Understanding the Ethnobotany, Chemistry, Pharmacology, and Distribution of Genus Hydnora (Aristolochiaceae)
Abstract
1. Introduction
2. Materials and Methods
3. Botanical Features and Taxonomy
4. Habitat and Ecology of Hydnora Species
5. Host Specificity Concerning Species Distribution
5.1. Finding the Hydnora Species in the Wild
5.2. Discovery of New Sites of Hydnora Species in Kenya
6. Approaches to Conservation Status
7. Ethnobotany
7.1. Species Uses
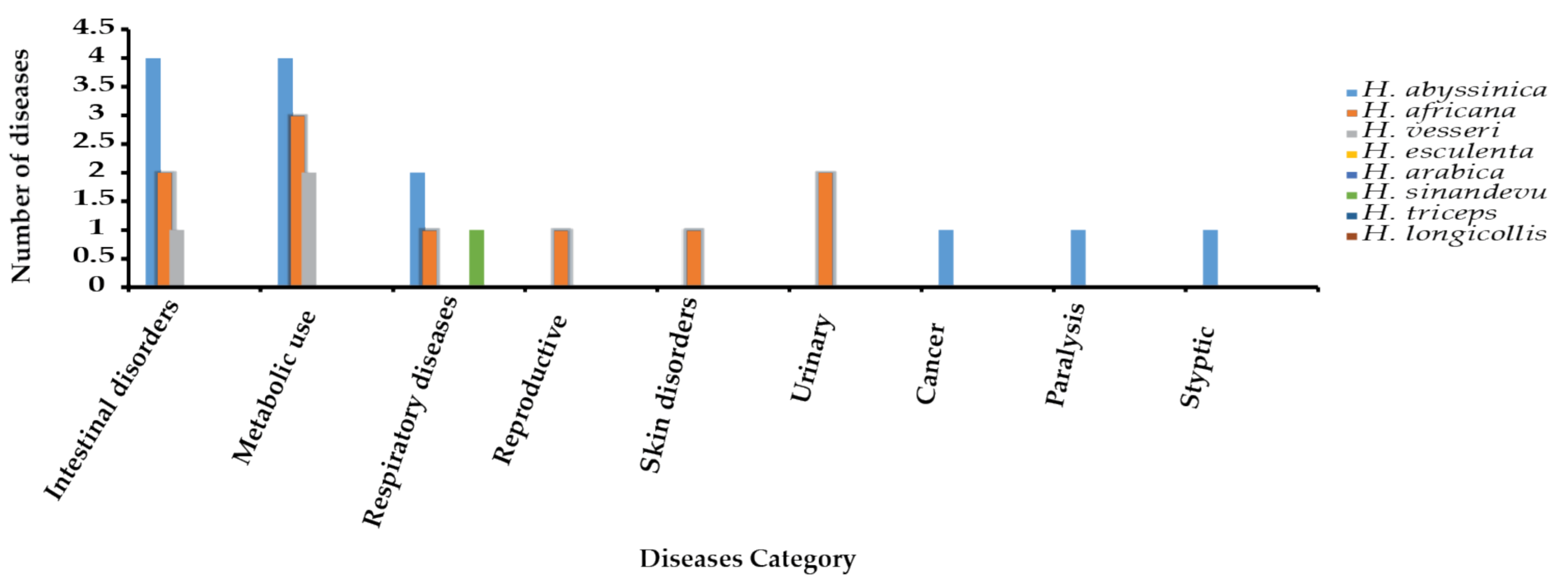
7.2. Summary of Uses
Most Used Species
7.3. Traditional Medicine
8. Chemical Composition and Biological Activities
8.1. Extraction Methods Used in Hydnora Species
8.2. Phytochemistry
8.3. Pharmacology of Hydnora Species
8.4. Antibacterial and Antifungal Activities
8.5. Antioxidant Activity
8.6. Antiproliferative Activity
8.7. Antidiarrheal Activity
9. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bolin, J.F.; Lupton, D.; Musselman, L.J. Hydnora arabica (Aristolochiaceae), a new species from the Arabian Peninsula and a key to Hydnora. Phytotaxa 2018, 338, 99. [Google Scholar] [CrossRef]
- Nickrent, D.; Blarer, A.; Qiu, Y.; Soltis, D.; Zanis, M. Paleoherb status of Hydnoraceae supported by multigene analyses. Botany 2001 PPP 2001, 89, 130–131. [Google Scholar]
- Jost, M.; Naumann, J.; Rocamundi, N.; Cocucci, A.A.; Wanke, S. The first plastid genome of the holoparasitic Genus Prosopanche (Hydnoraceae). Plants 2020, 9, 306. [Google Scholar] [CrossRef]
- Musselman, L.J.; Visser, J.H. Taxonomy and natural history of Hydnora (Hydnoraceae). Aliso J. Syst. Evol. 1989, 12, 317–326. [Google Scholar] [CrossRef]
- Bolin, J.F.; Maass, E.; Musselman, L.J. A new species of Hydnora (Hydnoraceae) from Southern Africa. Syst. Bot. 2011, 36, 255–260. [Google Scholar] [CrossRef]
- Bolin, J.F.; Musselman, L.J. Epitypification and ecological notes for the Malagasy holoparasite Hydnora esculenta (Hydnoraceae). Nord. J. Bot. 2013, 31, 286–290. [Google Scholar] [CrossRef]
- Williams, V.; Wojtasik, E.; Witkowski, E. Ethno-ecological evidence for Hydnora abyssinica occurring in Johannesburg and Durban traditional medicine markets. S. Afr. J. 2011, 77, 268–279. [Google Scholar] [CrossRef][Green Version]
- Bolin, J.F.; Maass, E.; Musselman, L.J. Pollination biology of Hydnora africana Thunb. (Hydnoraceae) in Namibia: Brood-site mimicry with insect imprisonment. Int. J. Plant. Sci. 2009, 170, 157–163. [Google Scholar] [CrossRef]
- Maass, E.; Musselman, L.J. Hydnora triceps (Hydnoraceae)-First record in Namibia and the first description of fruits. Dinteria 2004, 29, 1–10. [Google Scholar]
- JSTOR. Compilation of Hydnora sinandevu. 2020. Available online: https://plants.jstor.org/compilation/hydnora.sinandevu (accessed on 14 December 2020).
- Beentje, H.; Smith, S. Flora of Tropical East Africa. Syst. Geogr. Plants 2001, 71, 265–290. [Google Scholar] [CrossRef]
- Thorogood, C. Hydnora: The strangest plant in the world? PPP 2019, 1, 5–7. [Google Scholar] [CrossRef]
- Rookmaaker, L.; Svanberg, I. Bibliography of Carl Peter Thunberg (1743–1828). Sven. Linnésällskapets Årsskrift 1992, 1993, 7–71. [Google Scholar]
- Dold, T.; Cocks, M.; Sizane, N. Fine fare, rare remedy. Veld. Flora 2003, 89, 12–14. [Google Scholar]
- Hydnora africana Identification Information. 2020. Available online: https://www.biodiversitylibrary.org/page/46783843#page/83/mode/1up (accessed on 16 December 2020).
