Antimicrobial Activities of Sesquiterpene-Rich Essential Oils of Two Medicinal Plants, Lannea egregia and Emilia sonchifolia, from Nigeria
Abstract
1. Introduction
2. Results and Discussion
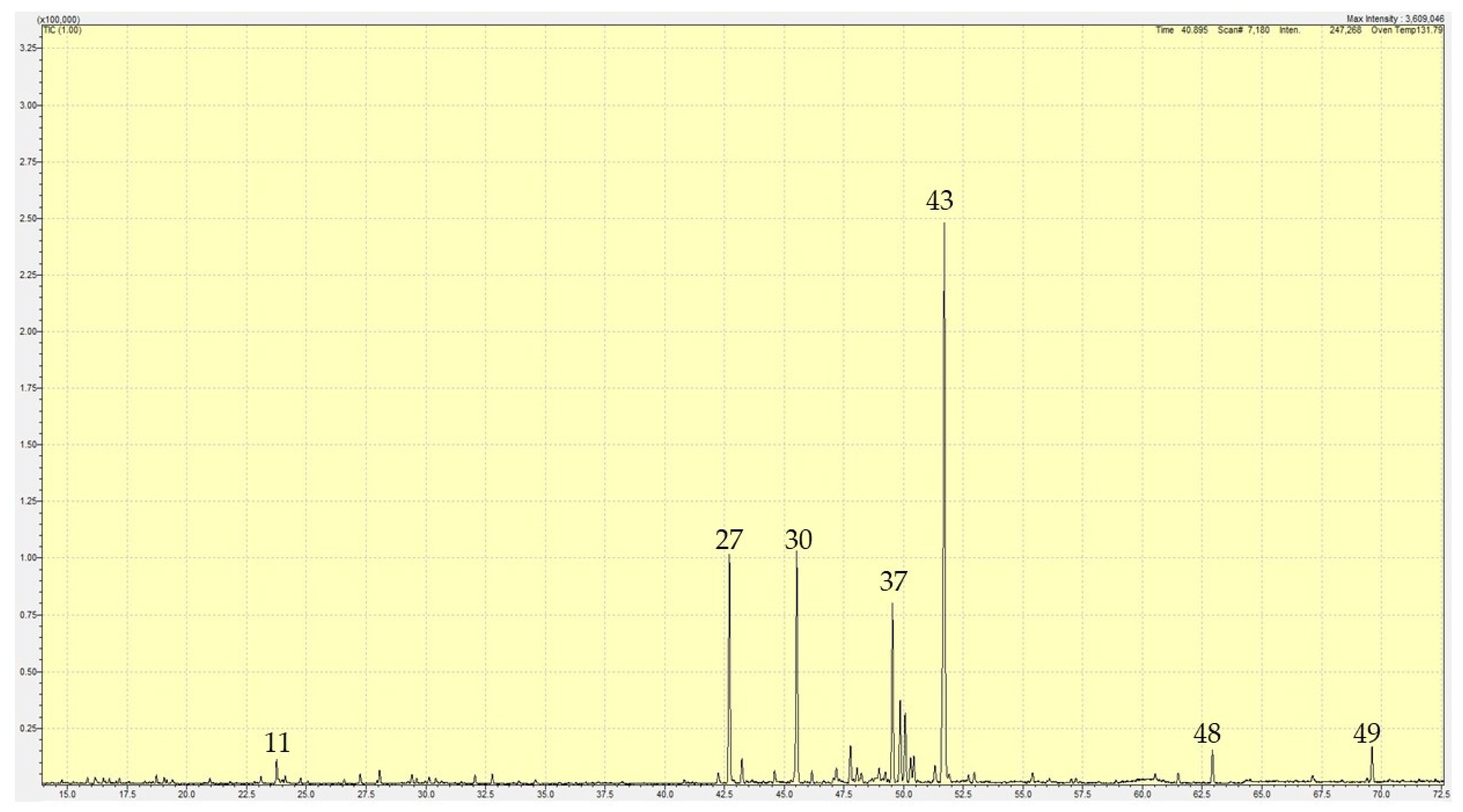
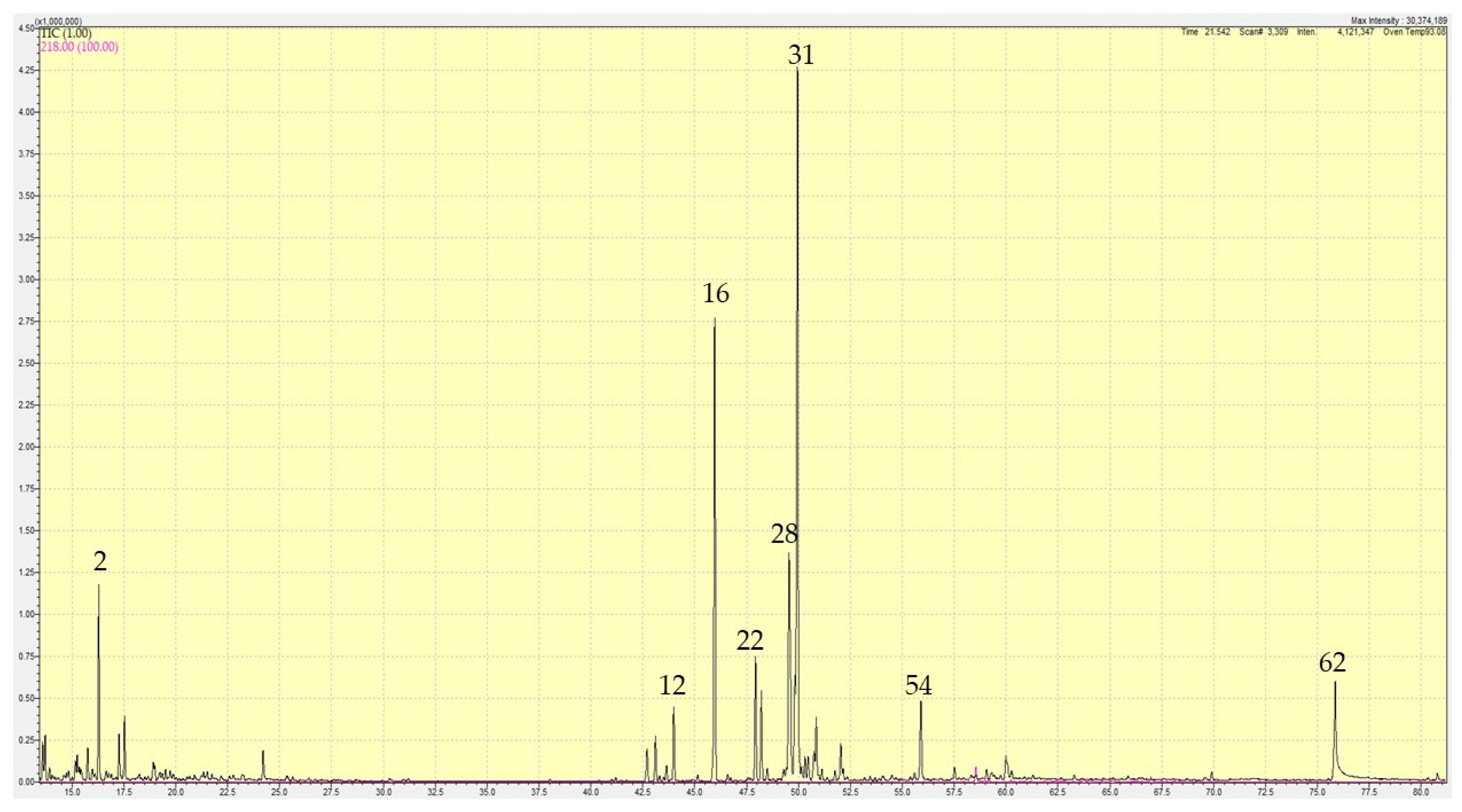
2.1. Essential Oil Compositions
2.1.1. Lannea egregia
2.1.2. Emilia sonchifolia
2.2. Antimicrobial Activity
3. Materials and Methods
3.1. Plant Materials
3.2. Isolation of Essential Oils
3.3. Gas Chromatography–Mass Spectrometry
3.4. Antibacterial and Antifungal Screening
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rai, M.; Acharya, D.; Ríos, J.L. Ethnomedicinal Plants: Revitalization of Traditional Knowledge of Herbs; Science Publishers: Enfield, NH, USA, 2011; ISBN 978-1-57808-696-2. [Google Scholar]
- Akram, M.; Tahir, I.M.; Shah, S.M.; Mahmood, Z.; Altaf, A.; Ahmad, K.; Munir, N.; Daniyal, M.; Nasir, S.; Mehboob, H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phytother. Res. 2018, 32, 811–822. [Google Scholar] [CrossRef]
- Shukla, S.; Mehta, A. Anticancer potential of medicinal plants and their phytochemicals: A review. Rev. Bras. Bot. 2015, 38, 199–210. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.A.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; De Castro, S.L.; Ferreira, V.F.; De Lacerda, M.V.G.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases–Part I. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Mukhtar, M.; Sarfraz, A. A review: Antifungal potentials of medicinal plants. J. Bioresour. Manag. 2015, 2, 23–31. [Google Scholar] [CrossRef]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016, 30, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Marston, A.; Maillard, M.; Hostettmann, K. Search for antifungal, molluscicidal and larvicidal compounds from African medicinal plants. J. Ethnopharmacol. 1993, 38, 209–214. [Google Scholar] [CrossRef]
- Adenubi, O.T.; Ahmed, A.S.; Fasina, F.O.; McGaw, L.J.; Eloff, J.N.; Naidoo, V. Pesticidal plants as a possible alternative to synthetic acaricides in tick control: A systematic review and meta-analysis. Ind. Crops Prod. 2018, 123, 779–806. [Google Scholar] [CrossRef]
- Calzetta, L.; Pistocchini, E.; Leo, A.; Roncada, P.; Ritondo, B.L.; Palma, E.; di Cave, D.; Britti, D. Anthelminthic medicinal plants in veterinary ethnopharmacology: A network meta-analysis following the PRISMA-P and PROSPERO recommendations. Heliyon 2020, 6, e03256. [Google Scholar] [CrossRef] [PubMed]
