Cutin Synthesis in Developing, Field-Grown Apple Fruit Examined by External Feeding of Labelled Precursors
Abstract
1. Introduction
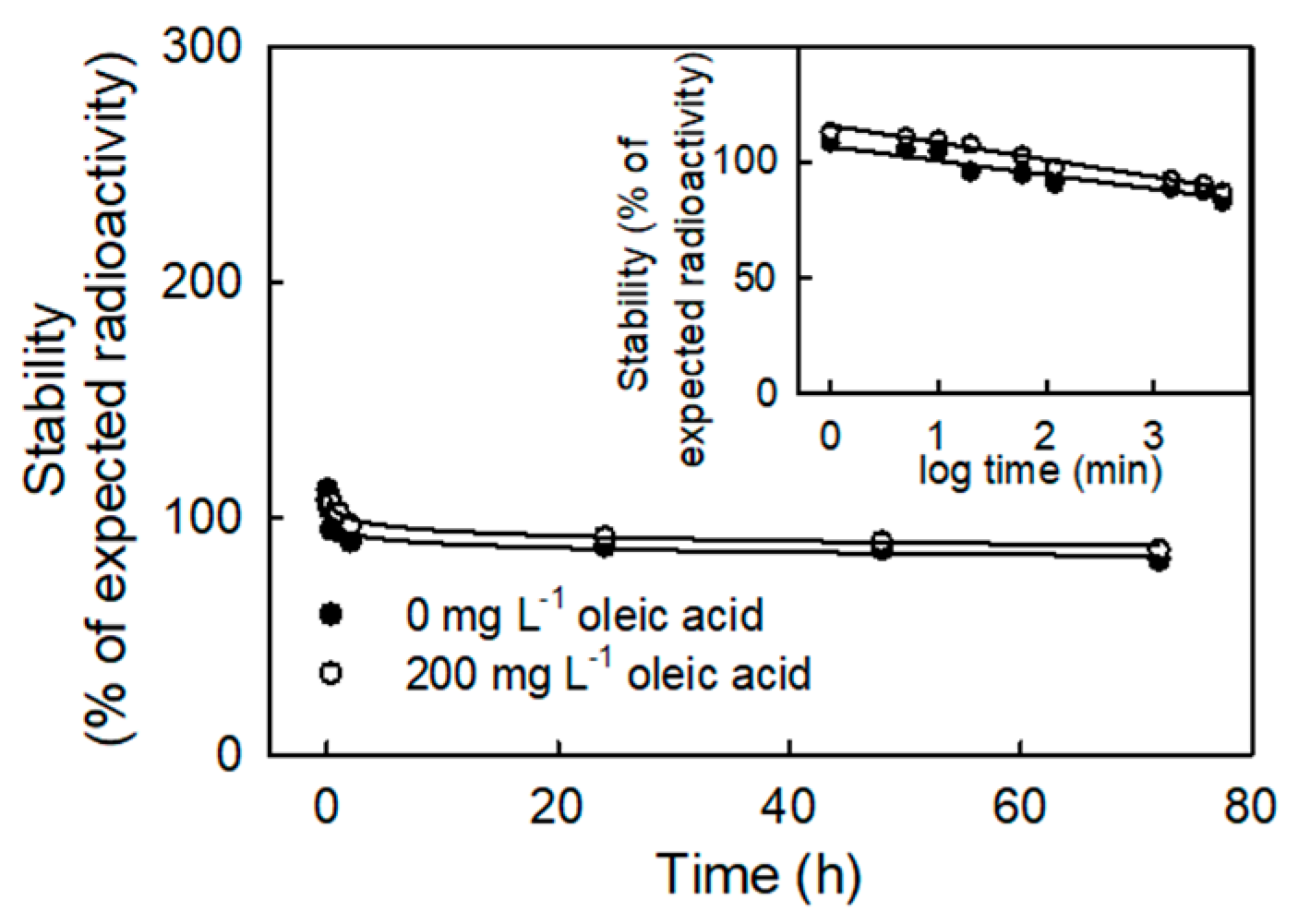
2. Results
3. Discussion
3.1. Incorporation into the Cutin Fraction Reflects Synthesis, but Not a Simple Partitioning
- (I)
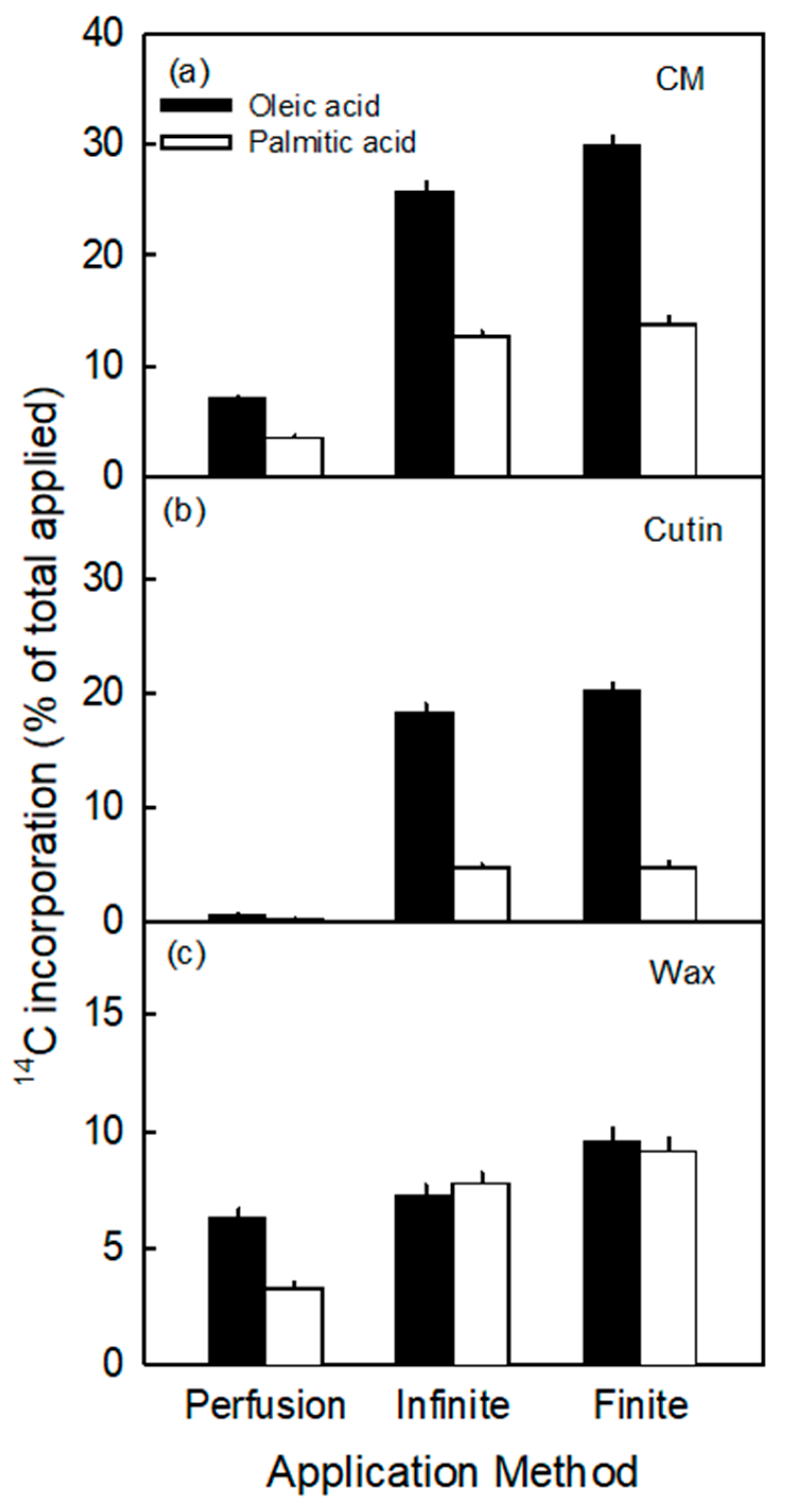
- The incorporation of the precursors in the cutin fraction was highly selective. It consistently favored oleic acid over palmitic acid, despite their very similar octanol/water partition coefficients (Koct/w) (i.e., for oleic acid logarithm of Koct/w is 7.64 and for palmitic acid it is 7.17) [35]. The value of log Koct/w