- Botha, J.; Witkowski, E.; Shackleton, C. An inventory of medicinal plants traded on the western boundary of the Kruger National Park, South Africa. Koedoe 2001, 44, 7–46. [Google Scholar] [CrossRef]
- Cunningham, A. An Investigation of the Herbal Medicine Trade in Natal/KwaZulu; Institute of Natural Resources, University of Natal: Cape Town, South Africa, 1988. [Google Scholar]
- Wojtasik, E. Ethnoecology, Trade and Distribution of the Parasitic Genera Hydnora and Sarcophyte Sold in South African Muthi Markets. Honours Dissertation, University of the Witwatersrand Johannesburg, Johannesburg, South Africa, 2009. [Google Scholar]
- El Ghazali, G.; El Tohami, M.; El Egami, A.; Abdalla, W.; Mohammed, M. Medicinal Plants of the Sudan. Part IV. Medicinal Plants of Northern Kordofan; Omdurman Islamic University Press: Khartoum, Sudan, 1997. [Google Scholar]
- Koko, W.S.; Mesaik, M.A.; Ranjitt, R.; Galal, M.; Choudhary, M.I. Immunosuppressive phenolic compounds from Hydnora abyssinica A. Braun. BMC Complement. Altern Med. 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Hafeez, A.I.A.; Ahmed, A.A.M.; Siror, S.H.A. Extraction and Analysis of Hydnora abyssinica Root (Tartose); Sudan University for Science and Technology: Khartoum, Sudan, 2016. [Google Scholar]
- Serjeant, R. Plants of Dhofar, the Southern Region of Oman, Traditional, Economic and Medicinal Uses; Miller, A.G., Morris, M., Eds.; Office of the Adviser for Conservation of the Environment, Diwan of Royal Court, Sultanate of Oman: Muscat, Oman, 1989; Volume 121, pp. 338–340. [Google Scholar]
- Belayneh, A.; Asfaw, Z.; Demissew, S.; Bussa, N.F. Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda, Eastern Ethiopia. J. Ethnobiol. Ethnomed. 2012, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatimi, M.; Ali, N.; Kilian, N.; Franke, K.; Arnold, N.; Kuhnt, C.; Schmidt, J.; Lindequist, U. Ethnobotany, chemical constituents and biological activities of the flowers of Hydnora abyssinica A. Br. (Hydnoraceae). Pharmazie 2016, 71, 222–226. [Google Scholar] [PubMed]
- Wintola, O.A.; Afolayan, A.J. Chemical constituents and biological activities of essential oils of Hydnora africana Thunb used to treat associated infections and diseases in South Africa. Appl. Sci. 2017, 7, 443. [Google Scholar] [CrossRef]
- Wintola, O.A.; Afolayan, A.J. The antibacterial, phytochemicals and antioxidants evaluation of the root extracts of Hydnora africana Thunb. used as antidysenteric in Eastern Cape Province, South Africa. BMC Complement. Altern Med. 2015, 15, 307. [Google Scholar] [CrossRef]
- Nethathe, B.; Ndip, R. Bioactivity of Hydnora africana on selected bacterial pathogens: Preliminary phytochemical screening. J. Microbiol. Res. 2011, 5, 2820–2826. [Google Scholar]
- Nghinaunye, T. Phytochemical, Antimicrobial and Cytotoxicity Evaluation of Rhizome Extracts of Hydnora Abyssinica from Acacia Nigrences Host; University of Namibia: Windhoek, Namibia, 2019. [Google Scholar]
- Beentje, H.; Luke, Q. Flora of Tropical East. Africa-Hydnoraceae (2002); CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Beentje, H. An Ecological and Floristic Study of the Forests of the Taita Hills, Kenya; National Museums of Kenya: Nairobi, Kenya, 1988. [Google Scholar]
- World Flora Database. Available online: http://www.worldfloraonline.org (accessed on 26 December 2020).
- Global Biodiversity Information Facility. Available online: https://www.gbif.org (accessed on 26 December 2020).
- The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries; Australian National Botanic Gardens. International Plant Names Index; The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries: Cambridge, MA, USA; Australian National Botanic Gardens: Canberra, Australia, 2010.
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 26 December 2020).
- JSTOR. Global Plants on JSTOR. Available online: https://plants.jstor.org/ (accessed on 16 December 2020).
- Royal Botanic Gardens, Kew. The Herbarium Catalogue. Available online: http://www.kew.org/herbcat (accessed on 20 December 2020).
- Royal Botanic Gardens, Kew. World Checklist of Selected Plant Families. Available online: http://wcsp.science.kew.org/ (accessed on 11 December 2020).
- Tropical Plant List. Available online: http://www.theplantlist.org/ (accessed on 11 November 2020).
- Inaturalist. A community for naturalists. Available online: https://www.inaturalist.org/ (accessed on 23 November 2020).
- Chase, M.W.; Christenhusz, M.; Fay, M.; Byng, J.; Judd, W.S.; Soltis, D.; Mabberley, D.; Sennikov, A.; Soltis, P.S.; Stevens, P.F. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. 2016, 181, 1–20. [Google Scholar]
- Cronquist, A.; Takhtadzhian, A.L. An Integrated System of Classification of Flowering Plants; Columbia University Press: New York, NY, USA, 1981. [Google Scholar]
- Takhtadzhian, A.L.; Takhtajan, L.A.; Takhtajan, A. Diversity and Classification of Flowering Plants; Columbia University Press: New York, NY, USA, 1997. [Google Scholar]
- Cocucci, A.; Cocucci, A. Prosopanche (Hydnoraceae): Somatic and Reproductive Structures, Biology, Systematics, Phylogeny and Potentialities as a Parasitic Weed; Junta de Andalucia: Cordoba, Spain, 1996; pp. 179–193.
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. 2009, 161, 105–121. [Google Scholar] [CrossRef]
- Naumann, J.; Der, J.P.; Wafula, E.K.; Jones, S.S.; Wagner, S.T.; Honaas, L.A.; Ralph, P.E.; Bolin, J.F.; Maass, E.; Neinhuis, C. Detecting and characterizing the highly divergent plastid genome of the nonphotosynthetic parasitic plant Hydnora visseri (Hydnoraceae). Genome Biol. Evol. 2016, 8, 345–363. [Google Scholar] [CrossRef]
- Massoni, J.; Forest, F.; Sauquet, H. Increased sampling of both genes and taxa improves resolution of phylogenetic relationships within Magnoliidae, a large and early-diverging clade of angiosperms. Mol. Phylogenet. Evol. 2014, 70, 84–93. [Google Scholar] [CrossRef]
- JSTOR. Hydnora longicolis Distribution Data. Available online: https://plants.jstor.org/fsi/img/size3/alukaplant/p/phase_01/p0002/p00758112.jpg (accessed on 16 December 2020).
