Potential Application of Some Lamiaceae Species in the Management of Diabetes
Abstract
1. Introduction
2. Method
3. Lamiaceae Species Used for Diabetes
3.1. Ajuga iva (L.) Schreb
3.1.1. Traditional Uses
3.1.2. Phytochemical Constituents
3.1.3. Antidiabetic Activity
3.2. Ballota nigra L.
3.2.1. Traditional Uses
3.2.2. Phytochemical Constituents
3.2.3. Antidiabetic Activity
3.3. Becium grandiflorum (Lam.) Pic. Serm.
3.3.1. Traditional Uses
3.3.2. Phytochemical Constituents
3.3.3. Antidiabetic Activity
3.4. Calamintha officinalis Moench
3.4.1. Traditional Uses
3.4.2. Phytochemical Constituents
3.4.3. Antidiabetic Activity
3.5. Coleus forskohlii (Willd.) Briq
3.5.1. Traditional Uses
3.5.2. Phytochemical Constituents
3.5.3. Antidiabetic Activity
3.6. Hyptis suaveolens (L.) Poit
3.6.1. Traditional Uses
3.6.2. Phytochemical Constituents
3.6.3. Antidiabetic Activity
3.7. Lavandula angustifolia Mill
3.7.1. Traditional Uses
3.7.2. Phytochemical Constituents
3.7.3. Antidiabetic Activity
3.8. Lavandula dentata L.
3.8.1. Traditional Uses
3.8.2. Phytochemical Constituents
3.8.3. Antidiabetic Activity
3.9. Lavandula multifida L.
3.9.1. Traditional Uses
3.9.2. Phytochemical Constituents
3.9.3. Antidiabetic Activity
3.10. Lavandula stoechas L.
3.10.1. Traditional Uses
3.10.2. Phytochemical Constituents
3.10.3. Antidiabetic Activity
3.11. Leonotis leonurus (L.) R.Br
3.11.1. Traditional Uses
3.11.2. Phytochemical Constituents
3.11.3. Antidiabetic Activity
3.12. Leonotis nepetifolia (L.) R.Br
3.12.1. Traditional Uses
3.12.2. Phytochemical Constituents
3.12.3. Antidiabetic Activity
3.13. Marrubium vulgare L.
3.13.1. Traditional Uses
3.13.2. Phytochemical Constituents
3.13.3. Antidiabetic Activity
3.14. Ocimum gratissimum L.
3.14.1. Traditional Uses
3.14.2. Phytochemical Constituents
3.14.3. Antidiabetic Activity
3.15. Ocimum sanctum L.
3.15.1. Traditional Uses
3.15.2. Phytochemical Constituents
3.15.3. Antidiabetic Activity
3.16. Ocimum basilicum L.
3.16.1. Traditional Uses
3.16.2. Phytochemical Constituents
3.16.3. Antidiabetic Activity
3.17. Ocimum canum L.
3.17.1. Traditional Uses
3.17.2. Phytochemical Constituents
3.17.3. Antidiabetic Activity
3.18. Rosmarinus officinalis L.
3.18.1. Traditional Uses
3.18.2. Phytochemical Constituents
3.18.3. Antidiabetic Activity
3.19. Salvia lavandulifolia Valh
3.19.1. Traditional Uses
3.19.2. Phytochemical Constituents
3.19.3. Antidiabetic Activity
3.20. Salvia officinalis L.
3.20.1. Traditional Uses
3.20.2. Phytochemical Constituents
3.20.3. Antidiabetic Activity
3.21. Salvia fruticosa Mill
3.21.1. Traditional Uses
3.21.2. Phytochemical Constituents
3.21.3. Antidiabetic Activity
3.22. Teucrium polium L.
3.22.1. Traditional Uses
3.22.2. Phytochemical Constituents
3.22.3. Antidiabetic Activity
3.23. Teucrium cubense Jacq
3.23.1. Traditional Uses
3.23.2. Phytochemical Constituents
3.23.3. Antidiabetic Activity
4. Antidiabetic Activity of Some Notable Chemical Constituents Isolated from Lamiaceae Species
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tripathy, J.P. Burden and risk factors of diabetes and hyperglycemia in India: Findings from the Global Burden of Disease Study 2016. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 381–387. [Google Scholar] [CrossRef] [PubMed]
- De Fronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19. [Google Scholar] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Dal, S.; Sigrist, S. The Protective Effect of Antioxidants Consumption on Diabetes and Vascular Complications. Diseases 2016, 4, 24. [Google Scholar] [CrossRef]
- Etsassala, N.G.E.R.; Badmus, J.A.; Waryo, T.; Marnewick, J.; Cupido, C.N.; Hussein, A.A.; Iwuoha, E. Alpha-Glucosidase and Alpha-Amylase Inhibitory Activities of Novel Abietane Diterpenes from Salvia africana-lutea. Antioxidants 2019, 8, 421. [Google Scholar] [CrossRef]
- Hussein, A.A. Chemistry of South African Lamiaceae: Structures and biological activity of terpenoids. Terpenes and Terpenoids 2018. [Google Scholar] [CrossRef]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res. Manag. 2018, 2018, 1–44. [Google Scholar] [CrossRef]
- Raja, R.R. Medicinally Potential Plants of Labiatae (Lamiaceae) Family: An Overview. Res. J. Med. Plant 2012, 6, 203–213. [Google Scholar] [CrossRef]
- Balogun, F.O.; Tshabalala, N.T.; Ashafa, A.O.T. Antidiabetic Medicinal Plants Used by the Basotho Tribe of Eastern Free State: A Review. J. Diabetes Res. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.-E.; Balahbib, A.; El-Shazly, M.; Chamkhi, I. Ethnomedicinal use, phytochemistry, pharmacology, and toxicology of Ajuga iva (L.) schreb. J. Ethnopharmacol. 2020, 258, 112875. [Google Scholar] [CrossRef] [PubMed]
- Eddouks, M.; Ajebli, M.; Hebi, M. Ethnopharmacological survey of medicinal plants used in Daraa-Tafilalet region (Province of Errachidia), Morocco. J. Ethnopharmacol. 2017, 198, 516–530. [Google Scholar] [CrossRef]
- Al-Aboudi, A.; Afifi, F.U. Plants used for the treatment of diabetes in Jordan: A review of scientific evidence. Pharm. Biol. 2010, 49, 221–239. [Google Scholar] [CrossRef]
- Chouitah, O.; Meddah, B.; Aoues, A.; Sonnet, P. Essential oil from the leaves of Ajuga iva: Chemical composition and anti-microbial activity. J. Essent. 2017, 20, 873–877. [Google Scholar]