is closely related to the logarithm of the cuticle/water partition coefficient (log Kcut/w) [36]. The two calculated log Kcut/w values are 7.47 for oleic acid and 7.01 for palmitic acid. The measured selectivity of incorporation in the cutin fraction is consistent with the composition of apple fruit cutin where C18 compounds dominate over C16 ones, with ratios ranging from 2.2:1 to 3.4:1 [37,38,39]. This ratio is close to that obtained in our study (range 3.8 and 4.2 for the infinite and finite dose feeding, respectively; Figure 2).
- (II)
- When the cuticle was extracted using a chloroform/methanol solution, the radiolabel associated with the cuticle remained in the cutin polymer. If the two precursors were only partitioned into the cutin, we would have expected both precursors to be extracted by the chloroform/methanol solution and, hence, to end up in the supernatant as part of the wax fraction. However, this was clearly not the case for the cutin fraction.
- (III)
- Kolattukudy and Walton [29] and Kolattukudy et al. [38] reported that externally applied oleic acid converts into 18-hydroxy-C18 or 10,18-dihydroxy-C18 or 9,10,18-trihydroxy-C18 acid and externally applied palmitic acid into 16-hydroxy-C16 or 10,16-dihydroxy-C16 acid before incorporation into the cutin.
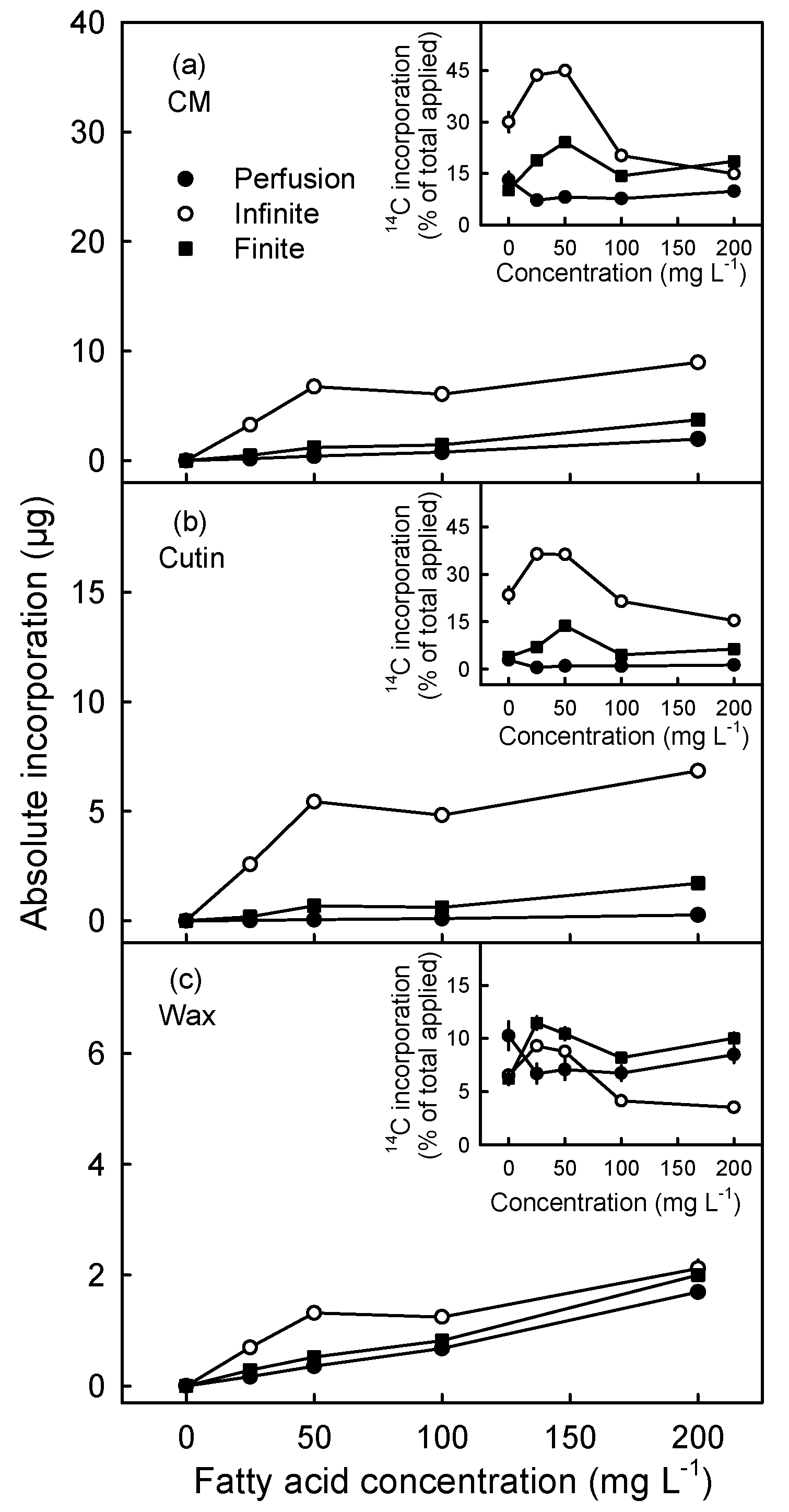