- Musselman, L.; Visser, J. The strangest plant in the world. Veld Flora 1986, 71, 109–111. [Google Scholar]
- Tennakoon, K.U.; Bolin, J.F.; Musselman, L.J.; Maass, E. Structural attributes of the hypogeous holoparasite Hydnora triceps Drège & Meyer (Hydnoraceae). Am. J. Bot. 2007, 94, 1439–1449. [Google Scholar]
- Elijah, M.M. A study of the plastid genome of Hydnora abyssinica and the phylogenetic reinstatement of Hydnoraceae. Ph.D. Thesis, Wuhan Botanical Garden Chinese Academy of Sciences, Wuhan, China, 2020. [Google Scholar]
- South African National Biodiversity Institute. Hydnora africana Thunb. Available online: http://pza.sanbi.org/hydnora-africana#:~:text=The%20fruit%20is%20extremely%20astringent,face%20wash%20also%20treat%20acne (accessed on 24 December 2020).
- IUCN Red List.org. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 24 December 2020).
- IUCN. East African Plants Red List Authority 2014. Saintpaulia ionantha. The IUCN Red List of Threatened Species. Plants 2014, 9, 456. [Google Scholar]
- IUCN. International Union for Conservation Guidelines for the Prevention of Biodiversity Loss Caused by Alien Invasive Species Sec3; IUCN: Gland, Switzerland, 2000. [Google Scholar]
- Nyafuono, J.F.; Remigius, Z.B.; Odyek, O. Taxonomy and ethnobotany of Hydnora in lake Mburo National Park (Uganda). ISR J. Plant. Sci. 2000, 48, 99–103. [Google Scholar] [CrossRef]
- Musselman, L.J.; Visser, J.H. Hydnora johannis in Southern Africa. Dinteria 1987, 19, 77–82. [Google Scholar]
- Musselman, L. The genus Hydnora (Hydnoraceae), 5. In Proceedings of the International Symposium of Parasitic Weeds, Nairobi, Kenya, 24–30 June 1991; CIMMYT: Mexico City, Mexico, 1991. [Google Scholar]
- Musselman, L. Parasitic angiosperms of Sudan: Orobanchaceae, Hydnoraceae, and Cuscuta. Notes R. Bot. Gard. Edinb. 1984, 42, 21–39. [Google Scholar]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa: Being an Account of Their Medicinal and Other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal; E. & S. Livingstone: Edinburgh, Scotland, 1962. [Google Scholar]
- Andriamparany, J.N. Diversity, Local Uses and Availability of Medicinal Plants and Wild Yams in the Mahafaly Region of South-Western Madagascar; Universität Kassel: Kassel, Germany, 2015. [Google Scholar]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A.J.P. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Koffi, E.; Sea, T.; Dodehe, Y.; Soro, S. Effect of solvent type on extraction of polyphenols from twenty three Ivorian plants. J. Anim. Plant. Sci. 2010, 5, 550–558. [Google Scholar]
- Saafaa Abdelaziz, M.A. Hydnora abyssinica: Quantitative analysis and antibacterial activity assessment of rhizome ethanoic extract. Int. J. Pharm. Sci. Rev. Res. 2017, 5, 1–11. [Google Scholar]
- Saadabi, A.; Ayoub, S.M.H. Comparative bioactivity of Hydnora abyssinica A. Braun against different groups of fungi and bacteria. J. Med. Plant. 2009, 3, 262–265. [Google Scholar]
- Onyancha, J.M.; Gikonyo, N.K.; Wachira, S.W.; Mwitari, P.G.; Gicheru, M.M. Anticancer activities and safety evaluation of selected Kenyan plant extracts against breast cancer cell lines. J. Pharmacogn. Phytother. 2018, 10, 21–26. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Adam, M.I.M.; Hamadnalla, H.M.Y. Phytochemical screening and antimicrobial activity of Hydnora abyssinica Root Extract. Environ. Sci. Policy 2019, 2, 4. [Google Scholar]
- Mosa, E.; Justin, D.; Hamam, S.; Mohamed, E.; Saad, M. Evaluation of phytochemical and antimicrobial activities of some Sudanese medicinal plants. World. J. Pharm Sci. 2014, 3, 1769–1776. [Google Scholar]
- Elshiekh, Y.H.; Ali, M.A.M. Preliminary phytochemical screening, antibacterial and antioxidant activities of Azanza garckeana (Fruits). GSCBPS 2020, 11, 125–129. [Google Scholar]
- Bisi-Johnson, M.A.; Obi, C.L.; Samuel, B.B.; Eloff, J.N.; Okoh, A.I. Antibacterial activity of crude extracts of some South African medicinal plants against multidrug-resistant etiological agents of diarrhoea. BMC Complement. Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Aeppli, C.; Berg, M.; Hofstetter, T.B.; Kipfer, R.; Schwarzenbach, R.P. Simultaneous quantification of polar and non-polar volatile organic compounds in water samples by direct aqueous injection-gas chromatography/mass spectrometry. J. Chromatogr. A 2008, 1181, 116–124. [Google Scholar] [CrossRef]
- Osman, E.E.; Galal, M. Antioxidant and antiglycation potential of some Sudanese medicinal plants and their isolated compounds. BLACPMA 2009, 8, 402–411. [Google Scholar]
- Maulana, T.; Falah, S.; Andrianto, D. Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity of Water and Ethanol Extract from Surian (Toona sinensis) Leaves; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 012021. [Google Scholar]
- Yagi, S.; Yagi, A.; Gadir, E.A.; Henry, M.; Chapleur, Y.; Laurain-Mattar, D. Toxicity of Hydnora johannis Becca. Dried roots and ethanol extract in rats. J. Ethnopharmacol. 2011, 137, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Alamin, A. Apport de la Chromatographie de Partage Centrifuge à l’Étude Phytochimique de 3 Plantes Utilisées en Médecine Traditionnelle Soudanaise. Ph.D. Thesis, Universite de Tours, Tours, France, 2016; pp. 110–113. [Google Scholar]
- Subhan, N.; Burrows, G.E.; Kerr, P.G.; Obied, H.K. Phytochemistry, ethnomedicine, and pharmacology of Acacia. Stud. Nat. Prod. Chem. 2018, 57, 247–326. [Google Scholar]
- Jackson Seukep, A.; Zhang, Y.-L.; Xu, Y.-B.; Guo, M.-Q. In vitro antibacterial and antiproliferative potential of Echinops lanceolatus Mattf. (Asteraceae) and identification of potential bioactive compounds. Pharmaceuticals 2020, 13, 59. [Google Scholar] [CrossRef]
- Sinisgalli, C.; Faraone, I.; Vassallo, A.; Caddeo, C.; Bisaccia, F.; Armentano, M.F.; Milella, L.; Ostuni, A. Phytochemical profile of Capsicum annuum L. cv Senise, incorporation into liposomes, and evaluation of cellular antioxidant activity. Antioxidants 2020, 9, 428. [Google Scholar] [CrossRef]
- World Health Organization. World Cancer Report: Cancer Research for Cancer Development; IARC: Lyon, France, 2020. [Google Scholar]
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Wintola, O.A.; Afolayan, A.J. Twenty Eight Days Oral Administration Assessment of Hydnora africana Thunb. Aqueous root extract on key metabolic markers of wistar rats. Int. J. Pharm. Sci. Res. 2018, 9, 1713–1722. [Google Scholar]
- Pawlowski, S.W.; Warren, C.A.; Guerrant, R. Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology 2009, 136, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Schiller, L.R. Antidiarrheal drug therapy. Curr. Gastroenterol. Rep. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Yassin, H.M.; El Badwi, S.M. Potential anti-diarrhoeal activity of aqueous root extract of Hydnora abyssinica in Rats. Available online: http://khartoumspace.uofk.edu/handle/123456789/9289 (accessed on 14 February 2021).