- Burkill, H.M. The Useful Plants of West Tropical Africa, vol. 1, Families A–D, 2nd ed.; Royal Botanic Gardens, Kew: Richmond, UK, 1985; ISBN 094764301X. [Google Scholar]
- Mabberley, D.J. Mabberley’s Plant-Book, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008; ISBN 978-0-521-82071-4. [Google Scholar]
- AbdulRahaman, A.A.; Kolawole, O.S.; Oladele, F.A. Leaf epidermal features as taxonomic characters in some Lannea species (Anacardiaceae). Phytol. Balc. 2014, 20, 227–231. [Google Scholar]
- Keay, R.W.J. Trees of Nigeria; Clarendon Press: Oxford, UK, 1989; ISBN 0198545606. [Google Scholar]
- Arbonnier, M. Trees, Shrubs and Lianas of West African Dry Zones; CIRAD, Margraf Publishers Gmbh, MNHN: Paris, France, 2004; ISBN 9782759203130. [Google Scholar]
- Koné, W.M.; Atindehou, K.K.; Dossahoua, T.; Betschart, B. Anthelmintic activity of medicinal plants used in northern Côte d’Ivoire against intestinal helminthiasis. Pharm. Biol. 2005, 43, 72–78. [Google Scholar] [CrossRef]
- Rafiu, B.O.; Sonibare, A.M.; Adesanya, E.O. Phytochemical screening, antimicrobial and antioxidant studies of Lannea egregia Engl. and K. Krause (Anacardiaceae) stem bark. J. Med. Plants Econ. Dev. 2019, 3, a62. [Google Scholar] [CrossRef]
- Neuwinger, H.D. African Traditional Medicine: A Dictionary of Plant Use and Applications; Medpharm Scientific Publishers: Stuttgart, Germany, 2000; ISBN 978-3887630867. [Google Scholar]
- Soladoye, M.O.; Amusa, N.A.; Raji-Esan, S.O.; Chukwuma, E.C.; Taiwo, A.A. Ethnobotanical survey of anti-cancer plants in Ogun State, Nigeria. Ann. Biol. Res. 2010, 1, 261–273. [Google Scholar]
- Idowu, P.A.; Ekemezie, L.C.; Olaiya, C.O. Phytochemical, antioxidant and antimicrobial studies of Lannea egregia Engl. & K. Krause (Anacardiaceae) extracts and chromatographic fractions. J. Phytomedicine Ther. 2020, 19, 348–363. [Google Scholar]
- Couto, V.M.; Vilela, F.C.; Dias, D.F.; dos Santos, M.H.; Soncini, R.; Nascimento, C.G.O.; Giusti-Paiva, A. Antinociceptive effect of extract of Emilia sonchifolia in mice. J. Ethnopharmacol. 2011, 134, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Muko, K.N.; Ohiri, F.C. A preliminary study on the anti-inflammatory properties of Emilia sonchifolia leaf extracts. Fitoterapia 2000, 71, 65–68. [Google Scholar] [CrossRef]