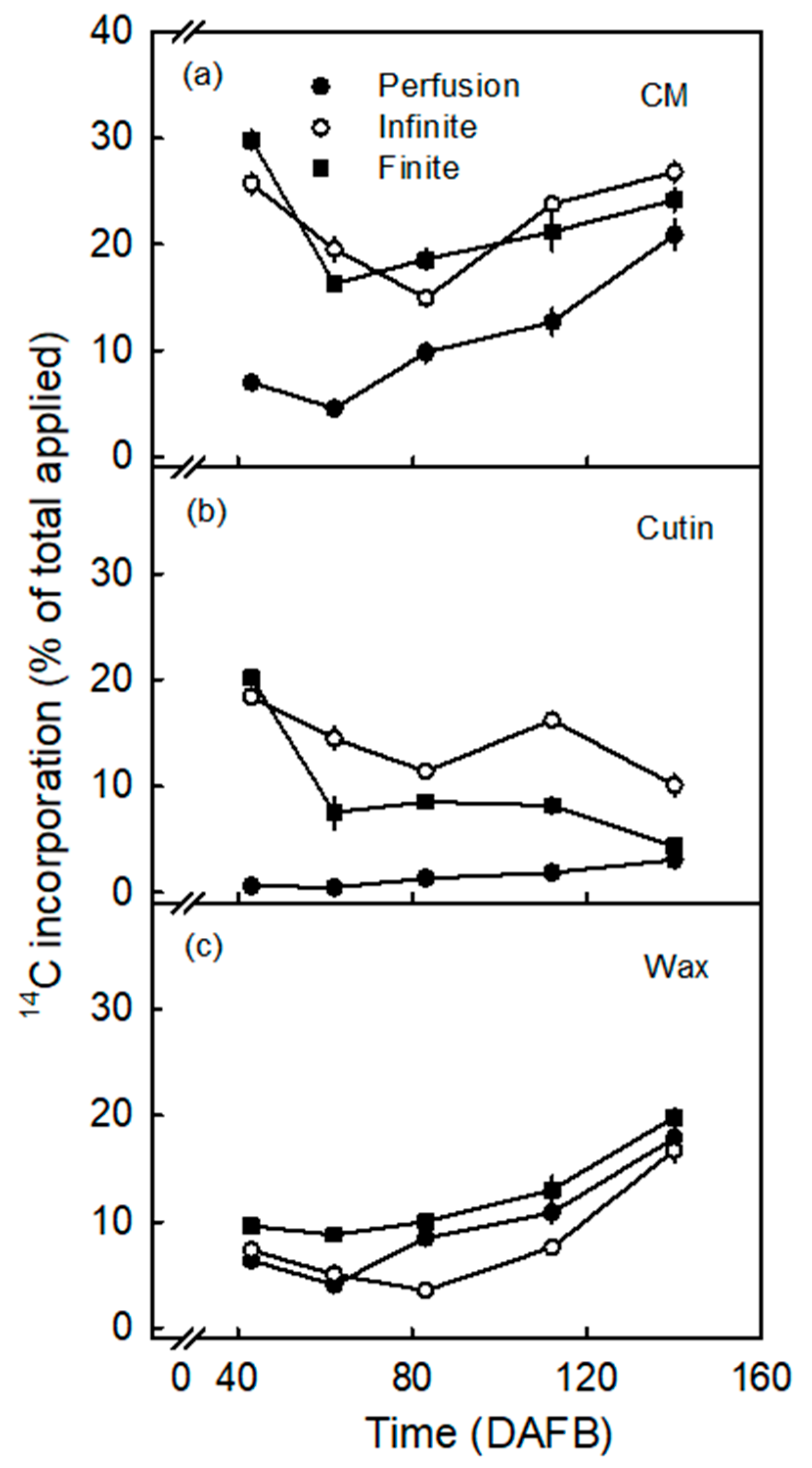
3.2. Incorporation Was Higher Following Infinite Dose Feeding Than Finite Dose Feeding—Both Methods Were Superior to Perfusion
3.3. Incorporation of Oleic Acid Exceeds That of Palmitic Acid
3.4. Conclusions
4. Materials and Methods
4.1. Plant Materials
4.2. In Vitro Laboratory Assay Using 14C
4.2.1. Preparation of Donor Solutions
4.2.2. Feeding Epidermal Skin Sample with 14C-Oleic and 14C-Palmitic Acid
- (1)
- In the finite dose method, 100 µL of donor solution containing 3 × 106 dpm mL−1 radioactivity was applied to the tube on the ES. After feeding, the glass jar was left open to allow evaporation of the water from the feeding solution. Within 15 h of feeding, the donor solution had formed a macroscopically visible dry deposit on the ES. Thereafter, the glass jar was closed.
- (2)
- In the infinite dose method, 300 µL of donor solution containing 1 × 106 dpm mL−1 radioactivity was applied to the tube on the ES. Immediately after feeding the glass jar was closed.
- (3)
- In the perfusion method, 100 µL of donor solution containing 3 × 106 dpm mL−1 radioactivity was applied directly to the cut surface of the ES. This application mimicked a perfusion of donor solution by injection as performed in experiments using intact fruit attached to a tree in the field.