- Fettach, S.; Mrabti, H.; Sayah, K.; Bouyahya, A.; Salhi, N.; Cherrah, Y.; El Abbes, F. Phenolic content, acute toxicity of Ajuga iva extracts and assessment of their antioxidant and carbohydrate digestive enzyme inhibitory effects. S. Afr. J. Bot. 2019, 125, 381–385. [Google Scholar] [CrossRef]
- Boudjelal, A.; Siracusa, L.; Henchiri, C.; Sarri, M.; Abderrahim, B.; Baali, F.; Ruberto, G. Antidiabetic effects of aqueous infu-sions of Artemisia herba-alba and Ajuga iva in alloxan-induced diabetic rats. Planta Med. 2015, 81, 696–704. [Google Scholar]
- El Hilaly, J.; Tahraoui, A.; Israili, Z.H.; Lyoussi, B. Hypolipidemic effects of acute and sub-chronic administration of an aqueous extract of Ajuga iva L. whole plant in normal and diabetic rats. J. Ethnopharmacol. 2006, 105, 441–448. [Google Scholar] [CrossRef]
- Wang, J.J.; Jin, H.; Zheng, S.-L.; Xia, P.; Cai, Y.; Ni, X.-J. Phytoecdysteroids from Ajuga iva act as potential antidiabetic agent against alloxan-induced diabetic male albino rats. Biomed. Pharmacother. 2017, 96, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. The Pharmacological Importance of Ballota nigra—A review. Ind. J. Pharm. Sci. Res. 2015, 5, 249–256. [Google Scholar]
- Nusier, M.K.; Bataineh, H.N.; Bataineh, Z.M.; Daradka, H.M. Effects of Ballota nigra on glucose and insulin in alloxan-diabetic albino rats. Neuro Endocrinol. Lett. 2007, 28, 470–472. [Google Scholar] [PubMed]
- Grayer, R.J.; Veitch, N.C. An 8-hydroxylated external flavone and its 8-O-glucoside from Becium grandiflorum. Phytochemistry 1998, 47, 779–782. [Google Scholar] [CrossRef]
- Beshir, K.; Shibeshi, W.; Ejigu, A.; Engidawork, E. In-vivo wound healing activity of 70% ethanol leaf extract of Becium gran-diflorum Lam. (Lamiaceae) in mice. Ethiop. Pharmarm. J. 2016, 32, 117–130. [Google Scholar] [CrossRef]
- Gebremeskel, L.; Tuem, K.B.; Teklu, T. Evaluation of Antidiabetic Effect of Ethanolic Leaves Extract of Becium grandiflorum Lam. (Lamiaceae) in Streptozotocin-Induced Diabetic Mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1481–1489. [Google Scholar] [CrossRef]
- Moattar, F.S.; Sariri, R.; Giahi, M.; Yaghmaee, P.; Ghafoori, H.; Jamalzadeh, L. Antioxidant and Anti-Proliferative Activity of Calamintha officinalis Extract on Breast Cancer Cell Line MCF-7. J. Biol. Sci. 2015, 15, 194–198. [Google Scholar] [CrossRef]
- Monforte, M.T.; Tzakou, O.; Nostro, A.; Zimbalatti, V.; Galati, E.M. Chemical composition and biological activities of Cala-mintha officinalis Moench essential oil. J. Med. Food 2011, 14, 297–303. [Google Scholar] [CrossRef]
- Khera, N.; Bhatia, A. Medicinal plants as natural antidiabetic agents. Int. J. Pharm. Life Sci. 2014, 5, 713–729. [Google Scholar]
- Monforte, M.T.; Lanuzza, F.; Pergolizzi, S.; Mondello, F.; Tzakou, O.; Galati, E.M. Protective effect of Calamintha officinalis Moench leaves against alcohol-induced gastric mucosa injury in rats. Macroscopic, histologic and phytochemical analysis. Phytother. Res. 2012, 26, 839–844. [Google Scholar] [CrossRef]
- Singh, P.P.; Jha, S.; Irchhaiya, R.; Fatima, A.; Agarwal, P. A review on phytochemical and pharmacological potential of Cala-mintha officinalis Moench. Int. J. Pharm. Sci. Res. 2012, 3, 1001–1004. [Google Scholar]
- Lemhadri, A.; Zeggwagh, N.A.; Maghrani, M.; Jouad, H.; Michel, J.B.; Eddouks, M. Hypoglycaemic effect of Calamintha offici-nalis Moench in normal and streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2004, 56, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Loftus, H.L.; Astell, K.J.; Mathai, M.L.; Su, X.Q. Coleus forskohlii Extract Supplementation in Conjunction with a Hypocaloric Diet Reduces the Risk Factors of Metabolic Syndrome in Overweight and Obese Subjects: A Randomized Controlled Trial. Nutrients 2015, 7, 9508–9522. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Silva, M.; Trujillo, X.; Trujillo-Hernández, B.; Sánchez-Pastor, E.; Urzúa, Z.; Mancilla, E.; Huerta, M. Effect of Chronic Administration of Forskolin on Glycemia and Oxidative Stress in Rats with and without Experimental Diabetes. Int. J. Med. Sci. 2014, 11, 448–452. [Google Scholar] [CrossRef]
- Henderson, S.; Magu, B.; Rasmussen, C.; Lancaster, S.; Kerksick, C.; Smith, P.; Melton, C.; Cowan, P.; Greenwood, M.; Earnest, C.P.; et al. Effects of Coleus forskohlii Supplementation on Body Composition and Hematological Profiles in Mildly Overweight Women. J. Int. Soc. Sports Nutr. 2005, 2, 54–62. [Google Scholar] [CrossRef]
- Patel, M.B. Forskolin: A successful therapeutic phytomolecule. East Cent. Afr. J. Pharm. Sci. 2010, 13, 25–32. [Google Scholar]
- Khan, B.A.; Akhtar, N.; Anwar, M.; Mahmood, T.; Khan, H.; Hussain, I.; Khan, K.A. Botanical description of Coleus forskohlii: A review. J. Med. Plant Res. 2012, 6, 4832–4835. [Google Scholar]
- Bhowal, M.; Mehta, D.M. Coleus forskholii: Phytochemical and pharmacological profile. Int. J. Pharm. Sci. 2017, 8, 3599–3618. [Google Scholar]
- Yokotani, K.; Chiba, T.; Sato, Y.; Umegaki, K. Coleus forskohlii Extract Attenuates the Hypoglycemic Effect of Tolbutamide in vivo via a Hepatic Cytochrome P450-Mediated Mechanism. J. Food Hyg. Soc. Jpn. (Shokuhin Eiseigaku Zasshi) 2014, 55, 73–78. [Google Scholar] [CrossRef]
- Khatun, S.; Chatterjee, N.C.; Cakilcioglu, U. Antioxidant activity of the medicinal plant Coleus forskohlii Briq. Afr. J. Biotechnol. 2011, 10, 2530–2535. [Google Scholar]
- Sharma, P.P.; Roy, R.K.; Anurag, D.G.; Vipin, K.S. Hyptis suaveolens (L.) poit: A phyto-pharmacological review. Int. J. Chem. Pharm. Anal. 2013, 4, 1–11. [Google Scholar]