- Bassey, M.E.; Effiong, E.O. Preliminary investigation of herbs used in paediatric care among the people of Akwa Ibom State, Nigeria. J. Nat. Prod. Plant Resour. 2011, 1, 33–42. [Google Scholar]
- Essien, G.E.; Nwidu, L.L.; Nwafor, P.A. Anti-inflammatory and analgesic potential of methanolic extract of Emilia sonchifolia (Compositae) leaves in rodents. Afr. J. Biomed. Res. 2009, 12, 199–207. [Google Scholar]
- Essien, G.E.; Thomas, P.S.; Udoette, I.M. In vitro antioxidant analysis and quantitative determination of phenolic and flavonoid contents of Emilia sonchifolia (L) D.C (Asteraceae) leaf extract and fractions. GSC Biol. Pharm. Sci. 2020, 11, 44–52. [Google Scholar] [CrossRef]
- George Kallivalappil, G.; Kuttan, G. Evaluation of the anti-inflammatory and urotoxicity ameliorative effects of γ-humulene containing active fraction of Emilia sonchifolia (L.) DC. Inflammopharmacology 2019, 27, 409–420. [Google Scholar] [CrossRef]
- Shylesh, B.S.; Padikkala, J. In vitro cytotoxic and antitumor property of Emilia sonchifolia (L.) DC in mice. J. Ethnopharmacol. 2000, 73, 495–500. [Google Scholar] [CrossRef]
- Gayathri Devi, D.; Lija, Y.; Cibin, T.R.; Biju, P.G.; Gayathri Devi, V.; Abraham, A. Evaluation of the protective effects of Emilia sonchifolia Linn. (DC.) on perchlorate-induced oxidative damage. J. Biol. Sci. 2006, 6, 887–892. [Google Scholar]
- Nworu, C.S.; Akah, P.A.; Okoye, F.B.C.; Esimone, C.O. Inhibition of pro-inflammatory cytokines and inducible nitric oxide by extract of Emilia sonchifolia L. aerial parts. Immunopharmacol. Immunotoxicol. 2012, 34, 925–931. [Google Scholar] [CrossRef]
- Shen, S.; Shen, L.; Zhang, J.; Li, G.; Li, Z.; Pan, R.; Si, J. Emiline, a new alkaloid from the aerial parts of Emilia sonchifolia. Phytochem. Lett. 2013, 6, 467–470. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Chen, H.-W.; Lee, C.-C.; He, B.-J.; Yang, Y.-C. Hepatotoxic pyrrolizidine alkaloids in Emilia sonchifolia from Taiwan. J. Food Compos. Anal. 2015, 42, 1–7. [Google Scholar] [CrossRef]
- Smitharani; Remya, K.; Baby, B.; Rasheed, S.; Azeem, A.; Kumar, S. Investigation on the wound healing activity of aqueous extract of Emilia sonchifolia (L.) DC. Int. J. Herb. Med. 2017, 5, 34–39. [Google Scholar]
