Glutacetine® Biostimulant Applied on Wheat under Contrasting Field Conditions Improves Grain Number Leading to Better Yield, Upgrades N-Related Traits and Changes Grain Ionome
Abstract
1. Introduction
2. Results
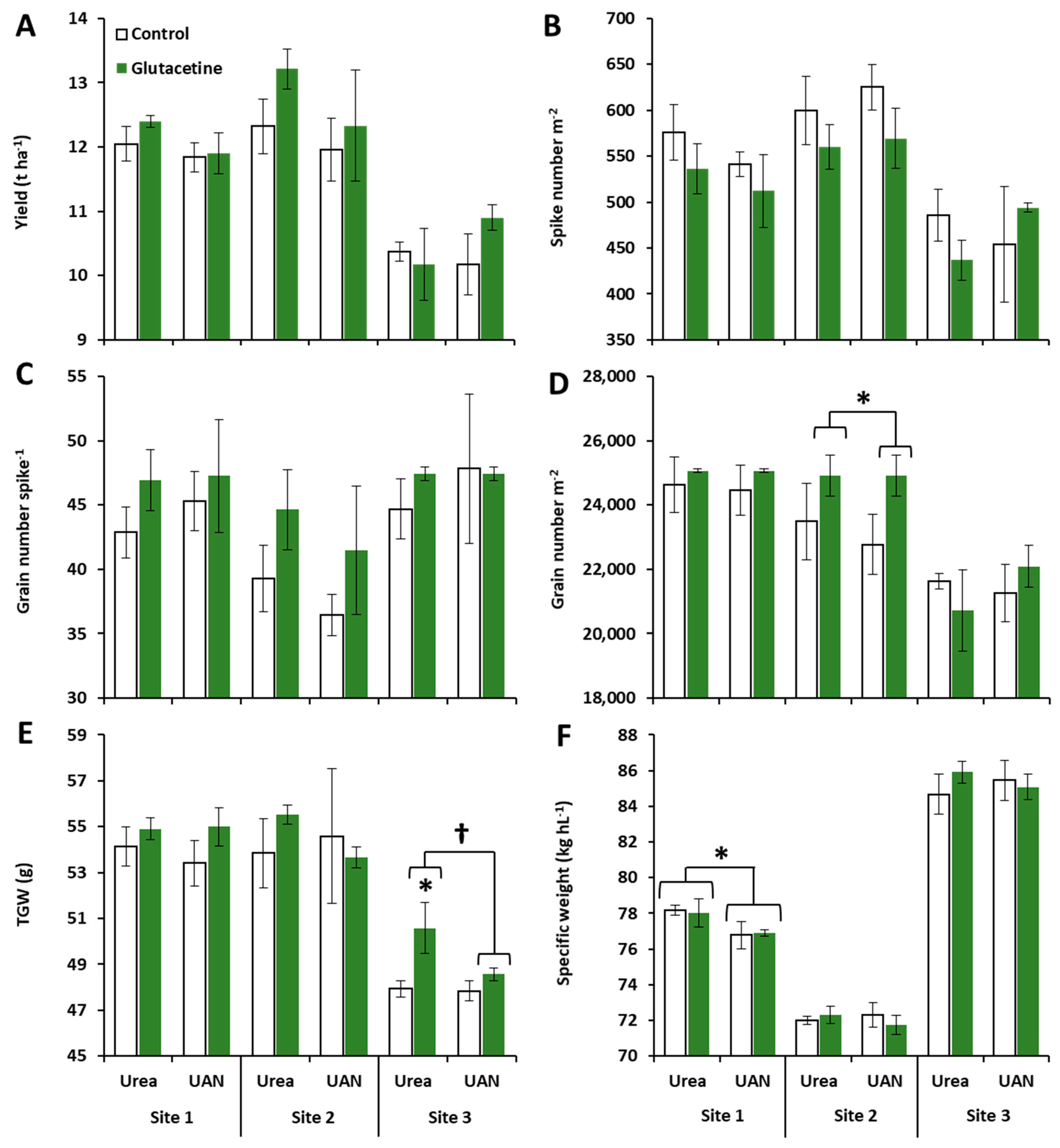
2.1. Effects of Site, N Fertilizer Forms and Glutacetine® on Grain Yield and Its Components