- Awolola, G.V.; Sofidiya, M.O.; Baijnath, H.; Noren, S.S.; Koorbanally, N.A. The phytochemistry and gastroprotective activities of the leaves of Ficus glumosa. S. Afr. J. Bot. 2019, 126, 190–195. [Google Scholar] [CrossRef]
- Williams, V.; Falcão, M.; Wojtasik, E. Hydnora abyssinica: Ethnobotanical evidence for its occurrence in southern Mozambique. S. Afr. J. Bot. 2011, 77, 474–478. [Google Scholar] [CrossRef][Green Version]
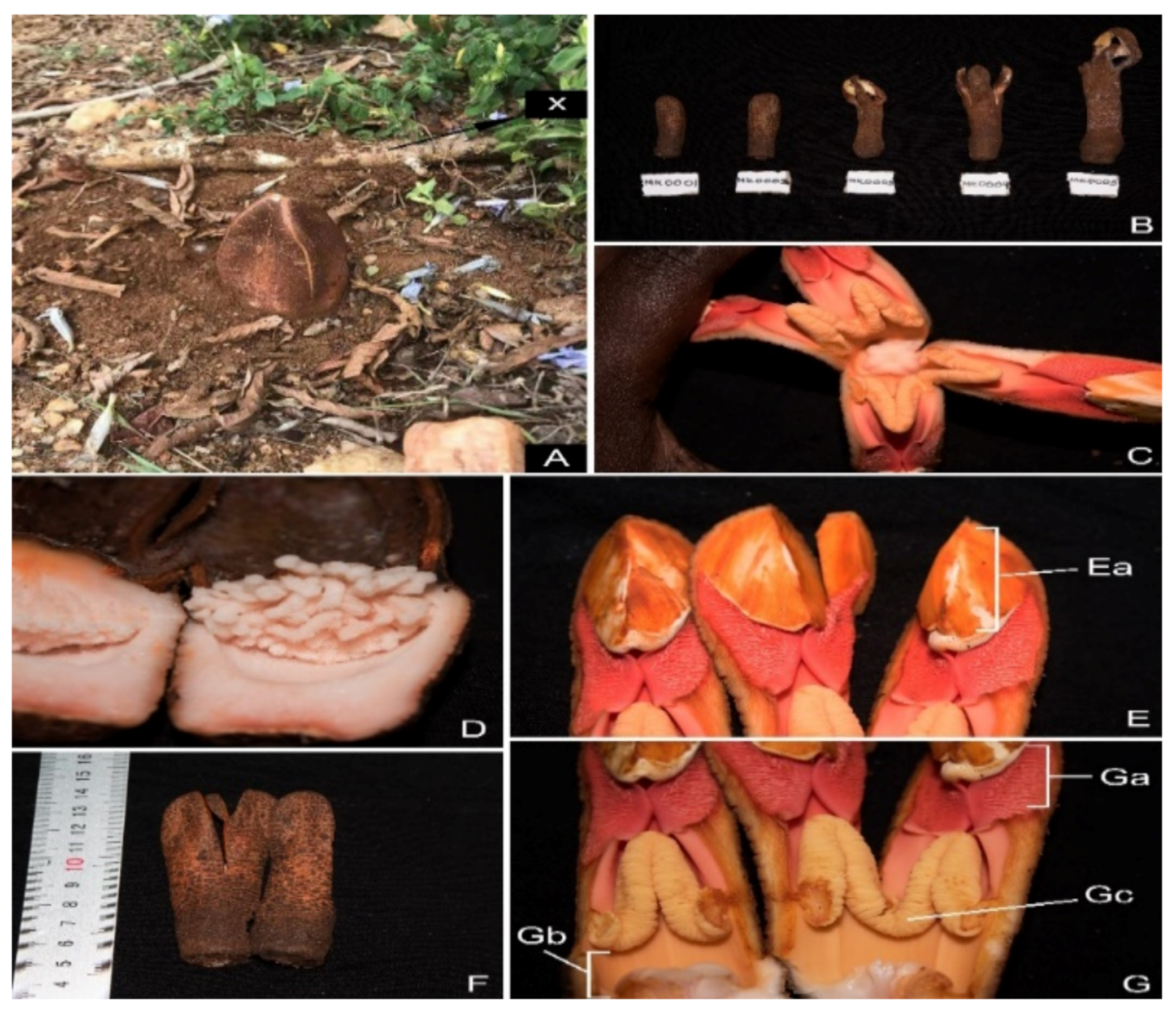
| Species | Ecological Conditions and Host | References |
|---|---|---|
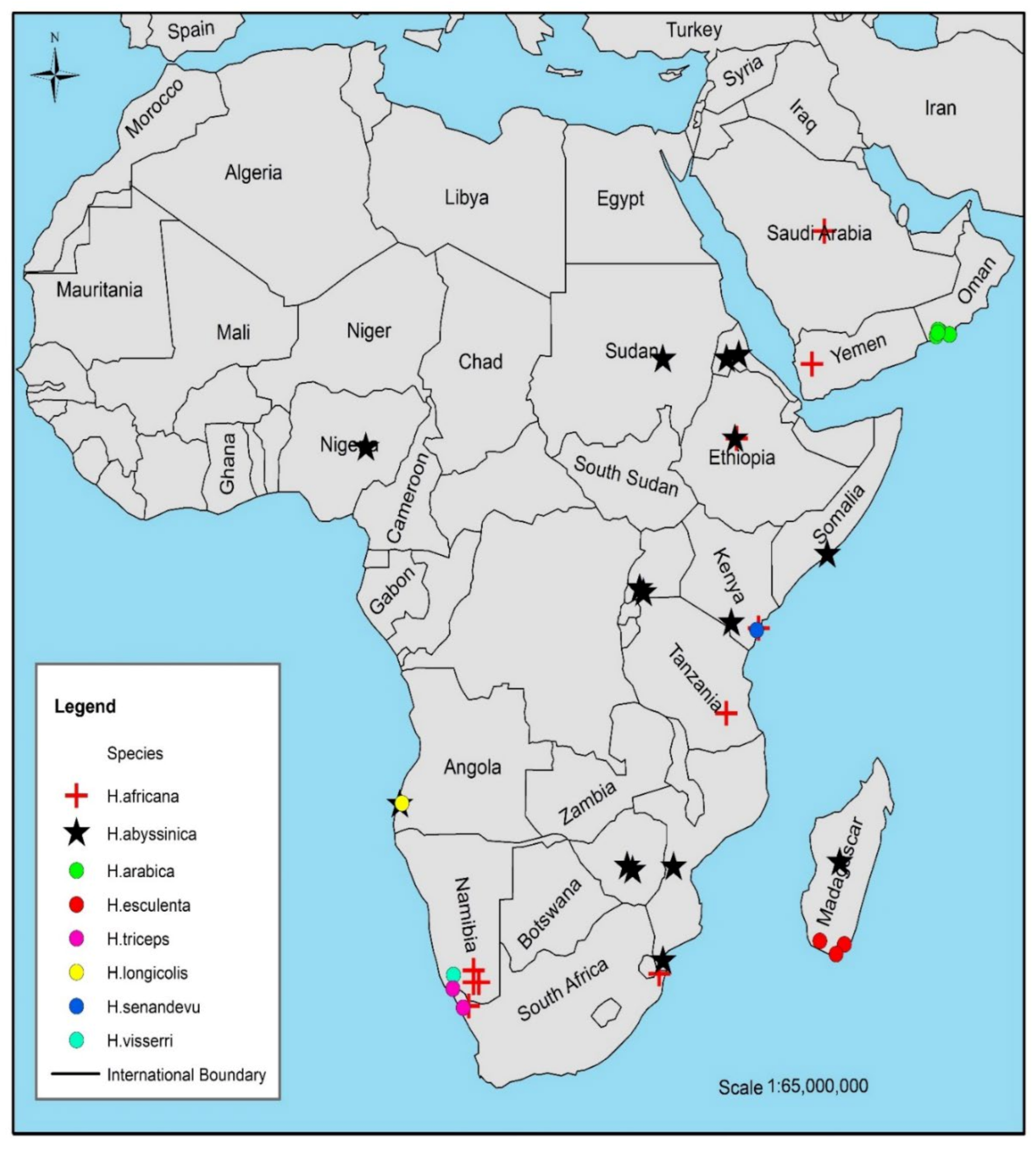
| H. abyssinica | Found in dry woodland, wooded grassland, or bushland Host plants include Acacia karoo Hayne, A. nilotica (L.) Delile, A luederitz Engl., A. tortilis (Forssk.) Hayne, A. seyal Delile, A. gerradii Benth., A. karoo Hayne, A. xanthophloea Benth., A. grandicornuta Geratner, A. tortilis subsp. heteracantha (Burch.) Brenan and A. nigrescens Oliv. | [6,40] |
| H. africana | Found in the semi-arid arid and dry regions Host plants are Euphorbia mauritanica L., E. tirucalli L., E. caputmedusae L., E. indecora N.E.Br. (=Euphorbia decussata E.Mey. ex Boiss), E. gregaria Marloth, E. gummifera Boiss, E. karrounsis (Bois.) N.E Br, E. lignosa Marloth, E. mauritanica L., and Albizzia lebbek (L.) Benth. | [8,12] |
| H. arabica | It grows above the ground surface only when flowering Host plants; Acacia tortilis (Forssk.) Hayne and Pithecellobium dulce (Roxb.) Benth. | [1] |
| H. sinandevu | Found in scattered tree grassland, Commiphora bushland, thicket, or forest margin between mangrove and forest Host plants; Commiphora campestris Engl. and C. africana (A.Rich.) Endl. roots, as well as on Pterocarps or Ostryodris | [10,11] |