- Ilondu, E.M. Biopesticidal potentials of plants extracts against Cochliobolus lunatus R.R. Nelson & F.A. Haasis. Anamorph: Curvularia lunatus (Wakker) Boedgin. J. Biopestic. 2020, 13, 53–62. [Google Scholar]
- Gebara, S.S.; de Oliveira Ferreira, W.; Ré-Poppi, N.; Simionatto, E.; Carasek, E. Volatile compounds of leaves and fruits of Mangifera indica var. coquinho (Anacardiaceae) obtained using solid phase microextraction and hydrodistillation. Food Chem. 2011, 127, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Montanari, R.M.; Barbosa, L.C.A.; Demuner, A.J.; Silva, C.J.; Andrade, N.J.; Ismail, F.M.D.; Barbosa, M.C.A. Exposure to Anacardiaceae volatile oils and their constituents induces lipid peroxidation within food-borne bacteria cells. Molecules 2012, 17, 9728–9740. [Google Scholar] [CrossRef]
- Kpoviessi, D.S.S.; Gbaguidi, F.A.; Kossouoh, C.; Agbani, P.; Yayi-Ladekan, E.; Sinsin, B.; Moudachirou, M.; Accrombessi, G.C.; Quetin-Leclercq, J. Chemical composition and seasonal variation of essential oil of Sclerocarya birrea (A. Rich.) Hochst subsp birrea leaves from Benin. J. Med. Plant Res. 2011, 5, 4640–4646. [Google Scholar]
- Owolabi, M.S.; Ogundajo, A.L.; Dosoky, N.S.; Setzer, W.N. Chemical composition and antimicrobial potential of essential oils of leaf and stem bark of Haematostaphis barteri Hook. f. (Anacardiaceae). J. Essent. Oil Bear. Plants 2020, 23, 583–593. [Google Scholar] [CrossRef]
- Pandit, S.S. Genetic Analysis of Alphonso Mango Flavor Biogenesis. Ph.D. thesis, National Chemical Laboratory, Pune, India, 2008. [Google Scholar]
- Hiwilepo-van Hal, P. Processing of Marula (Sclerocarya birrea subsp. caffra) Fruits: A Case Study on Health-Promoting Compounds in Marula Pulp. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2013. [Google Scholar]
- Joshi, R.K. Volatile constituents of Emilia sonchifolia from India. Nat. Prod. Commun. 2018, 13, 1355–1356. [Google Scholar] [CrossRef]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef]
- Van Vuuren, S.; Holl, D. Antimicrobial natural product research: A review from a South African perspective for the years 2009–2016. J. Ethnopharmacol. 2017, 208, 236–252. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Mondello, L. FFNSC 3; Shimadzu Scientific Instruments: Columbia, MD, USA, 2016. [Google Scholar]
- NIST. NIST17; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017.
