. Chem. Ecol. 2018, 44, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.X.; Zhou, S.X.; Li, W.J.; Jiang, C.Y.; Yang, W.; Han, C.X.; Zhang, C.; Shao, H. Chemical composition and allelopathic, phytotoxic and pesticidal activities of Atriplex cana LEDEB. (Amaranthaceae) essential oil. Chem. Biodivers. 2019, 16, e1800595. [Google Scholar] [CrossRef] [PubMed]
- Souza-Alonso, P.; Gonzalez, L.; Lopez-Nogueira, A.; Cavaleiro, C.; Pedrol, N. Volatile organic compounds of Acacia longifolia and their effects on germination and early growth of species from invaded habitats. Chem. Ecol. 2018, 34, 126–145. [Google Scholar] [CrossRef]
- Sun, H.Z.; He, X.L. A comparative study on germination characteristics of three species from Seriphidium (Bess.) Poljak. J. Northwest A F Univ. Nat. Sci. Ed. 2007, 35, 198–202. (In Chinese) [Google Scholar]
- Hussain, A. The genus Artemisia (Asteraceae): A review on its ethnomedicinal prominence and taxonomy with emphasis on foliar anatomy, morphology, and molecular phylogeny. Proc. Pak. Acad. Sci. B Life Environ. Sci. 2020, 57, 1–28. [Google Scholar]
- Rizvi, S.A.H.; Ling, S.; Zeng, X. Seriphidium brevifolium essential oil: A novel alternative to synthetic insecticides against the dengue vector Aedes albopictus. Environ. Sci. Pollut. Res. 2020, 27, 31863–31871. [Google Scholar] [CrossRef] [PubMed]
- Boutkhil, S.; Idrissi, M.E.; Chakir, S.; Derraz, M.; Amechrouq, A.; Chbicheb, A.; Badaoui, K.E. Antibacterial and antifungal activity of extracts and essential oils of Seriphidium herba-alba (Asso) Soják and their combination effects with the essential oils of Dysphania ambrosioides (L.) Mosyakin & Clemants. Acta Bot. Gallica 2011, 158, 425–433. [Google Scholar]
- Gilani, S.A.; Fujii, Y.; Sugano, M.; Watanabe, K.N. Chemotypic variations and phytotoxic studies of essential oils of endemic medicinal plant, Seriphidium kurramense, from pakistan. J. Med. Plants Res. 2010, 4, 309–315. [Google Scholar]
- Abd-ElGawad, A.M.; El Gendy, A.E.N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Omer, E.A.; Dar, B.A.; Al-Taisan, W.A.A.; Elshamy, A.I. Essential oil enriched with oxygenated constituents from invasive plant Argemone ochroleuca exhibited potent phytotoxic effects. Plants 2020, 9, 998. [Google Scholar] [CrossRef]
- Assaeed, A.; Elshamy, A.; El Gendy, A.E.N.; Dar, B.; Al-Rowaily, S.; Abd-ElGawad, A. Sesquiterpenes-rich essential oil from above ground parts of Pulicaria somalensis exhibited antioxidant activity and allelopathic effect on weeds. Agronomy 2020, 10, 399. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Abd El-Gawad, A.M.; El Gendy, A.E.N.G.; Assaeed, A.M. Chemical characterization of Euphorbia heterophylla L. essential oils and their antioxidant activity and allelopathic potential on Cenchrus echinatus L. Chem. Biodivers. 