- Nayak, S.; Kar, D.M. Evaluation of antidiabetic and antioxidant activity of aerial parts of Hyptis suaveolens Poit. Afr. J. Pharm. Pharmacol. 2013, 7, 1–7. [Google Scholar] [CrossRef]
- Jayakumar, S.V.; Ganesh, S.K. New enolic type bioactive constituents from Hyptis suaveolens (L.) Poit. Asian J. Plant Sci. Res. 2012, 2, 403–408. [Google Scholar]
- Mishra, S.B.; Verma, A.; Mukerjee, A.; Vijayakumar, M. Anti-hyperglycemic activity of leaves extract of Hyptis suaveolens L. Poit in streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2011, 4, 689–693. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L.). A review. Herba Polonica 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Issa, A.; Mohammad, M.; Hudaib, M.; Tawah, K.; Rjai, T.A.; Oran, S.; Bustanji, Y. A potential role of Lavandula angustifolia in the management of diabetic dyslipidemia. J. Med. Plant Res. 2011, 5, 3876–3882. [Google Scholar]
- Bakhsha, F.; Mazandarani, M.; Aryaei, M.; Jafari, S.Y.; Bayate, H. Phytochemical and Anti-oxidant Activity of Lavandula angustifolia Mill. Essential oil on Preoperative Anxiety in Patients undergoing Diagnostic Curettage. Int. J. Women’s Health Reprod. Sci. 2014, 2, 268–271. [Google Scholar] [CrossRef]
- Mirjalili, M.H.; Salehi, P.; Vala, M.M.; Ghorbanpour, M. The effect of drying methods on yield and chemical constituents of the essential oil in Lavandula angustifolia Mill. (Lamiaceae). Plant Physiol. Rep. 2019, 24, 96–103. [Google Scholar] [CrossRef]
- Sanna, M.D.; Les, F.; López, V.; Galeotti, N. Lavender (Lavandula angustifolia Mill.) Essential Oil Alleviates Neuropathic Pain in Mice with Spared Nerve Injury. Front. Pharmacol. 2019, 10, 472. [Google Scholar] [CrossRef]
- Yadikar, N.; Bobakulov, K.; Li, G.; Aisa, H.A. Seven new phenolic compounds from Lavandula angustifolia. Phytochem. Lett. 2018, 23, 149–154. [Google Scholar] [CrossRef]
- Chhetri, B.K.; Ali, N.A.A.; Setzer, W.N. A Survey of Chemical Compositions and Biological Activities of Yemeni Aromatic Medicinal Plants. Medicines 2015, 2, 67–92. [Google Scholar] [CrossRef] [PubMed]
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical survey of medicinal plants used in the traditional treatment of diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Renaud, E.N.C.; Charles, D.J.; Simon, J.E. Essential Oil Quantity and Composition from 10 Cultivars of Organically Grown Lavender and Lavandin. J. Essent. Oil Res. 2001, 13, 269–273. [Google Scholar] [CrossRef]
- Martins, R.D.P.; Gomes, R.A.D.S.; Granato, A.C.; Okura, M.H. Chemical characterization of Lavandula dentata L. essential oils grown in Uberaba-MG. Ciência Rural 2019, 49, 49. [Google Scholar] [CrossRef]
- Almohawes, Z.N.; AlRuhaimi, H.S. Effect of Lavandula dentata extract on Ovalbumin-induced Asthma in Male Guinea Pigs. Braz. J. Biol. 2020, 80, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; De Tommasi, N.; Pescitelli, G.; Di Bari, L.; Morelli, I.; Braca, A. Structure and absolute configuration of new diter-penes from Lavandula multifida. J. Nat. Prod. 2002, 65, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Sosa, S.; Altinier, G.; Politi, M.; Braca, A.; Morelli, I.; Della Loggia, R. Extracts and constituents of Lavandula multifida with topical anti-inflammatory activity. Phytomedicine 2005, 12, 271–277. [Google Scholar] [CrossRef]
- El Hilaly, J.; Lyoussi, B. Hypoglycaemic effect of the lyophilised aqueous extract of Ajuga iva in normal and streptozotocin diabetic rats. J. Ethnopharmacol. 2002, 80, 109–113. [Google Scholar] [CrossRef]
- Benbelaid, F.; Bendahou, M.; Khadir, A.; Abdoune, M.A.; Bellahsene, C.; Zenati, F.; Bouali, W.; Abdelouahid, D.E. Antimi-crobial activity of essential Oil of Lavandula multifida L. J. Microbiol. Biotech. Res. 2012, 2, 244–247. [Google Scholar]
- Zoubi, Y.E.; Bousta, D.; Farah, A. A Phytopharmacological review of a Mediterranean plant: Lavandula stoechas L. Clin. Phytosci. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Khalid, M.; Akhtar, J.; Siddiqui, H.H.; Ahmad, U.; Ahsan, F.; Khan, M.M.; Ahamd, M.; Ali, A. Lavandula stoe-chas (Ustukhuddus): A miracle plant. J. Innov. Pharm. Biol. Sci. 2016, 3, 96–102. [Google Scholar]
- Boufellous, M.; Lrhorfi, L.A.; Berrani, A.; Haoud, H.E.; Zaher, A.; Bouhaddioui, M.B.; Bengueddour, R. Phytochemical screen-ing of a medicinal plant: Lavandula stoechas (Lamiaceae). J. Pharmacogn. Phytochem. 2017, 6, 56–62. [Google Scholar]
- Sebai, H.; Selmi, S.; Rtibi, K.; Souli, A.; Gharbi, N.; Sakly, M. Lavender (Lavandula stoechas L.) essential oils attenuate hyper-glycemia and protect against oxidative stress in alloxan-induced diabetic rats. Lipids Health Dis. 2013, 12, 189. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Sunmonu, T.O. In vivo Studies on Antidiabetic Plants Used in South African Herbal Medicine. J. Clin. Biochem. Nutr. 2010, 47, 98–106. [Google Scholar] [CrossRef] [PubMed]
- El-Ansari, M.A.I.; Aboutabl, E.A.; Farrag, A.R.H.; Sharaf, M.; Hawas, U.W.; Soliman, G.M.; El-Saeed, G. Phytochemical and pharmacological studies on Leonotis leonurus. Pharm. Biol. 2009, 47, 894–902. [Google Scholar] [CrossRef]
- Mazimba, O. Leonotis leonurus: A herbal medicine review. J. Pharmacogn. Phytochem. 2015, 3. Available online: https://www.phytojournal.com/vol3Issue6/Issue_march_2015/3-6-21.1.pdf (accessed on 20 October 2020).