| H. visseri | Found in succulent karoo and Nama-Karoo vegetation in Namibia and the Northern Cape region of South Africa Host plants; Euphorbia gregaria Marloth, and Euphorbia gummifera Boiss. | [5] |
| H. esculenta | Found in semi-arid and dry areas in East Madagascar Mostly parasitizing Fabaceae species, Albizzia tulearensis R.Vig., and Pithecellobium dulce (Roxb.) Benth. | [7] |
| H. triceps | It is found in South Africa and Namibia Host plants are Euphorbia dregeana E. Mey. ex Boiss., and Zygophyllum orbiculatum Welw. ex Oliv. | [6,9] |
| H. longicollis | Found in Angola Host plants include Zygophyllum orbiculatum Welw. ex Oliv., Euphorbia damarana L.C.Leach, and other Euphorbia species | [5] |
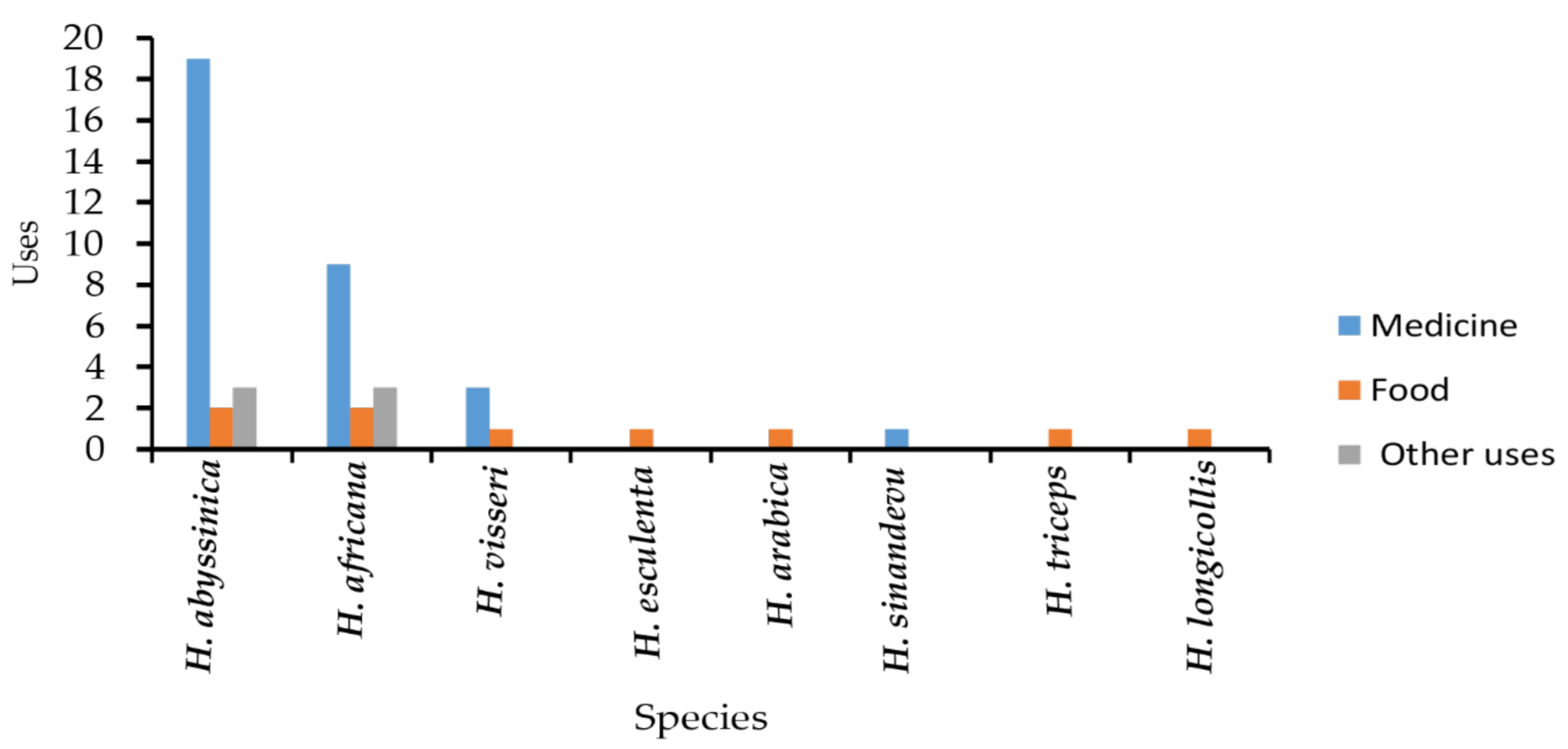
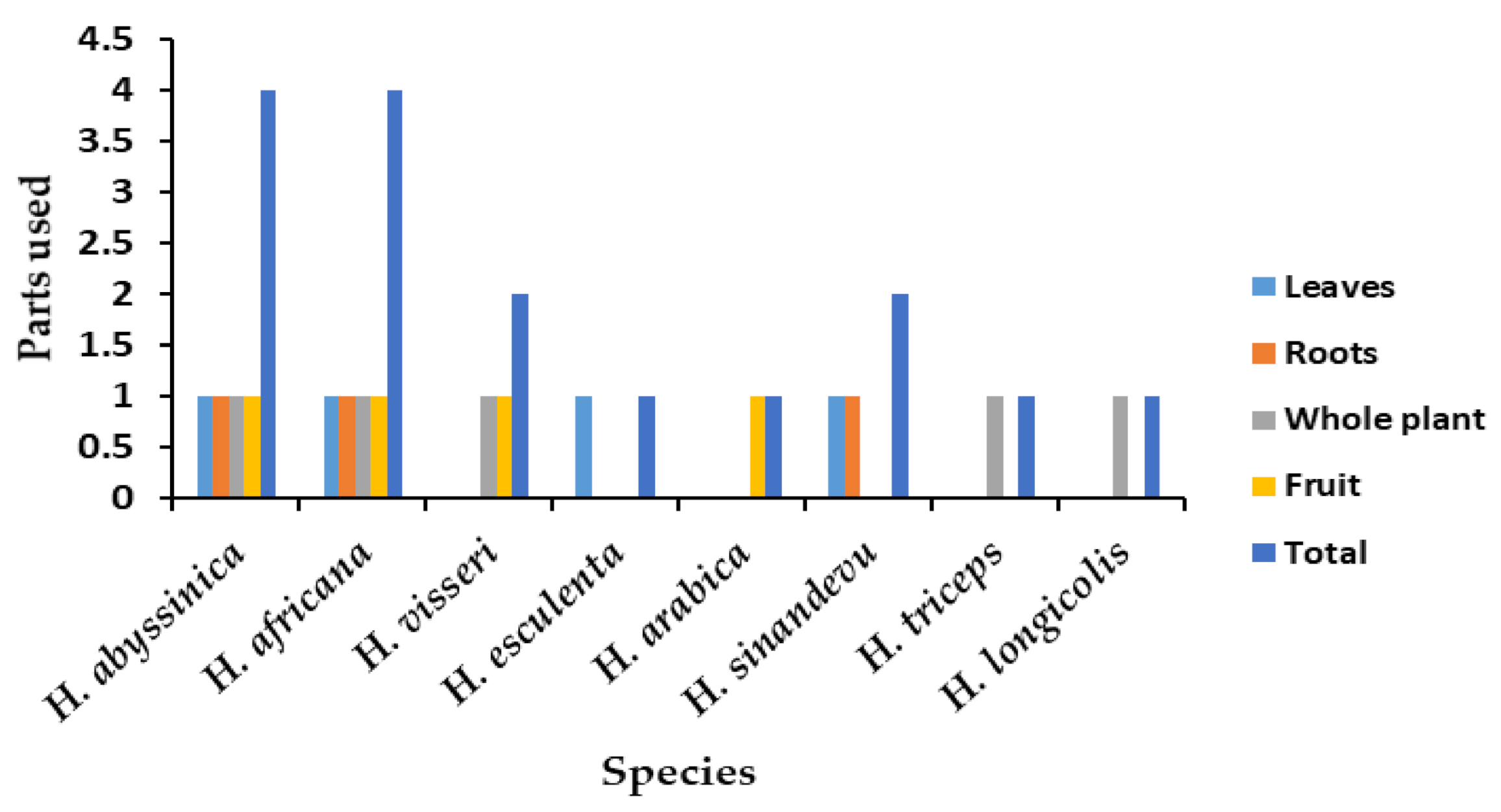
| Species | Use | References |
|---|---|---|
| H. africana | Food, tanning leather, fishing nets preservation, treat dysentery, chronic diarrhea, stomach crumps, stop bleeding, kidney and bladder, treat acne, inflamed throat | [14,51] |
| H. abyssinica | Food, treat dysentery, diarrhea, cholera, swelling of tonsillitis, charcoal, tanning leather, paralysis, diabetes, hiccups, fever, insomnia, hypertension, measles, hemorrhoids, throat inflammation, styptic gastric ulcer, and cancer | [6,24,52,53] |
| H. visseri | Food, treatment of diarrhea, hypertension, and diabetes | [5] |
| H. esculenta | Source of food | [4,7] |
| H. arabica | Source of food (fruits) | [1] |
| H. triceps | Food for wild animals | [9] |
| H. sinandevu | Treatment of throat infections | [51] |
| H. longicollis | Food for wild animals | [5] |
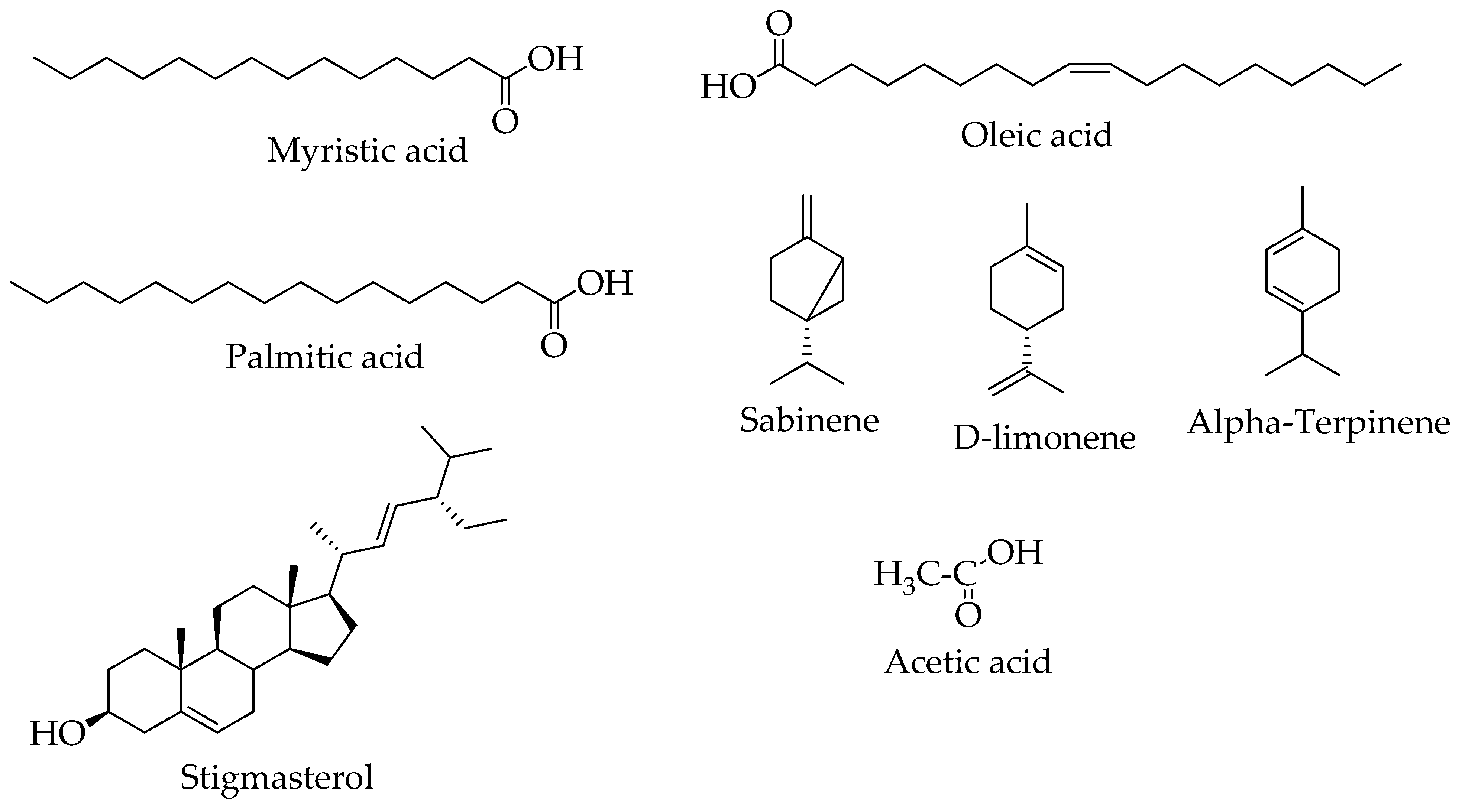