2019, 16, e1900051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.X.; Wei, C.X.; Zhang, C.; Han, C.X.; Nigora, K.; Shao, H. Chemical composition, phytotoxic, antimicrobial and insecticidal activity of the essential oils of Dracocephalum integrifolium. Toxins 2019, 11, 598. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Vokou, D.; Douvli, P.; Blionis, G.T.; Halley, J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Braguini, W.L.; Kelmer–Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of four monoterpenes of germination, primary root growth, and mitochondrial respiration of maize. J. Chem. Ecol. 2000, 26, 611–624. [Google Scholar] [CrossRef]
- Martino, L.D.; Mancini, E.; Almeida, L.F.R.; Feo, V.D. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef]
- Barton, A.F.M.; Clarke, B.R.; Dell, B.; Knight, A.R. Post-emergent herbicidal activity of cineole derivatives. J. Pest. Sci. 2014, 87, 531–541. [Google Scholar] [CrossRef]
- Barney, J.N.; Hay, A.G.; Weston, L.A. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J. Chem. Ecol. 2005, 31, 247. [Google Scholar] [CrossRef]
- Viros, J.; Fernandez, C.; Wortham, H.; Gavinet, J.; Ormeo, E. Litter of Mediterranean species as a source of volatile organic compounds. Atmos. Environ. 2020, 242, 117815. [Google Scholar]
- Yosef Friedjung, A.; Choudhary, S.P.; Dudai, N.; Rachmilevitch, S. Physiological conjunction of allelochemicals and desert plants. PLoS ONE 2013, 8, e81580. [Google Scholar] [CrossRef][Green Version]
- Barney, J.N.; Sparks, J.P.; Greenberg, J.; Whitlow, T.H.; Guenther, A. Biogenic volatile organic compounds from an invasive species: Impacts on plant–plant interactions. Plant. Ecol. 2009, 203, 195–205. [Google Scholar] [CrossRef][Green Version]
- Dragull, K.; Beck, J.J.; Merrill, G.B. Essential oil yield and composition of Pistacia vera ‘Kerman’ fruits, peduncles and leaves grown in California. J. Sci. Food Agric. 2010, 90, 664–668. [Google Scholar] [CrossRef]
- Giuliani, C.; Ascrizzi, R.; Tani, C.; Bottoni, M.; Bini, L.M.; Flamini, G.; Fico, G. Salvia uliginosa Benth.: Glandular trichomes as bio-factories of volatiles and essential oil. Flora 2017, 233, 12–21. [Google Scholar] [CrossRef]
- Zhigzhitzhapova, S.V.; Radnaeva, L.D.; Gao, Q.B.; Chen, S.L.; Zhang, F.Q. Chemical composition of volatile organic compounds of Artemisia vulgaris L. (Asteraceae) from the Qinghai–Tibet Plateau. Ind. Crops Prod. 2016, 83, 462–469. [Google Scholar] [CrossRef]
- Uitterhaegen, E.; Burianova, K.; Ballas, S.; Veronese, T.; Merah, O.; Talou, T.; Stevens, C.V.; Evon, P.; Simon, V. Characterization of volatile organic compound emissions from self-bonded boards resulting from a coriander biorefinery. Ind. Crops Prod. 2018, 122, 57–65. [Google Scholar] [CrossRef]
- Najar, B.; Ferri, B.; Cioni, P.; Pistelli, L. Volatile emission and essential oil composition of Sambucus nigra L. organs during different developmental stages. Plant. Biosyst. 2020. [Google Scholar] [CrossRef]