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Dissertation, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. [Google Scholar]
- Sahm, D.H.; Washington, J.A. Antibacterial susceptibility tests: Dilution methods. In Manual of Clinical Microbiology; Balows, A., Hausler, W.J., Herrmann, K.L., Isenberg, H.D., Shamody, H.J., Eds.; American Society for Microbiology: Washington, DC, USA, 1991. [Google Scholar]
- EUCAST Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [CrossRef]
| Emilia sonchifolia Extract (Geographical Source) | Phytochemicals Identified | Biological Activity | Ref. |
|---|---|---|---|
| Methanol plant extract (Kerala, India) | None identified | In vitro cytotoxicity (L-929 murine lung fibroblast, IC50 = 15 μg/mL) | [28] |
| Aqueous leaf extract (Nsukka, Nigeria) | None identified | Anti-inflammatory (mouse paw edema assay, ED50 = 780 mg/kg) | [23] |
| Ethanol plant extract (Kerala, India) | None identified | Inhibition of perchlorate oxidative stress (rat model) | [29] |
| Methanol leaf extract (Ibiono, Nigeria) | None identified | Analgesic (acetic acid writing, formalin hind paw, and hot plate assays, mouse model) | [25] |
| CH3OH/CH2Cl2 (1:1) extract of aerial parts (Nsukka, Nigeria) | Quercetrin, chlorophyll, caffeic acid derivative | Anti-inflammatory (inhibition of pro-inflammatory cytokines, mouse model) | [30] |
| Ethanol extract of aerial parts (Liuzhou, China) | Emiline (pyrrolidine alkaloid) | Neuroprotective (in vitro PC12 cells) | [31] |
| Aqueous HCl (0.5 N) plant extract (Taiwan) | Pyrrolizidine alkaloids: senecionine, seneciphylline, integerrimine, senkirkine, otosenine, neosenkirkine, petasitenine, acetylsenkirkine, desacetyldoronine, acetylpetasitenine, and doronine | None carried out, but pyrrolizidine alkaloids known to be hepatotoxic. | [32] |
| Aqueous plant extract (Kerala, India) | None identified | Wound-healing activity (rat model) | [33] |
| Ethanol leaf extract (Abraka, Nigeria) | None identified | Antifungal activity (Curvularia lunatus, MIC = 72 mg/mL) | [34] |
| Methanol leaf extract (Uyo, Nigeria) | None identified | Antioxidant (FRAP and DPPH assays) | [26] |
| Sr. No. | RT | RI(calc) | RI(db) | Compound | Ave % | St Dev |
|---|---|---|---|---|---|---|
| 1 | 15.838 | 977 | 978 | β-Pinene | 0.21 | 0.06 |
| 2 | 16.152 | 983 | 986 | 6-Methylhept-5-en-2-one | 0.38 | 0.04 |
| 3 | 16.494 | 989 | 991 | 2-Pentylfuran | 0.25 | 0.02 |
| 4 | 18.725 | 1024 | 1025 | p-Cymene | 0.34 | 0.03 |
| 5 | 19.044 | 1028 | 1030 | Limonene | 0.24 | 0.02 |
| 6 | 19.149 | 1030 | 1031 | β-Phellandrene | 0.11 | 0.01 |
| 7 | 20.961 | 1057 | 1058 | γ-Terpinene | 0.22 | 0.01 |
| 8 | 21.811 | 1070 | 1069 | cis-Linalool oxide | 0.12 | 0.02 |
| 9 | 22.81 | 1085 | 1086 | Terpinolene | 0.08 | 0.02 |
| 10 | 23.099 | 1089 | 1091 | p-Cymenene | 0.32 | 0.04 |
| 11 | 23.752 | 1099 | 1099 | Linalool | 1.12 | 0.01 |