- Odeyemi, S.; Bradley, G. Medicinal Plants Used for the Traditional Management of Diabetes in the Eastern Cape, South Africa: Pharmacology and Toxicology. Molecules 2018, 23, 2759. [Google Scholar] [CrossRef]
- Pushpan, R.; Nishteswar, K.; Kumari, H. Ethno medicinal claims of Leonotis nepetifolia (L.) R. Br: A review. Int. J. Res. Ayurveda Pharm. 2012, 3, 783–785. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, V.; Malhotra, H.; Singh, S. Medicinal plants with potential anti-arthritic activity. J. Intercult. Ethnopharmacol. 2015, 4, 147–179. [Google Scholar] [CrossRef]
- Dhawan, N.G.; Khan, A.S.; Srivastava, P. A general appraisal of Leonotis nepetifolia (L) R. Br: An essential medicinal plant. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 118–121. [Google Scholar]
- Li, J.; Fronczek, F.R.; Ferreira, D.; Burandt, C.L., Jr.; Setola, V.; Roth, B.L.; Zjawiony, J.K. Bis-spirolabdane diterpenoids from Leonotis nepetaefolia. J. Nat. Prod. 2012, 75, 728–734. [Google Scholar] [CrossRef]
- Okach, D.O.; Nyunja, A.R.O.; Opande, G. Phytochemical screening of some wild plants from Lamiaceae and their role in traditional medicine in Uriri District-Kenya. Int. J. Herb. Med. 2013, 1, 135–143. [Google Scholar]
- Da Silva Almeida, J.R.G.; de Menezes Barbosa, J.; Cavalcante, N.B.; Delange, D.M. A review of the chemical composition and biological activity of Leonotis nepetifolia (Linn.) R. Br. (lion’s ear). Rev. Cuba. Plantas Med. 2018, 23. Available online: http://www.revplantasmedicinales.sld.cu/index.php/pla/article/view/687 (accessed on 20 October 2020).
- Lodhi, S.; Vadnere, G.P.; Sharma, V.K.; Usman, M. Marrubium vulgare L.: A review on phytochemical and pharmacological aspects. J. Intercult. Ethnopharmacol. 2017, 6, 429. [Google Scholar] [CrossRef]
- Vergara-Galicia, J.; Aguirre-Crespo, F.; Tun-Suarez, A.; Aguirre-Crespo, A.; Estrada-Carrillo, M.; Jaimes-Huerta, I.; Flo-res-Flores, A.; Estrada-Soto, S.; Ortiz-Andrade, R. Acute hypoglycemic effect of ethanolic extracts from Marrubium vulgare. Phytopharmacology 2012, 3, 54–60. [Google Scholar]
- Boudjelal, A.; Henchiri, C.; Siracusa, L.; Sari, M.; Ruberto, G. Compositional analysis and in vivo anti-diabetic activity of wild Algerian Marrubium vulgare L. infusion. Fitoterapia 2012, 83, 286–292. [Google Scholar] [CrossRef]
- Zawiślak, G.R.A.Ż.Y.N.A. The chemical composition of the essential oil of Marrubium vulgare L. from Poland. Farmacia 2012, 60, 287–292. [Google Scholar]
- Villanueva, J.R.; Esteban, J.M.; Villanueva, L.R. A Reassessment of the Marrubium vulgare L. Herb’s Potential Role in Diabetes Mellitus Type 2: First Results Guide the Investigation toward New Horizons. Medicines 2017, 4, 57. [Google Scholar] [CrossRef]
- Shaheen, F.; Rasool, S.; Shah, Z.A.; Soomro, S.; Jabeen, A.; Mesaik, M.A.; Choudhary, M.I. Chemical constituents of Marrubium vulgare as potential inhibitors of nitric oxide and respiratory burst. Nat. Prod. Commun. 2014, 9, 1934578X1400900705. [Google Scholar] [CrossRef]
- Aćimović, M.; Jeremić, K.; Salaj, N.; Gavarić, N.; Kiprovski, B.; Sikora, V.; Zeremski, T. Marrubium vulgare L.: A phytochemical and pharmacological overview. Molecules 2020, 25, 2898. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef]
- Amri, B.; Martino, E.; Vitulo, F.; Corana, F.; Ben Kâab, L.B.; Rui, M.; Rossi, D.; Mori, M.; Rossi, S.; Collina, S. Marrubium vulgare L. Leave Extract: Phytochemical Composition, Antioxidant and Wound Healing Properties. Molecules 2017, 22, 1851. [Google Scholar] [CrossRef] [PubMed]
- Elberry, A.A.; Harraz, F.M.; Ghareib, S.A.; Gabr, S.A.; Nagy, A.A.; Abdel-Sattar, E. Methanolic extract of Marrubium vulgare ameliorates hyperglycemia and dyslipidemia in streptozotocin-induced diabetic rats. Int. J. Diabetes Mellit. 2015, 3, 37–44. [Google Scholar] [CrossRef]
- Idm’hand, E.; Msanda, F.; Cherifi, K. Ethnopharmacological review of medicinal plants used to manage diabetes in Morocco. Clin. Phytosci. 2020, 6, 1–32. [Google Scholar] [CrossRef]
- Ohtera, A.; Miyamae, Y.; Nakai, N.; Kawachi, A.; Kawada, K.; Han, J.; Isoda, H.; Neffati, M.; Akita, T.; Maejima, K.; et al. Identification of 6-octadecynoic acid from a methanol extract of Marrubium vulgare L. as a peroxisome proliferator-activated receptor γ agonist. Biochem. Biophys. Res. Commun. 2013, 440, 204–209. [Google Scholar] [CrossRef]
- Silva, M.K.D.N.; Carvalho, V.R.D.A.; Matias, E.F.F. Chemical Profile of Essential oil of Ocimum gratissimum L. and Evaluation of Antibacterial and Drug Resistance-modifying Activity by Gaseous Contact Method. Pharmacogn. J. 2015, 8, 4–9. [Google Scholar] [CrossRef]