- Santonja, M.; Bousquet-Mélou, A.; Greff, S.; Ormeño, E.; Fernandez, C. Allelopathic effects of volatile organic compounds released from Pinus halepensis needles and roots. Ecol. Evol. 2019, 9, 8201–8213. [Google Scholar] [CrossRef]
- Ercoli, L.; Masoni, A.; Pampana, S.; Arduini, I. Allelopathic effects of rye, brown mustard and hairy vetch on redroot pigweed, common lambsquarter and knotweed. Allelopath. J. 2007, 19, 249–256. [Google Scholar]
- Tang, J.S.; Jiang, C.Y.; Liu, Y.; Zhang, X.Y.; Shao, H.; Zhang, C. Allelopathic potential of volatile organic compounds released by Xanthium sibiricum Patrin ex Widder. Allelopath. J. 2019, 47, 233–242. [Google Scholar] [CrossRef]
- Evans, H.; Crocoll, C.; Bajpai, D.; Callaway, R.M. Volatile chemicals from leaf litter are associated with invasiveness of a neotropical weed in Asia. Ecology 2011, 92, 316–324. [Google Scholar]
- Jiang, C.Y.; Zhou, S.X.; Zokir, T.; Mei, Y.; Jin, G.Z.; Han, C.X.; Zhang, C.; Shao, H. Chemical composition and phytotoxic activity of the essential oil of Artemisia sieversiana growing in Xinjiang, China. Nat. Prod. Res. 2020. [Google Scholar] [CrossRef]
- Preston, C.A.; Betts, H.; Baldwin, I.T. Methyl jasmonate as an allelopathic agent: Sagebrush inhibits germination of a neighboring tobacco, Nicotiana attenuata. J. Chem. Ecol. 2002, 28, 2343–2369. [Google Scholar] [CrossRef]
- Sainz, P.; Andres, M.F.; Martinez-Diaz, R.A.; Bailen, M.; Navarro-Rocha, J.; Diaz, C.E.; Gonzalez-Coloma, A. Chemical composition and biological activities of Artemisia pedemontana subsp. Assoana essential oils and hydrolate. Biomolecules 2019, 9, 558. [Google Scholar] [CrossRef]
- Tsubo, M.; Nishihara, E.; Nakamatsu, K.; Cheng, Y.; Shinoda, M. Plant volatiles inhibit restoration of plant species communities in dry grassland. Basic Appl. Ecol. 2012, 13, 76–84. [Google Scholar] [CrossRef]
- Arroyo, A.I.; Pueyo, Y.; Pellissier, F.; Ramos, J.; Espinosa-Ruiz, A.; Millery, A. Phytotoxic effects of volatile and water soluble chemicals of Artemisia herba-alba. J. Arid Environ. 2018, 151, 1–8. [Google Scholar] [CrossRef]
- Luo, Y.; Du, Z.; Yan, Z.; Zhao, X.; Li, M.H. Artemisia halodendron litters have strong negative allelopathic effects on earlier successional plants in a semi-arid sandy dune region in china. Front. Plant. Sci. 2020, 11, 961. [Google Scholar] [CrossRef]
- Ahmad, K.; Ali, A.; Afridi, W.A.; Somayya, R.; Ullah, M.J. Antimicrobial, hemagglutination and phytotoxic activity of crude ethanolic and aqueous extracts of Seriphidium kurramense. J. Tradit. Chin. Med. 2018, 38, 433–438. [Google Scholar] [CrossRef]
- Williamson, G.B.; Richardson, D. Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988, 14, 181–187. [Google Scholar] [CrossRef]
- Andreani, S.; Barboni, T.; Desjobert, J.M.; Paolini, J.; Costa, J.; Muselli, A. Essential oil composition and chemical variability of Xanthium italicum Moretti from Corsica. Flavour Frag. J. 2012, 27, 227–236. [Google Scholar] [CrossRef]