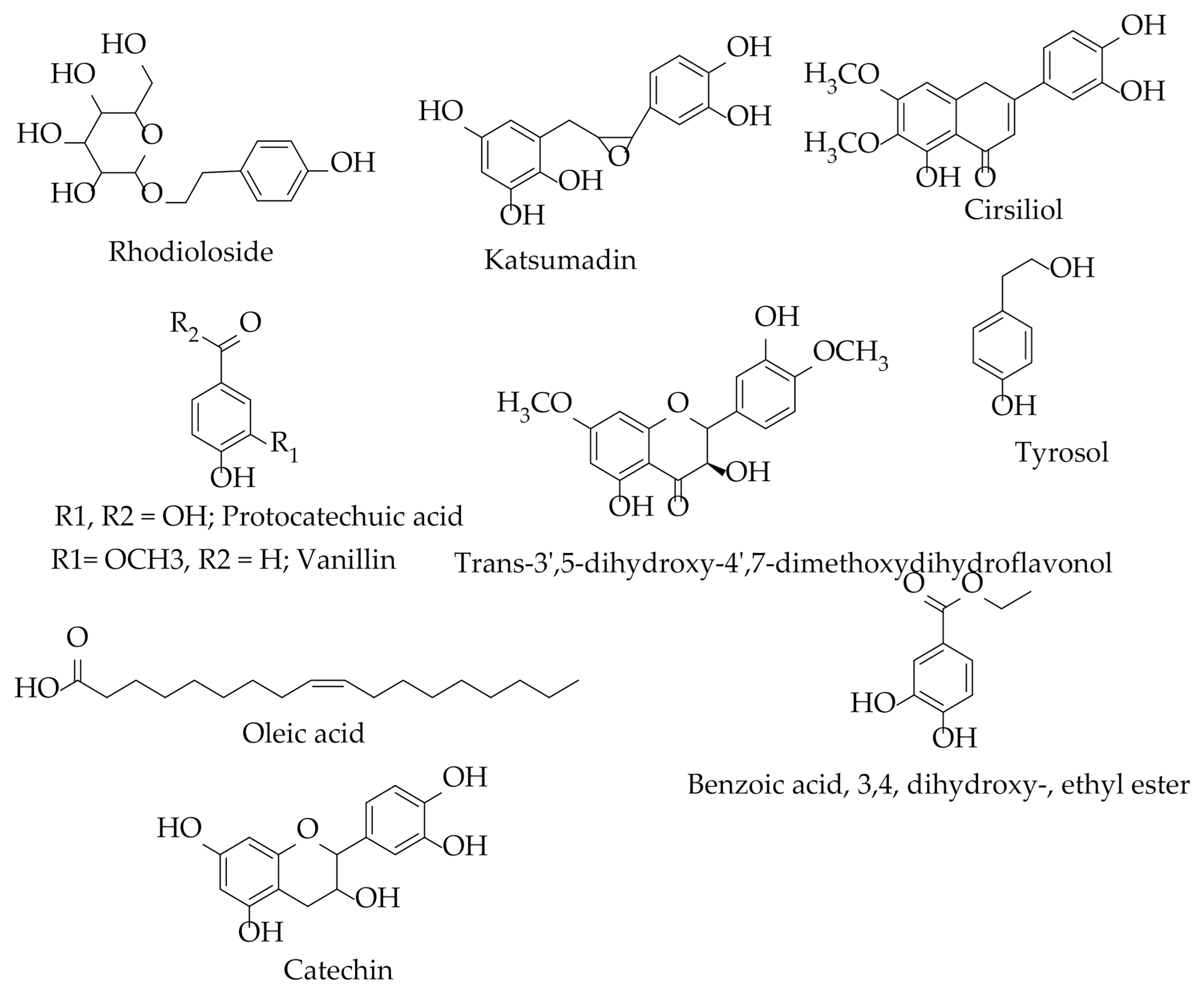
| Chemical Classes | Compounds | Plant/Part(s) | Characterization Method | References |
|---|---|---|---|---|
| Phenylpropanoids | Katsumadin | H. abyssinica rhizomes | NMR | [74] |
| Flavonoids | Catechin | H. abyssinica (whole plant and roots) | NMR | [20,73,74] |
| Cirsiliol | ||||
| Trans 3′ 5-dihydroxy- 4′ 7-dimethoxydihydroflavonol | ||||
| Esters | 2-hydroxyhexadecyl ester | H.abyssinica | NMR/ GC-MS | [20,26] |
| Benzoic acid, 3, 4, dihydroxy-, ethyl ester | ||||
| Fatty acids | Myristic acid | H. abyssinica (whole plant and roots) | ||
| Oleic acid | H. abyssinica roots | [74] | ||
| Palmitic acid | H. abyssinica roots | GC-MS | ||
| Phenolic acids/derivative | Tyrosol | H. abyssinica (plant and roots) | NMR | [20,72,74] |
| Protocatechuic acid | ||||
| Aldehydes | Vanillin | |||
| Monoterpenes | Rhodioloside (glycoside) | H. abyssinica rhizomes | NMR | [74] |
| Sabinene | H. abyssinica flowers | GC-MS | [24] | |
| D-limonene | ||||
| γ-Terpinene | ||||
| α-Terpinene | ||||
| Sterols | stigmasterol | H. abyssinica roots | [74] | |
| Organic acids | Acetic acid | H. abyssinica flowers | GC-MS | [24] |
| Plant/Part Investigated | Assay Method | Results | References |
|---|---|---|---|
| H. abyssinica flowers | Agar diffusion | All extracts showed low activity on yeast, Gram-positive, and Gram-negative bacteria | [24] |
| H. abyssinica rhizome | Agar disc diffusion/well diffusion | In both assays, the methanol and methanol-dichloromethane extracts exhibited moderate to high activities against bacteria tests. Their highest activity was against Candida albicans. Water extract showed relatively low activity. Generally, the inhibition activities were dose-dependent | [29] |