- Okoduwa, S.I.R.; Umar, I.A.; James, D.B.; Inuwa, H.M. Anti-Diabetic Potential of Ocimum gratissimum Leaf Fractions in Fortified Diet-Fed Streptozotocin Treated Rat Model of Type-2 Diabetes. Medicines 2017, 4, 73. [Google Scholar] [CrossRef]
- Omodamiro, O.D.; Jimoh, M.A. Antioxidant and antibacterial activities of Ocimum gratissimum. AJPCT 2015, 3, 10–19. [Google Scholar]
- Prabhu, K.S.; Lobo, R.; Shirwaikar, A. Ocimum gratissimum: A Review of its Chemical, Pharmacological and Ethnomedicinal Properties. Open Complement. Med. J. 2009, 1, 1–15. [Google Scholar] [CrossRef]
- Pandey, S. Phytochemical constituents, pharmacological and traditional uses of Ocimum gratissimum L in tropics. Indo Am. J. Pharm. 2017, 4, 4234–4242. [Google Scholar]
- Oboh, G. Antioxidant and antimicrobial properties of ethanolic extract of Ocimum gratissimum leaves. J. Pharmacol Toxicol. 2010, 5, 396–402. [Google Scholar]
- Venuprasad, M.; Kandikattu, H.K.; Razack, S.; Khanum, F. Phytochemical analysis of Ocimum gratissimum by LC-ESI–MS/MS and its antioxidant and anxiolytic effects. S. Afr. J. Bot. 2014, 92, 151–158. [Google Scholar] [CrossRef]
- Ezuruike, U.F.; Prieto, J.M. The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. J. Ethnopharmacol. 2014, 155, 857–924. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.M.; Da Silva, D.; Sola-Penna, M.; Camargo, L.M.D.M.; Celestrini, D.D.M.; Tinoco, L.; Costa, S.S. Identification of chicoric acid as a hypoglycemic agent from Ocimum gratissimum leaf extract in a biomonitoring in vivo study. Fitoterapia 2014, 93, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Onyechi, A.U.; Ibeanu, V.N.; Maduforo, A.N.; Ugwuonah, A.U.; Nsofor, L.D. Effects of Corchorus olitorius, Myrianthus arboreus and Annona muricata aqueous leaves extracts on body weight, blood glucose levels and lipid profile of alloxan-induced diabetic rats. J. Dietit. Assoc. Niger. 2018, 9, 2635–3326. [Google Scholar]
- Shimada, H.; Kuma, C.; Iseri, T.; Matsumura, S.I.; Kawase, A.; Matsuura, M.; Iwaki, M. Inhibitory effect of Ocimum gratissimum leaf extract on postprandial increase of blood glucose. Nat. Prod. Commun. 2019, 14, 1934578X19883728. [Google Scholar] [CrossRef]
- Antora, R.A.; Salleh, R.M. Antihyperglycemic effect of Ocimum plants: A short review. Asian Pac. J. Trop. Biomed. 2017, 7, 755–759. [Google Scholar] [CrossRef]
- Mondal, S.; Mirdha, B.R.; Mahapatra, S.C. The science behind sacredness of Tulsi (Ocimum sanctum Linn.). Indian J. Physiol. Pharmacol. 2010, 53, 291–306. [Google Scholar]
- Jayant, S.K.; Srivastava, N. Effect of Ocimum sanctum against alloxan induced diabetes and biochemical alterations in rats. Integr. Obes. Diabetes 2015, 2, 1–4. [Google Scholar] [CrossRef][Green Version]
- Pattanayak, P.; Behera, P.; Das, D.; Panda, S.K. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn. Rev. 2010, 4, 95–105. [Google Scholar] [CrossRef]
- Patil, R.; Patil, R.; Ahirwar, B.; Ahirwar, D. Isolation and characterization of antidiabetic component (bioactivity—Guided fractionation) from Ocimum sanctum L. (Lamiaceae) aerial part. Asian Pac. J. Trop. Med. 2011, 4, 278–282. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Marenah, L.; Ali, L.; Rokeya, B.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Ocimum sanctum leaf extracts stimulate insulin secretion from perfused pancreas, isolated islets and clonal pancreatic β-cells. J. Endocrinol. 2006, 189, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chang, C. Basil (Ocimum basilicum L.) Oils: Essential oils in food preservation. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–238. [Google Scholar]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chem. 2016, 196, 1066–1074. [Google Scholar] [CrossRef]
- Ugwu, M.N.; Umar, I.A.; Utu-Baku, A.B.; Dasofunjo, K.; Ukpanukpong, R.U.; Yakubu, O.E.; Okafor, A.I. Antioxidant status and organ function in streptozotocin-induced diabetic rats treated with aqueous, methanolic and petroleum ether extracts of Ocimum basilicum leaf. J. Appl. Pharm. Sci. 2013, 3, S75. [Google Scholar]
- Ch, M.; Naz, S.; Sharif, A.; Akram, M.; Saeed, M. Biological and Pharmacological Properties of the Sweet Basil (Ocimum basilicum). Br. J. Pharm. Res. 2015, 7, 330–339. [Google Scholar] [CrossRef]
- Purushothaman, B.; Srinivasan, R.P.; Suganthi, P.; Ranganathan, B.; Gimbun, J.; Shanmugam, K. A Comprehensive Review on Ocimum basilicum. J. Nat. Remedies 2018, 18, 71–85. [Google Scholar] [CrossRef]
- Marwat, S.K.; Khan, M.S.; Ghulam, S.; Anwar, N.; Mustafa, G.; Usman, K. Phytochemical constituents and pharmacological activities of sweet Basil-Ocimum basilicum L. (Lamiaceae). Asian J. Chem. 2011, 23, 3773. [Google Scholar]