- Esmaeili, A.; Rashidi, B.; Rezazadeh, S. Biological Activities of various extracts and chemical composition of Trigonella monantha C. A. Mey. subsp monantha grown in Iran. Iran. J. Pharm. Res. 2012, 11, 1127–1136. [Google Scholar] [PubMed]

| Number | Compounds | Percentage (%) | Average Percentage (%) | |||
|---|---|---|---|---|---|---|
| Stem | Leaf | Flower | Flowering Shoot | |||
| 1 | Santolina triene | 0.89 | 1.88 | 3.84 | 2.17 | 2.20 |
| 2 | α-Pinene | 1.86 | 1.43 | 1.91 | 1.6 | 1.70 |
| 3 | Camphene | 3.17 | 3.89 | 4.36 | 4.95 | 4.09 |
| 4 | β-Pinene | 0.78 | 0.9 | 1.17 | 0.92 | 0.94 |
| 5 | Artemiseole | 1.28 | 1.92 | 1.78 | 1.47 | 1.61 |
| 6 | β-Myrcene | - | - | 0.23 | - | 0.06 |
| 7 | 2-Carene | 0.45 | - | 0.45 | 0.59 | 0.37 |
| 8 | α-Terpinene | - | 0.43 | - | - | 0.11 |
| 9 | o-Cymene | 1.02 | 1.18 | 0.59 | 1.35 | 1.04 |
| 10 | Eucalyptol | 43 | 36.66 | 19.52 | 38.68 | 34.47 |
| 11 | γ-Terpinene | 0.82 | 0.74 | 0.83 | 0.87 | 0.82 |
| 12 | Terpinolene | - | - | 0.24 | 0.24 | 0.12 |
| 13 | Linalool | 0.39 | 0.52 | 0.66 | 0.61 | 0.55 |
| 14 | cis-2-p-Menthen-1-ol | 0.43 | 0.34 | 0.23 | - | 0.25 |
| 15 | Camphor | 21.55 | 24.91 | 21.64 | 23.35 | 22.86 |
| 16 | Nerol oxide | 1.9 | 1.67 | 1.43 | 1.79 | 1.70 |
| 17 | Pinocarvone | 0.29 | - | - | - | 0.07 |
| 18 | borneol | 5.92 | 3.5 | 2.02 | 4.06 | 3.88 |
| 19 | Lavandulol | 0.23 | 0.31 | 0.4 | 0.27 | 0.30 |
| 20 | 2-Caren-4-ol | 3.44 | 3.16 | 2.58 | 2.85 | 3.01 |
| 21 | α-Terpineol | 2.13 | 2.83 | 2.64 | 1.72 | 2.33 |
| 22 | cis-Carveol | 0.27 | 0.25 | 0.27 | - | 0.20 |
| 23 | Nerol | 2.11 | 2.82 | 3.41 | 1.36 | 2.43 |
| 24 | Carvone | 2.41 | 2.57 | 3.72 | 1.73 | 2.61 |
| 25 | (+)-trans-Chrysanthenyl acetate | - | - | - | 0.36 | 0.09 |
| 26 | cis-Geraniol | - | - | 0.28 | - | 0.07 |
| 27 | Bornyl acetate | 0.49 | 0.79 | 1.63 | 0.76 | 0.92 |
| 28 | Lavandulol acetate | - | - | 1.09 | 0.34 | 0.36 |
| 29 | α-Terpineol acetate | 2.46 | 1.32 | 1.87 | - | 1.41 |
| 30 | δ-Elemene | - | - | - | 0.96 | 0.24 |
| 31 | Eugenol | 0.23 | 0.4 | 0.47 | - | 0.28 |
| 32 | trans-Carveyl acetate | - | - | 0.34 | - | 0.09 |
| 33 | Nerol acetate | 0.56 | 2.28 | 9.03 | 2.13 | 3.50 |
| 34 | Geranyl acetate | - | - | 0.89 | - | 0.22 |
| 35 | Germacrene D | - | - | 0.55 | - | 0.14 |
| 36 | γ-Elemene | - | - | 1.29 | - | 0.32 |
| 37 | Spathulenol | 0.3 | - | 0.91 | - | 0.30 |
| 38 | Globulol | - | - | 0.26 | - | 0.07 |
| Monoterpene hydrocarbons | 8.99 | 10.45 | 13.62 | 12.69 | 11.44 | |
| Oxygenated monoterpenes | 85.35 | 81.46 | 60.58 | 77.89 | 76.32 | |
| Sesquiterpene hydrocarbons | 0.00 | - | 0.55 | 0.96 | 0.38 | |
| Oxygenated sesquiterpenes | 0.3 | - | 1.17 | - | 0.37 | |
| Others | 3.74 | 4.79 | 16.61 | 3.59 | 7.18 | |
| Total identified | 98.38 | 96.7 | 92.53 | 95.13 | 95.69 | |
| Oil yield (%, V/W) | 0.31 | 0.65 | 0.84 | 0.75 | 0.64 | |
| Test Plants | Organs and Major Constituents | Regression Equation | R2 | IC50 |