| H. abyssinica rhizome | Cup-plate agar diffusion | This sample showed promising antibacterial activity against the four bacteria and fungi strains assayed; however, it showed no inhibition against Sesbania leptocarpa | [67] |
| H. abyssinica rhizome | Disk diffusion | The crude extract had the highest activity of >20 mm minimum inhibition diameter | [63] |
| H. abyssinica root | Disk diffusion | The methanol extract exhibited low antibacterial activity. No activity was observed at 6.5 and 12.5 mg/mL sample concentrations on Bacillus subtillis | [21] |
| H. abyssinica root | Cup-plate agar diffusion | Methanol, chloroform, and petroleum ether exhibited partial to high antibacterial activity on all strains tested except on Pseudomonas aeruginosa where no activity was observed. High inhibition zones were observed in methanol extract. The activities were dose-dependent | [66] |
| H. abyssinica root | Cup-plate agar diffusion | The extracts’ activity increased with an increase in concentration. The water extract exhibited higher inhibition against all fungi and bacteria strains with >16 and >6 mm, respectively. A weak activity was observed in chloroform | [64] |
| H. abyssinica root | Broth microdilution | The water extract exhibited high activity against Enterococcus faecalis, Bacillus cereus, and Bacillus subtilis with MIC values of 16 and 64 µg/mL, respectively. Low activity was observed in all other antibacterial tests | [20] |
| H. africana | Agar well diffusion | The MIC50 of methanol, acetone, ethanol, and ethyl acetate extracts ranged from 0.078–2.5 mg/mL. Methanol extract had no inhibition against H. pylori | [27] |