| 12 | 23.979 | 1102 | 1104 | Hotrienol | 0.13 | 0.02 |
| 13 | 24.116 | 1104 | 1104 | Nonanal | 0.30 | 0.02 |
| 14 | 24.759 | 1113 | 1112 | (E)-2,4-Dimethylhepta-2,4-dienal | 0.26 | 0.04 |
| 15 | 26.577 | 1139 | 1139 | (Z)-3-Ethylidene-1-methyl-1,4-cycloheptadiene | 0.22 | 0.02 |
| 16 | 27.252 | 1149 | --- | Unidentified | 0.44 | 0.02 |
| 17 | 28.064 | 1160 | 1169 | p-Dimethoxybenzene | 0.54 | 0.06 |
| 18 | 29.26 | 1177 | 1172 | Lavandulol | 0.12 | 0.02 |
| 19 | 29.416 | 1180 | 1180 | Terpinen-4-ol | 0.44 | 0.05 |
| 20 | 30.132 | 1190 | 1192 | Methyl salicylate | 0.30 | 0.00 |
| 21 | 30.415 | 1194 | 1195 | α-Terpineol | 0.26 | 0.05 |
| 22 | 32.05 | 1218 | 1219 | β-Cyclocitral | 0.42 | 0.03 |
| 23 | 32.783 | 1228 | --- | Unidentified | 0.47 | 0.05 |
| 24 | 34.591 | 1255 | 1257 | Carvenone | 0.25 | 0.01 |
| 25 | 40.81 | 1347 | 1349 | α-Cubebene | 0.20 | 0.02 |
| 26 | 42.245 | 1369 | 1367 | Cyclosativene | 0.61 | 0.06 |
| 27 | 42.704 | 1376 | 1375 | α-Copaene | 11.39 | 0.22 |
| 28 | 43.231 | 1384 | 1382 | β-Bourbonene | 1.25 | 0.06 |
| 29 | 44.584 | 1404 | --- | Unidentified | 0.61 | 0.05 |
| 30 | 45.524 | 1419 | 1417 | (E)-Caryophyllene | 12.25 | 0.10 |
| 31 | 46.156 | 1429 | 1430 | β-Copaene | 0.57 | 0.02 |
| 32 | 47.186 | 1446 | 1447 | Geranyl acetone | 0.62 | 0.06 |
| 33 | 47.774 | 1455 | 1454 | α-Humulene | 1.85 | 0.03 |
| 34 | 48.052 | 1460 | 1457 | allo-Aromadendrene | 0.65 | 0.04 |
| 35 | 48.978 | 1474 | 1478 | γ-Muurolene | 0.57 | 0.03 |
| 36 | 49.235 | 1478 | 1479 | α-Amorphene | 0.41 | 0.06 |
| 37 | 49.533 | 1483 | 1476 | Selina-4,11-diene | 9.29 | 0.03 |
| 38 | 49.853 | 1488 | 1492 | β-Selinene | 4.26 | 0.04 |
| 39 | 50.065 | 1492 | 1492 | Valencene | 3.86 | 0.09 |
| 40 | 50.295 | 1495 | 1497 | α-Selinene | 1.24 | 0.08 |
| 41 | 50.425 | 1497 | 1497 | α-Muurolene | 1.35 | 0.06 |
| 42 | 51.313 | 1512 | 1512 | γ-Cadinene | 0.88 | 0.05 |
| 43 | 51.704 | 1519 | 1521 | α-Panasinsen | 34.90 | 0.25 |
| 44 | 52.716 | 1536 | 1538 | α-Cadinene | 0.37 | 0.05 |
| 45 | 52.96 | 1540 | 1541 | α-Calacorene | 0.54 | 0.02 |
| 46 | 55.41 | 1581 | 1577 | Caryophyllene oxide | 0.60 | 0.05 |
| 47 | 61.503 | 1688 | 1694 | Acorenone B | 0.55 | 0.06 |
| 48 | 62.926 | 1714 | 1715 | Pentadecanal | 1.82 | 0.12 |
| 49 | 69.609 | 1840 | 1841 | Phytone | 1.81 | 0.06 |
| Total identified | 98.48 |
| Sr. No. | RT | RI(calc) | RI(db) | Compound | Ave % | St Dev |
|---|---|---|---|---|---|---|
| 1 | 13.703 | 924 | 921 | Tricyclene | 1.08 | 0.16 |
| 2 | 16.285 | 971 | 974 | β-Pinene | 4.87 | 0.04 |
| 3 | 19.510 | 1027 | 1024 | Limonene | 0.25 | 0.01 |
| 4 | 41.005 | 1343 | 1345 | 7-epi-Silphiperfol-5-ene | 0.07 | 0.00 |
| 5 | 41.195 | 1346 | 1345 | α-Cubebene | 0.11 | 0.00 |
| 6 | 41.445 | 1350 | 1350 | α-Longipinene | 0.04 | 0.00 |
| 7 | 42.700 | 1369 | 1369 | Cyclosativene | 1.19 | 0.02 |
| 8 | 43.105 | 1375 | 1374 | α-Copaene | 1.46 | 0.01 |