- Ezeani, C.; Ezenyi, I.; Okoye, T.; Okoli, C. Ocimum basilicum extract exhibits antidiabetic effects via inhibition of hepatic glucose mobilization and carbohydrate metabolizing enzymes. J. Intercult. Ethnopharmacol. 2017, 6, 22–28. [Google Scholar] [CrossRef]
- Tshilanda, D.D.; Inkoto, C.L.; Mpongu, K.; Mata, S.; Mutwale, P.K.; Tshibangu, D.S.-T.; Bongo, G.N.; Koto-Te-Nyiwa, N.; Mpiana, P.T. Microscopic Studies, Phytochemical and Biological Screenings of Ocimum canum. Int. J. Pharm. Chem. 2019, 5, 61. [Google Scholar] [CrossRef]
- Rai, S.; Ghosh, H.; Basheer, M. Phytochemical characterization and antioxidative property of Ocimum canum: Effect of etha-nolic extract of leaves and seeds on basic immunologic and metabolic status of male rats. J. Immunol. Biol. 2016, 1, 2. [Google Scholar] [CrossRef]
- De Oliveira, J.R.; Camargo, S.E.A.; De Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Sancheti, G.; Goyal, P.K. Role of rosemary leaf extract against various doses of gamma radiation. Trees Life J. 2007, 2. [Google Scholar]
- Naimi, M.; Vlavcheski, F.; Shamshoum, H.; Tsiani, E. Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives. Nutrients 2017, 9, 968. [Google Scholar] [CrossRef] [PubMed]
- Emam, M. Comparative evaluation of antidiabetic activity of Rosmarinus officinalis L. and Chamomile recutita in streptozotocin induced diabetic rats. Agric. Biol. J. N. Am. 2012, 3, 247–252. [Google Scholar] [CrossRef]
- Etsassala, N.G.; Adeloye, A.O.; El-Halawany, A.; Hussein, A.A.; Iwuoha, E.I. Investigation of In-Vitro Antioxidant and Elec-trochemical Activities of Isolated Compounds from Salvia chamelaeagnea PJ Bergius Extract. Antioxidants 2019, 8, 98. [Google Scholar] [CrossRef]
- Ngo, Y.L.; Lau, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef]
- Khalil, O.A.; Ramadan, K.S.; Danial, E.N.; Alnahdi, H.S.; Ayaz, N.O. Antidiabetic activity of Rosmarinus officinalis and its relationship with the antioxidant property. Afr. J. Pharm. Pharmaco. 2012, 6, 1031–1036. [Google Scholar]
- Tu, Z.; Moss-Pierce, T.; Ford, P.; Jiang, T. Rosemary (Rosmarinus officinalis L.). Extract regulates glucose and lipid metabolism by activating AMPK and PPAR pathways in Hep G2 cells. J. Agric. Food Chem. 2013, 61, 2803–2810. [Google Scholar] [CrossRef]
- Escudero, J.; Perez, L.; Rabanal, R.M.; Valverde, S. Diterpenoids from Salvia oxyodon and Salvia lavandulifolia. Phytochemistry 1983, 22, 585–587. [Google Scholar] [CrossRef]
- Jiménez, J.; Risco, S.; Ruiz, T.; Zarzuelo, A. Hypoglycemic Activity of Salvia lavandulifolia. Planta Medica 1986, 52, 260–262. [Google Scholar] [CrossRef]
- Craft, J.D.; Satyal, P.; Setzer, W.N. The Chemotaxonomy of Common Sage (Salvia officinalis) Based on the Volatile Constituents. Medicines 2017, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, Pharmacology, and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease, and Cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Eidi, M. Antidiabetic effects of sage (Salvia officinalis L.) leaves in normal and streptozotocin-induced diabetic rats. Diabetes Metab. Syndr. Clin. Res. Rev. 2009, 3, 40–44. [Google Scholar] [CrossRef]
- Ahmad, G.; Mahdi, E. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar]
- Behradmanesh, S.; Derees, F.; Rafieian-Kopaei, M. Effect of Salvia officinalis on diabetic patients. J. Ren. Inj. Prev. 2013, 2, 51–54. [Google Scholar]
- Boukhary, R.; Aboul-Ela, M.; Al-Hanbali, O.; El-Lakany, A. Chemical Constituents from Salvia fruticosa libanotica. Pharmacogn. J. 2017, 10, 45–48. [Google Scholar] [CrossRef]
- Boukhary, R.; Raafat, K.; Ghoneim, A.I.; Aboul-Ela, M.; El-Lakany, A. Anti-Inflammatory and Antioxidant Activities of Salvia fruticosa: An HPLC Determination of Phenolic Contents. Evid. Based Complement. Altern. Med. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Perfumi, M.; Arnold, N.; Tacconi, R. Hypoglycemic activity of Salvia fruticosa Mill from Cyprus. J. Ethnopharmacol. 1991, 34, 135–140. [Google Scholar] [CrossRef]
- Mahdizadeh, R.; Moein, S.; Soltani, N.; Malekzadeh, K.; Mahmoodreza, M. Study the molecular mechanism of salvia species in prevention of diabete. IJPSR 2018, 9, 4512–4521. [Google Scholar] [CrossRef]
- Bahramikia, S.; Yazdanparast, R. Phytochemistry and Medicinal Properties of Teucrium polium L. (Lamiaceae). Phytother. Res. 2012, 26, 1581–1593. [Google Scholar] [CrossRef]
- Movahedi, A.; Basir, R.; Rahmat, A.; Charaffedine, M.; Othman, F. Remarkable anticancer activity of Teucrium polium on hepatocellular carcinogenic rats. Evid. Based Complement. Altern. Med. 2014, 2014, 726724. [Google Scholar] [CrossRef] [PubMed]
- Kawashty, S.; El-Din, E.G.; Saleh, N. The flavonoid chemosystematics of two Teucrium species from Southern Sinai, Egypt. Biochem. Syst. Ecol. 1999, 27, 657–660. [Google Scholar] [CrossRef]