|---|---|---|---|---|
| A. retroflexus root | stem | y = −11.508x2 + 93.403x − 82.097 | 0.9882 | 1.824 |
| leaf | y = −9.5325x2 + 79.559x − 60.805 | 0.9848 | 1.767 | |
| flower | y = −11.779x2 + 93.393x − 76.567 | 0.9789 | 1.735 | |
| flowering shoot | y = −5.8052x2 + 61.363x − 56.398 | 0.8291 | 2.186 | |
| eucalyptol | y = 9.3438x − 18.531 | 0.9327 | 6.264 | |
| camphor | y = 5.9152x2 − 22.147x + 20.625 | 0.8819 | 4.782 | |
| mixture | y = 8.4375x − 7.9375 | 0.9196 | 6.867 | |
| A. retroflexus shoot | stem | y = −9.4749x2 + 80.749x − 67.239 | 0.969 | 1.856 |
| leaf | y = −8.705x2 + 74.203x − 54.044 | 0.9746 | 1.769 | |
| flower | y = −9.65x2 + 82.18x − 70.342 | 0.9835 | 1.879 | |
| flowering shoot | y = −5.2372x2 + 59.054x − 58.134 | 0.8253 | 2.300 | |
| eucalyptol | y = 2.6388x2 − 9.2084x − 5.0955 | 0.8247 | 6.636 | |
| camphor | y = 0.8189x2 − 2.4932x + 10.955 | 0.9145 | 8.593 | |
| mixture | y = 7.1338x − 27.898 | 0.9718 | 10.920 | |
| P. annua root | stem | y = −7.9365x2 + 69.349x − 47.888 | 0.9572 | 1.770 |
| leaf | y = −7.1368x2 + 61.667x − 30.127 | 0.9836 | 1.593 | |
| flower | y = −6.3977x2 + 61.779x − 45.954 | 0.8971 | 1.945 | |
| flowering shoot | y = 26.641x − 19.025 | 0.9214 | 2.591 | |
| eucalyptol | y = 13.236x − 6.6472 | 0.9108 | 4.280 | |
| camphor | y = 2.1033x2 − 1.6868x + 23.79 | 0.8723 | 3.954 | |
| mixture | y = 8.1633x + 19.125 | 0.9023 | 4.782 | |
| P. annua shoot | stem | y = −8.0478x2 + 72.872x − 61.388 | 0.9209 | 1.947 |
| leaf | y = −8.732x2 + 73.998x − 52.504 | 0.9859 | 1.744 | |
| flower | y = −9.6585x2 + 85.289x − 84.178 | 0.9599 | 2.048 | |
| flowering shoot | y = −6.9597x2 + 71.954x − 80.814 | 0.8953 | 2.354 | |
| eucalyptol | y = 11.934x − 14.689 | 0.9772 | 5.421 | |
| camphor | y = 8.0984x + 13.475 | 0.9201 | 4.51 | |
| mixture | y = 9.3115x + 8.0656 | 0.945 | 4.504 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Zokir, T.; Mei, Y.; Lei, L.; Shi, K.; Zou, T.; Zhang, C.; Shao, H. Allelopathic Effect of Serphidium kaschgaricum (Krasch.) Poljak. Volatiles on Selected Species. Plants 2021, 10, 495. https://doi.org/10.3390/plants10030495
Zhou S, Zokir T, Mei Y, Lei L, Shi K, Zou T, Zhang C, Shao H. Allelopathic Effect of Serphidium kaschgaricum (Krasch.) Poljak. Volatiles on Selected Species. Plants. 2021; 10(3):495. https://doi.org/10.3390/plants10030495
Chicago/Turabian StyleZhou, Shixing, Toshmatov Zokir, Yu Mei, Lijing Lei, Kai Shi, Ting Zou, Chi Zhang, and Hua Shao. 2021. "Allelopathic Effect of Serphidium kaschgaricum (Krasch.) Poljak. Volatiles on Selected Species" Plants 10, no. 3: 495. https://doi.org/10.3390/plants10030495
APA StyleZhou, S., Zokir, T., Mei, Y., Lei, L., Shi, K., Zou, T., Zhang, C., & Shao, H. (2021). Allelopathic Effect of Serphidium kaschgaricum (Krasch.) Poljak. Volatiles on Selected Species. Plants, 10(3), 495. https://doi.org/10.3390/plants10030495