4.2.3. Sample Preparation and Analysis of Radioactivity
4.3. Experiments
4.4. In Vivo Assay Using 13C
4.4.1. 13C Fatty Acid Solution Preparation
4.4.2. Feeding 13C Fatty Acid
4.4.3. Sample Processing and 13C Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knoche, M.; Lang, A. Ongoing growth challenges fruit skin integrity. Crit. Rev. Plant Sci. 2017, 36, 190–215. [Google Scholar] [CrossRef]
- Faust, M.; Shear, C.B. Russeting of apples, an interpretive review. HortScience 1972, 7, 233–235. [Google Scholar]
- Grimm, E.; Peschel, S.; Becker, T.; Knoche, M. Stress and strain in the sweet cherry skin. J. Am. Soc. Hort. Sci. 2012, 137, 383–390. [Google Scholar] [CrossRef]
- Kerstiens, G. Cuticular water permeability and its physiological significance. J. Exp. Bot. 1996, 47, 1813–1832. [Google Scholar] [CrossRef]
- Kerstiens, G. Water transport in plant cuticles: An update. J. Exp. Bot. 2006, 57, 2493–2499. [Google Scholar] [CrossRef] [PubMed]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-S. Penetration of cuticles by plant pathogens. In Plant Pathogenesis and Resistance, 1st ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 3–48. [Google Scholar]
- Serrano, M.; Coluccia, F.; Torres, M.; L’Haridon, F.; Métraux, J.-P. The cuticle and plant defense to pathogens. Front. Plant Sci. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Considine, J.A.; Kriedemann, P.E. Fruit splitting in grapes: Determination of the critical turgor pressure. Crop Pasture Sci. 1972, 23, 17–23. [Google Scholar] [CrossRef]
- Swift, J.G.; May, P.; Lawton, E.A. Concentric cracking of grape berries. Vitis 1974, 13, 30–35. [Google Scholar]
- Hampson, C.R.; Kemp, H. Characteristics of important commercial apple cultivars. In Apples: Botany, Production and Uses, 1st ed.; Ferree, D.C., Warrington, I.J., Eds.; CABI Publishing: Wallinford, UK, 2003; pp. 61–89. [Google Scholar]
- Grimm, E.; Khanal, B.P.; Winkler, A.; Knoche, M.; Köpcke, D. Structural and physiological changes associated with the skin spot disorder in apple. Postharvest Biol. Technol. 2012, 64, 111–118. [Google Scholar] [CrossRef]
- Faust, M.; Shear, C.B. Fine structure of the fruit surface of three apple cultivars. J. Am. Soc. Hort. Sci. 1972, 97, 351–355. [Google Scholar]
- Khanal, B.P.; Grimm, E.; Knoche, M. Russeting in apple and pear: A plastic periderm replaces a stiff cuticle. AOB Plants 2013, 5, 1–12. [Google Scholar] [CrossRef]
- Stösser, R.; Neubeller, J. Histologische und chemische Untersuchung der “Halswelke” bei der Hauszwetsche (Prunus domestica L.)/ Histological and chemical investigations on the shrivelling in plums (Prunus domestica L.) cv. ‘Hauszwetsche’. Gartenbauwissenschaft 1985, 50, 97–104. [Google Scholar]
- Lai, X.; Khanal, B.P.; Knoche, M. Mismatch between cuticle deposition and area expansion in fruit skins allows potentially catastrophic buildup of elastic strain. Planta 2016, 244, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Skene, D.S. Growth stresses during fruit development in Cox’s Orange Pippin apples. J. Hort. Sci. 1980, 55, 27–32. [Google Scholar] [CrossRef]
- Skene, D.S. The development of russet, rough russet and cracks on the fruit of the apple Cox’s Orange Pippin during the course of the season. J. Hort. Sci. 1982, 57, 165–174. [Google Scholar] [CrossRef]
- Maguire, K.M. Factors Affecting Mass Loss of Apples. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 1998. [Google Scholar]