| H. africana | Dilution microplate | The sample showed moderate activity against all bacteria strains. The activity was both time and concentration-dependent | [69] |
| H. africana | Agar well diffusion | Acetone and ethanol extract exhibited moderate to high MIC as compared to ciprofloxacin (drug). The aqueous extract showed no activity. The ethanol extract was highly active on both Escherichia coli and Pseudomonas aureginosa. Acetone had high activity against Shigella sonnei | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkala, E.M.; Mutungi, M.M.; Mutinda, E.S.; Oulo, M.A.; Wanga, V.O.; Mwachala, G.; Hu, G.-W. Understanding the Ethnobotany, Chemistry, Pharmacology, and Distribution of Genus Hydnora (Aristolochiaceae). Plants 2021, 10, 494. https://doi.org/10.3390/plants10030494
Mkala EM, Mutungi MM, Mutinda ES, Oulo MA, Wanga VO, Mwachala G, Hu G-W. Understanding the Ethnobotany, Chemistry, Pharmacology, and Distribution of Genus Hydnora (Aristolochiaceae). Plants. 2021; 10(3):494. https://doi.org/10.3390/plants10030494
Chicago/Turabian StyleMkala, Elijah Mbandi, Moses Mutuse Mutungi, Elizabeth Syowai Mutinda, Millicent Akinyi Oulo, Vincent Okelo Wanga, Geoffrey Mwachala, and Guang-Wan Hu. 2021. "Understanding the Ethnobotany, Chemistry, Pharmacology, and Distribution of Genus Hydnora (Aristolochiaceae)" Plants 10, no. 3: 494. https://doi.org/10.3390/plants10030494
APA StyleMkala, E. M., Mutungi, M. M., Mutinda, E. S., Oulo, M. A., Wanga, V. O., Mwachala, G., & Hu, G.-W. (2021). Understanding the Ethnobotany, Chemistry, Pharmacology, and Distribution of Genus Hydnora (Aristolochiaceae). Plants, 10(3), 494. https://doi.org/10.3390/plants10030494