| 9 | 43.305 | 1378 | 1374 | Isoledene | 0.18 | 0.00 |
| 10 | 43.640 | 1384 | 1387 | β-Bourbonene | 0.50 | 0.01 |
| 11 | 43.865 | 1387 | 1385 | α-Bourbonene | 0.08 | 0.00 |
| 12 | 43.985 | 1389 | 1389 | β-Elemene | 2.38 | 0.05 |
| 13 | 44.170 | 1392 | 1390 | Sativene | 0.04 | 0.01 |
| 14 | 44.895 | 1403 | 1398 | Cyperene | 0.06 | 0.00 |
| 15 | 45.140 | 1407 | 1409 | α-Gurjunene | 0.20 | 0.01 |
| 16 | 45.960 | 1420 | 1417 | (E)-Caryophyllene | 15.72 | 0.35 |
| 17 | 46.585 | 1430 | 1430 | β-Copaene | 0.23 | 0.01 |
| 18 | 46.725 | 1432 | 1432 | trans-α-Bergamotene | 0.10 | 0.01 |
| 19 | 47.485 | 1444 | 1447 | Isogermacrene D | 0.05 | 0.00 |
| 20 | 47.580 | 1446 | 1445 | Myltayl-4(12)-ene | 0.14 | 0.00 |
| 21 | 47.700 | 1448 | 1444 | 6,9-Guaiadiene | 0.09 | 0.00 |
| 22 | 47.925 | 1452 | 1454 | (E)-β-Farnesene | 3.96 | 0.03 |
| 23 | 48.205 | 1456 | 1452 | α-Humulene | 2.97 | 0.03 |
| 24 | 48.490 | 1461 | 1464 | 9-epi-(E)-Caryophyllene | 0.42 | 0.05 |
| 25 | 48.620 | 1463 | 1465 | cis-Muurola-4(14),5-diene | 0.04 | 0.02 |
| 26 | 49.285 | 1474 | 1476 | Selina-4,11-diene | 0.49 | 0.03 |
| 27 | 49.410 | 1476 | 1479 | γ-Muurolene | 0.41 | 0.01 |
| 28 | 49.540 | 1478 | 1475 | γ-Gurjunene | 8.58 | 0.36 |
| 29 | 49.605 | 1479 | 1483 | α-Amorphene | 2.81 | 0.57 |
| 30 | 49.820 | 1482 | 1484 | Germacrene D | 3.53 | 0.10 |
| 31 | 49.945 | 1484 | 1481 | γ-Himachalene | 25.16 | 0.78 |
| 32 | 50.040 | 1486 | 1487 | Aristolochene | 0.61 | 0.13 |
| 33 | 50.140 | 1488 | 1492 | δ-Selinene | 0.52 | 0.03 |
| 34 | 50.305 | 1490 | 1489 | β-Selinene | 0.81 | 0.02 |
| 35 | 50.455 | 1493 | 1496 | Valencene | 0.97 | 0.03 |
| 36 | 50.745 | 1498 | 1498 | α-Selinene | 1.19 | 0.03 |
| 37 | 50.855 | 1499 | 1500 | α-Muurolene | 2.11 | 0.04 |
| 38 | 50.965 | 1501 | 1505 | α-Cuprenene | 0.16 | 0.03 |
| 39 | 51.120 | 1504 | 1505 | (E,E)-α-Farnesene | 0.38 | 0.05 |
| 40 | 51.375 | 1508 | 1505 | β-Bisabolene | 0.13 | 0.04 |
| 41 | 51.590 | 1512 | 1509 | Tridecanal | 0.06 | 0.01 |
| 42 | 51.755 | 1514 | 1513 | γ-Cadinene | 0.35 | 0.01 |
| 43 | 51.925 | 1517 | 1514 | Cubebol | 0.08 | 0.01 |
| 44 | 52.030 | 1519 | 1522 | δ-Cadinene | 1.29 | 0.04 |
| 45 | 52.140 | 1521 | 1520 | 7-epi-α-Selinene | 0.41 | 0.03 |
| 46 | 52.255 | 1523 | 1521 | trans-Calamenene | 0.05 | 0.01 |
| 47 | 52.345 | 1524 | 1521 | β-Sesquiphellandrene | 0.16 | 0.02 |
| 48 | 53.165 | 1538 | 1537 | α-Cadinene | 0.10 | 0.01 |
| 49 | 53.445 | 1543 | 1544 | α-Calacorene | 0.18 | 0.01 |
| 50 | 53.680 | 1544 | 1545 | trans-Cadinene ether | 0.11 | 0.00 |
| 51 | 53.845 | 1547 | 1548 | α-Elemol | 0.04 | 0.00 |
| 52 | 54.680 | 1561 | 1564 | β-Calacorene | 0.05 | 0.01 |
| 53 | 55.580 | 1576 | 1577 | Spathulenol | 0.32 | 0.02 |
| 54 | 55.890 | 1581 | 1582 | Caryophyllene oxide | 3.05 | 0.03 |
| 55 | 57.510 | 1609 | 1608 | Humulene epoxide II | 0.39 | 0.01 |
| 56 | 59.300 | 1641 | 1638 | τ-Cadinol | 0.21 | 0.01 |