- Khanal, B.P.; Grimm, E.; Finger, S.; Blume, A.; Knoche, M. Intracuticular wax fixes and restricts strain in leaf and fruit cuticles. New Phytol. 2013, 200, 134–143. [Google Scholar] [CrossRef]
- Khanal, B.P.; Knoche, M.; Bußler, S.; Schlüter, O. Evidence for a radial strain gradient in apple fruit cuticles. Planta 2014, 240, 891–897. [Google Scholar] [CrossRef]
- Heredia, A. Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochim. Biophys. Acta. 2003, 1620, 1–7. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Biopolyester membranes of plants: Cutin and suberin. Science 1980, 208, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Lendzian, K.J.; Schönherr, J. In-vivo study of cutin synthesis in leaves of Clivia miniata Reg. Planta 1983, 158, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Kolattukudy, P.E. Biosynthesis of wax in Brassica oleracea. Biochemistry 1965, 4, 1844–1855. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Cutin biosynthesis in Vicia faba leaves. Plant Physiol. 1970, 46, 759–760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kolattukudy, P.E. Biosynthesis of a lipid polymer, cutin: The structural component of plant cuticle. Biochem. Biophys. Res. Commun. 1970, 41, 299–305. [Google Scholar] [CrossRef]
- Kolattukudy, P.E.; Walton, T.J. Structure and biosynthesis of the hydroxy fatty acids of cutin in Vicia faba leaves. Biochemistry 1972, 11, 1897–1907. [Google Scholar] [CrossRef]
- Kolattukudy, P.E.; Walton, T.J.; Kushwaha, R.P.S. Biosynthesis of the C18 family of cutin acids: ω-hydroxyoleic acid, ω-hydroxy-9,10-epoxystearic acid, 9,10,18-trihydroxystearic acid, and their ∆12-unsaturated analogs. Biochemistry 1973, 12, 4488–4498. [Google Scholar] [CrossRef] [PubMed]
- Knoche, M.; Lownds, N.K.; Bukovac, M.J. Factors affecting the absorption of gibberellin A3 by sour cherry leaves. Crop Prot. 1992, 11, 57–63. [Google Scholar] [CrossRef]
- Knoche, M.; Petracek, P.D.; Bukovac, M.J. Finite dose diffusion studies: I. Characterizing cuticular penetration in a model system using NAA and isolated tomato fruit cuticles. Pest Manag. Sci. 2000, 56, 1005–1015. [Google Scholar] [CrossRef]
- Knoche, M.; Khanal, B.P.; Brüggenwirth, M.; Thapa, S. Patterns of microcracking in apple fruit skin reflect those of the cuticular ridges and of the epidermal cell walls. Planta 2018, 248, 293–306. [Google Scholar] [CrossRef]
- Schönherr, J.; Riederer, M. Foliar penetration and accumulation of organic chemicals in plant cuticles. Rev. Environ. Contam. Toxicol. 1989, 108, 1–70. [Google Scholar]
- Sangster, J. LOGKOW: A databank of evaluated octanol-water partition coefficients. GDF Databanks Bull. 1997, 1, 6–8. [Google Scholar]
- Kerler, F.; Schönherr, J. Accumulation of lipophilic chemicals in plant cuticles: Prediction from octanol/water partition coefficients. Arch. Environ. Contam. Toxicol. 1988, 17, 1–6. [Google Scholar] [CrossRef]
- Holloway, P.J. Cutins of Malus Pumila fruits and leaves. Phytochemistry 1973, 12, 2913–2920. [Google Scholar] [CrossRef]
- Kolattukudy, P.E.; Walton, T.J.; Kushwaha, R.P.S. Epoxy acids in the lipid polymer, cutin and their role in the biosynthesis of cutin. Biochem. Biophys. Res. Commun. 1971, 42, 739–744. [Google Scholar] [CrossRef]