- Tabatabaie, P.S.; Yazdanparast, R. Teucrium polium extract reverses symptoms of streptozotocin-induced diabetes in rats via rebalancing the Pdx1 and FoxO1 expressions. Biomed. Pharmacother. 2017, 93, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Zapata-Bustos, R.; Romo-Yañez, J.; Camarillo-Ledesma, P.; Gómez-Sánchez, M.; Salazar-Olivo, L.A. The antidiabetic plants Tecoma stans (L.) Juss. ex Kunth (Bignoniaceae) and Teucrium cubense Jacq (Lamiaceae) induce the incor-poration of glucose in insulin-sensitive and insulin-resistant murine and human adipocytes. J. Ethnopharmacol. 2010, 127, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Du, Y.J.; Song, H.C. Alpha-glucosidase and alpha-amylase inhibitory activities of guava leaves. Food Chem. 2010, 123, 6–13. [Google Scholar] [CrossRef]
- Firdous, S.M. Phytochemicals for treatment of diabetes. EXCLI J. 2014, 13, 451–453. [Google Scholar]
- Fatima, N.; Hafizur, R.M.; Hameed, A.; Ahmed, S.; Nisar, M.; Kabir, N. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cells of pancreas. Eur. J. Nutr. 2017, 56, 591–601. [Google Scholar] [CrossRef]
- Jadhav, R.; Puchchakayala, G. Hypoglycemic and antidiabetic activity of flavonoids: Boswellic acid, ellagic acid, quercetin, rutin on streptozotocin-nicotinamide induced type 2 diabetic rats. Group 2012, 1, 100. [Google Scholar]
- Wu, P.P.; Zhang, K.; Lu, Y.J.; He, P.; Zhao, S.Q. In vitro and in vivo evaluation of the antidiabetic activity of ursolic acid deriv-atives. Eur. J. Med. Chem. 2014, 80, 502–508. [Google Scholar] [CrossRef]
- Guzmán-Ávila, R.; Flores-Morales, V.; Paoli, P.; Camici, G.; Ramírez-Espinosa, J.J.; Cerón-Romero, L.; Navarrete-Vázquez, G.; Hidalgo-Figueroa, S.; Yolanda Rios, M.; Villalobos-Molina, R.; et al. Ursolic acid derivatives as potential anti-diabetic agents: In vitro, in vivo, and in silico studies. Drug Dev. Res. 2018, 79, 70–80. [Google Scholar] [CrossRef]
- Zang, Y.; Igarashi, K.; Li, Y. Antidiabetic effects of luteolin and luteolin-7-O-glucoside on KK-A y mice. Biosci. Biotechnol. Biochem. 2016, 80, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Osigwe, C.C.; Akah, P.A.; Nworu, C.S.; Okoye, F.B. Apigenin: A methanol fraction component of Newbouldia laevis leaf, as a potential antidiabetic agent. J. Phytopharmacol. 2017, 6, 38–44. [Google Scholar]
- Sadeghi, H.M.; Mansourabadi, A.; Rezvani, M.E.; Ghobadi, M.; Razavi, N.; Bagheri, M. Salvigenin has potential to ameliorate Streptozotocin-induced diabetes mellitus and heart complications in Rats. J. Adv. Med. 2016, 1–12. [Google Scholar] [CrossRef]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical Basis of the Antidiabetic Activity of Oleanolic Acid and Related Pentacyclic Triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Wu, H.; Liu, J.; Hu, J.-X.; Liao, N.; Peng, J.; Cao, P.-P.; Liang, X.; Hai, C. Antidiabetic Effect of Oleanolic Acid: A Promising Use of a Traditional Pharmacological Agent. Phytother. Res. 2011, 25, 1031–1040. [Google Scholar] [CrossRef]

| Plant Species | Part of the Plant | Mode of Preparation | Geographic Location | Antidiabetic Activity | References |
|---|---|---|---|---|---|
| Ajuga iva (L.) Schreb | Aerial parts (Leaf and stem) | Decoction, infusion, and raw Powder | Morocco and Algeria | Hypoglycaemic, hypolipidemic, alpha-glucosidase and alpha-amylase activities | [13,14,17,19,20] |
| Ballota nigra L. | Aerial part | Hypoglycemic activity | [21] | ||
| Becium grandiflorum (Lam.) Pic.Serm | Leaves | Powder | Ethiopia | Antihyperglycemic activity | [24,25] |
| Calamintha officinalis Moench | Stem, leaves, and seeds | Decoction (aqueous) | Morocco | Hypoglycaemic activity | [28] |
| Coleus forskohlii (Willd.) Briq. | All plant | West Africa and India | Hypoglycaemic activityWeight-loss | [33,35] | |
| Hyptis suaveolens (L.) Poit. | Aerial part | Indonesia | Antihyperglycemic activity | [41,43] | |
| Lavandula angustifolia Mill. | Aerial part | Jordan | Inhibition of hormone sensitive lipase and pancreatic lipase activities | [45] | |
| Lavandula dentata L. | Leaves and stem | Decoction, infusion, and raw | Mediterranean or Saharan regions | Hypolipidemic and hypoglycaemic activities | [51,54] |
| Lavandula multifida L. | leaves and stems | decoction | Morocco | Antihypolipidemic and hypoglycaemic activities | [55,56,58,60] |
| Lavandula stoechas L. | Leaves | Decoction | Morocco, Tunisia | Hypoglycaemic activity | |
| Leonotis leonurus (L.) R. Br. | Leaves and stems | South Africa | Antihyperglycemic, hypoglycaemic and antilipidemic activities | [63,64,66] | |
| Leonotis nepetifolia (L.) R.Br. | Leaves | South Africa, India | Antidiabetic activity | [68,69,72] | |