| 57 | 59.430 | 1643 | 1640 | τ-Muurolol | 0.13 | 0.00 |
| 59 | 60.055 | 1654 | 1652 | Himachalol | 0.49 | 0.05 |
| 59 | 59.980 | 1653 | --- | Unidentified | 0.86 | 0.05 |
| 60 | 60.255 | 1658 | 1658 | neo-Intermedeol | 0.32 | 0.00 |
| 61 | 69.900 | 1839 | 1841 | Phytone | 0.27 | 0.01 |
| 62 | 75.845 | 1960 | 1959 | Palmitic acid | 5.24 | 1.25 |
| 63 | 80.780 | 2066 | 2071 | Dibenzyl disulfide | 0.23 | 0.00 |
| Total identified | 97.60 |
| Organism | Lannea egregia EO | Emilia sonchifolia EO | (–)-β-Pinene | (±)-Linalool | (E)-Caryophyllene | Caryophyllene Oxide | Positive Control a |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| Bacillus cereus | 312.5 | 625 | 312.5 | 312.5 | 312.5 | 312.5 | 1.22 |
| Staphylococcus aureus | 312.5 | 1250 | 256.3 | 312.5 | 312.5 | 78.1 | 0.61 |
| Staphylococcus epidermidis | 312.5 | 156.3 | 312.5 | 312.5 | 312.5 | 312.5 | <19.5 |
| Streptococcus pyogenes | 625 | 312.5 | 625 | 312.5 | 312.5 | 625 | <19.5 |
| Molds | |||||||
| Aspergillus fumigatus | 156.3 | 156.3 | 156.3 | 156.3 | 156.3 | 156.3 | <19.5 |
| Aspergillus niger | 156.3 | 156.3 | 78.1 | 1250 | 1250 | 156.3 | 1.56 |
| Cryptococcus neoformans | 312.5 | 625 | 312.5 | 312.5 | 312.5 | 312.5 | 0.78 |
| Microsporum canis | 312.5 | 312.5 | 312.5 | 312.5 | 312.5 | 312.5 | <19.5 |
| Microsporum gypseum | 312.5 | 312.5 | 312.5 | 312.5 | 312.5 | 156.3 | <19.5 |
| Trichophyton mentagrophytes | 156.3 | 312.5 | 156.3 | 625 | 625 | 156.3 | <19.5 |
| Trichophyton rubrum | 312.5 | 312.5 | 312.5 | 312.5 | 312.5 | 312.5 | <19.5 |
| Yeast | |||||||
| Candida albicans | 156.3 | 312.5 | 156.3 | 156.3 | 156.3 | 312.5 | 1.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogundajo, A.L.; Ewekeye, T.; Sharaibi, O.J.; Owolabi, M.S.; Dosoky, N.S.; Setzer, W.N. Antimicrobial Activities of Sesquiterpene-Rich Essential Oils of Two Medicinal Plants, Lannea egregia and Emilia sonchifolia, from Nigeria. Plants 2021, 10, 488. https://doi.org/10.3390/plants10030488
Ogundajo AL, Ewekeye T, Sharaibi OJ, Owolabi MS, Dosoky NS, Setzer WN. Antimicrobial Activities of Sesquiterpene-Rich Essential Oils of Two Medicinal Plants, Lannea egregia and Emilia sonchifolia, from Nigeria. Plants. 2021; 10(3):488. https://doi.org/10.3390/plants10030488
Chicago/Turabian StyleOgundajo, Akintayo L., Tolulope Ewekeye, Olubunmi J. Sharaibi, Moses S. Owolabi, Noura S. Dosoky, and William N. Setzer. 2021. "Antimicrobial Activities of Sesquiterpene-Rich Essential Oils of Two Medicinal Plants, Lannea egregia and Emilia sonchifolia, from Nigeria" Plants 10, no. 3: 488. https://doi.org/10.3390/plants10030488
APA StyleOgundajo, A. L., Ewekeye, T., Sharaibi, O. J., Owolabi, M. S., Dosoky, N. S., & Setzer, W. N. (2021). Antimicrobial Activities of Sesquiterpene-Rich Essential Oils of Two Medicinal Plants, Lannea egregia and Emilia sonchifolia, from Nigeria. Plants, 10(3), 488. https://doi.org/10.3390/plants10030488