- Walton, T.J.; Kolattukudy, P.E. Determination of the structures of cutin monomers by a novel depolymerization procedure and combined gas chromatography and mass spectrometry. Biochemistry 1972, 11, 1885–1897. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Cutin from Plants. In Biopolymers Online; Steinbüchel, A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P.; et al. Acyl-lipid metabolism. Arab. Book 2013, 11, e0161. [Google Scholar] [CrossRef]
- Schaeffer, S.M.; Christian, R.; Castro-Velasquez, N.; Hyden, B.; Lynch-Holm, V.; Dhingra, A. Comparative ultrastructure of fruit plastids in three genetically diverse genotypes of apple (Malus × domestica Borkh.) during development. Plant Cell Rep. 2017, 36, 1627–1640. [Google Scholar] [CrossRef]
- Benning, C. Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annu. Rev. Cell Dev. Biol. 2009, 25, 71–91. [Google Scholar] [CrossRef]
- He, M.; Qin, C.X.; Wang, X.; Ding, N.Z. Plant unsaturated fatty acids: Biosynthesis and regulation. Front. Plant Sci. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Li, N.; Xu, C.C.; Li-Beisson, Y.H.; Philippar, K. Fatty acid and lipid transport in plant cells. Trends Plant Sci. 2016, 2, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Kolattukudy, P.E.; Croteau, R.; Brown, L. Structure and biosynthesis of cuticular lipids: Hydroxylation of palmitic acid and decarboxylation of C(28), C(30), and C(32) acids in Vicia faba flowers. Plant Physiol. 1974, 54, 670–677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walton, T.J.; Kolattukudy, P.E. Enzymatic conversion of 16-hydroxypalmitic acid into 10,16-dihydroxypalmitic acid in Vicia Faba epidermal extracts. Biochem. Biophys. Res. Commun. 1972, 46, 16–21. [Google Scholar] [CrossRef]
- Silcox, D.; Holloway, P.J. A simple method for the removal and assessment of foliar deposits of agrochemicals using cellulose acetate film stripping. Aspects Appl. Biol. 1986, 11, 13–17. [Google Scholar]
- Gearing, J.N. The study of diet and trophic relationships through natural abundance 13C. In Carbon Isotope Techniques, 1st ed.; Coleman, D.C., Fry, B., Eds.; Academic Press: London, UK, 1991; pp. 201–208. [Google Scholar]

| Incorporation (% of Total Applied) | |||||
|---|---|---|---|---|---|
| Precursor | Stage (DAFB) | Method | CM | Cutin | Wax |
| 13C | 65 | Perfusion | 4.0 ± 0.6 | 1.9 ± 0.3 | 2.1 ± 0.5 |
| Infinite | 17.0 ± 2.0 | 10.1 ± 1.7 | 6.9 ± 1.1 | ||
| Finite | 15.4 ± 2.6 | 10.6 ± 1.7 | 4.9 ± 2.0 | ||
| 14C | 62 | Perfusion | 4.5 ± 0.3 | 0.5 ± 0.0 | 4.1 ± 0.3 |
| Infinite | 19.5 ± 1.1 | 14.5 ± 1.0 | 5.1 ± 0.2 | ||
| Finite | 16.3 ± 0.7 | 7.5 ± 0.5 | 8.8 ± 0.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, Y.; Khanal, B.P.; Sauheitl, L.; Knoche, M. Cutin Synthesis in Developing, Field-Grown Apple Fruit Examined by External Feeding of Labelled Precursors. Plants 2021, 10, 497. https://doi.org/10.3390/plants10030497
Si Y, Khanal BP, Sauheitl L, Knoche M. Cutin Synthesis in Developing, Field-Grown Apple Fruit Examined by External Feeding of Labelled Precursors. Plants. 2021; 10(3):497. https://doi.org/10.3390/plants10030497
Chicago/Turabian StyleSi, Yiru, Bishnu P. Khanal, Leopold Sauheitl, and Moritz Knoche. 2021. "Cutin Synthesis in Developing, Field-Grown Apple Fruit Examined by External Feeding of Labelled Precursors" Plants 10, no. 3: 497. https://doi.org/10.3390/plants10030497
APA StyleSi, Y., Khanal, B. P., Sauheitl, L., & Knoche, M. (2021). Cutin Synthesis in Developing, Field-Grown Apple Fruit Examined by External Feeding of Labelled Precursors. Plants, 10(3), 497. https://doi.org/10.3390/plants10030497