| Marrubium vulgare L. | Leaves | Decoction | Morocco, Mexico, and Algeria | Hypoglycaemic, antihyperlipidemic, alpha-glucosidase and alpha-amylase activities | [74,75,78,80] |
| Ocimum gratissimum L. | Leaves | Infusion, food vegetable | Western Africa, Nigeria | Antihyperglycemic and hypoglycaemic activities | [86,96] |
| Ocimum sanctum L. | India | Hypoglycaemic activity | [98,100] | ||
| Ocimum basilicum L. | Pakistan, Asia | Hyperlipidemia and hypoglycaemic activities | [103,104,106] | ||
| Ocimum canum L. | Leaves | Ghana | Antihyperglycemic activity | [109,111] | |
| Rosmarinus officinalis L. | Kingdom of Saudi Arabia | Alpha-glucosidase and hypoglycaemic activities | [114,115] | ||
| Salvia lavandulifolia Valh | Leaves | Spain | Hypoglycaemic activity | [119,121] | |
| Salvia officinalis L. | Leaves | Decoction and infusion | Asia, Latin America | Hypoglycaemic and hypolipidemic effects | [123,126] |
| Salvia fruticosa Mill. | Aerial part | Tea | Jordan | Hypoglycaemic activity | [15,127] |
| Teucrium polium L. | Dried aerial parts | Decoction and powder | South Iran | Hypoglycaemic activity | [130,131,132,134] |
| Teucrium cubense Jacq. | Mexican, Saudi Arabia, and North Africa | Hypoglycaemic activity | [15,135] |
| Compounds | Plant Source | Biological Activity/Mode of Action | References |
|---|---|---|---|
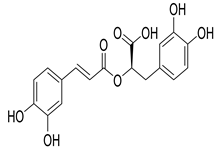 Rosmarinic acid | C. officinalis Rosemary S. lavandulifolia O. canum | Hypoglycaemic effect Significant antidiabetic effects in different in in vivo models and insulin-like effects in insulin target cells in in vitro models of type 2 diabetes | [30,63] |
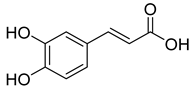 Caffeic acid | C. officinalis S. officinalis | Hypoglycaemic effect | [30] |
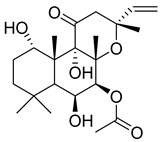 Forskolin | C. forskohlii | Glucose-induced insulin secretion Decreases fasting blood glucose levels Enhances the glucose-mediated stimulus Induces cells to release insulin Decreases basal glucose in healthy rats Attenuates the severity of hyperglycaemia in diabetic Rats | [33] |
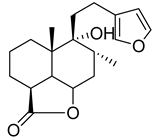 Marrubiin | L. leonurus | Increases the level of insulin and glucose transporter-2 gene expressions in INS-1 cells | [137] |
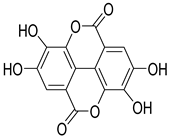 Ellagic acid | S. officinalis | Stimulates insulin secretion and decreases glucose intolerance | [138] |
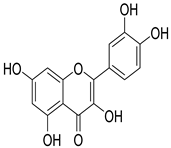 Quercetin | S. officinalis | Stimulate β-cells to release more insulin | [139] |
 Rutin | S. officinalis | Stimulates β-cells to produce more insulin | [139] |
 Ursolic acid | S. officinalis | Hypoglycaemic effect Stimulate glucose uptake | [140,141] |
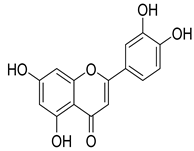 Luteolin | T. polium | Antidiabetic effects and hypoglycaemic effect | [142] |
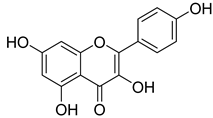 Apigenin | T. polium | Reduces blood glucose The hypoglycaemic effect in diabetic rats Stimulate the synthesis of glycogen in muscles Antihyperglycemic and Insulinmimetic activities | [143] |
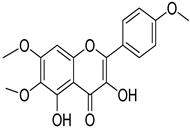 Salvigenin | T. polium | Improves diabetes through decreasing blood glucose, lipid profile, HbA1c. Increased insulin secretion | [144] |
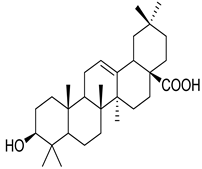 Oleanolic acid | H. suaveolens | Improves insulin response Significant blood glucose-lowering and weight loss effect | [145] |
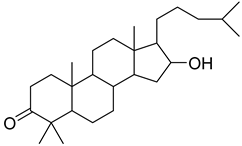 16-hydroxy-4,4,10,13-tetramethyl-17-(4-methyl-pentyl)-hexadecahydro-cyclopenta[a]phenanthren-3-one | O. sanctum | Antihyperglycemic activity | [96] |
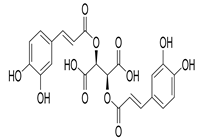 Chicoric acid | O. gratissimum | Significant decrease in the glycemic levels in diabetic mice | [93] |
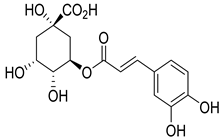 Chlorogenic acid | O. gratissimum | Significant hypoglycaemic activity in streptozotocin-induced diabetic rats | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etsassala, N.G.E.R.; Hussein, A.A.; Nchu, F. Potential Application of Some Lamiaceae Species in the Management of Diabetes. Plants 2021, 10, 279. https://doi.org/10.3390/plants10020279
Etsassala NGER, Hussein AA, Nchu F. Potential Application of Some Lamiaceae Species in the Management of Diabetes. Plants. 2021; 10(2):279. https://doi.org/10.3390/plants10020279
Chicago/Turabian StyleEtsassala, Ninon G.E.R., Ahmed A. Hussein, and Felix Nchu. 2021. "Potential Application of Some Lamiaceae Species in the Management of Diabetes" Plants 10, no. 2: 279. https://doi.org/10.3390/plants10020279
APA StyleEtsassala, N. G. E. R., Hussein, A. A., & Nchu, F. (2021). Potential Application of Some Lamiaceae Species in the Management of Diabetes. Plants, 10(2), 279. https://doi.org/10.3390/plants10020279