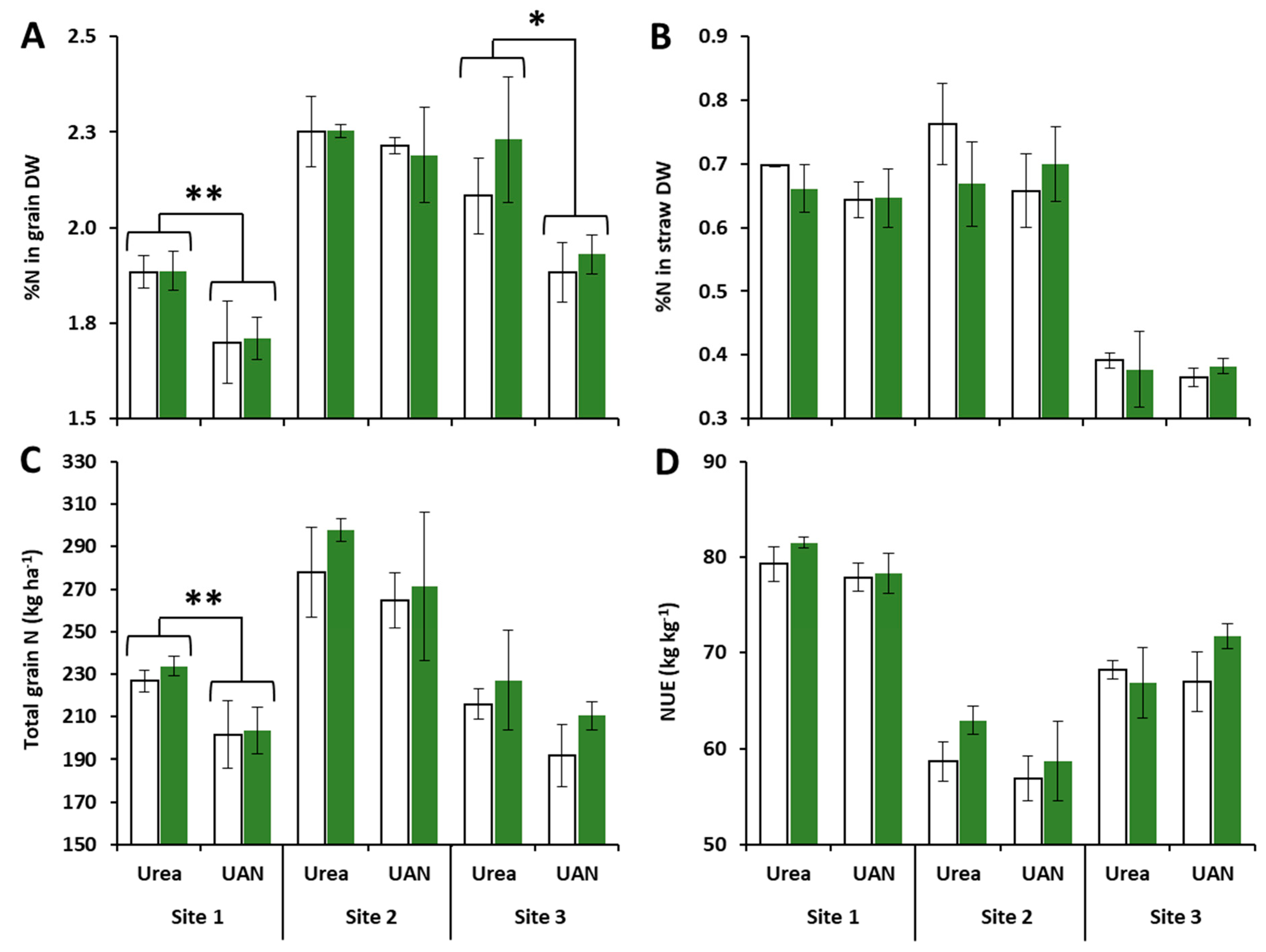
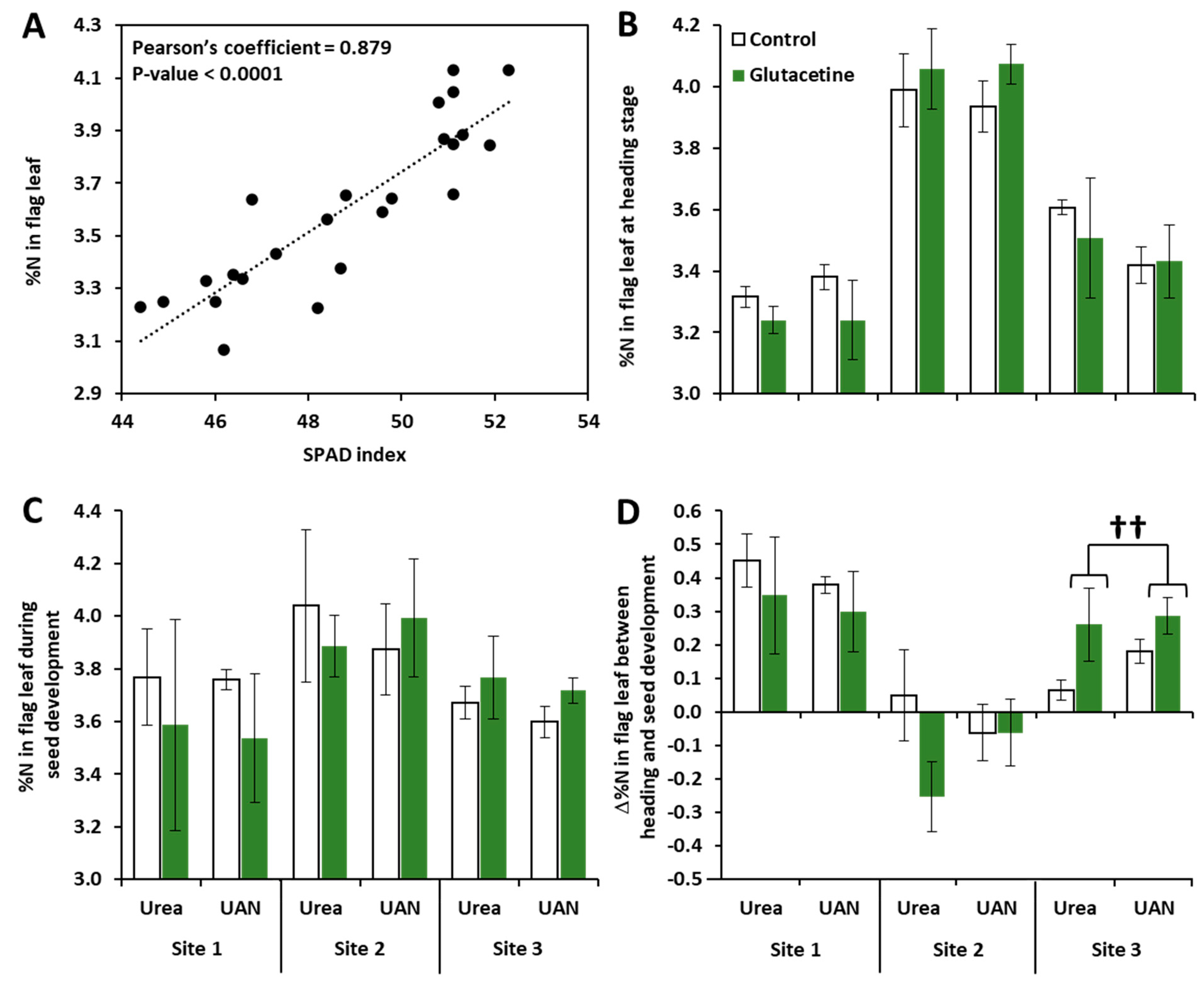
2.2. Impacts of Site, N Fertilizer Forms and Glutacetine® on N-Related Traits
2.3. Effects of Site, N fertilizer Forms and Glutacetine® on the Grain Ionome
2.4. Effects of Site, N fertilizer Forms and Glutacetine® on the Straw Ionome
3. Discussion
3.1. Glutacetine® Improves Grain Yield by Increasing Grain Number per Spike and per Square Meter
3.2. Glutacetine® Affects NUE and Total Grain N Differently
3.3. Glutacetine® Induces Changes in the Grain and Straw Ionomes
4. Materials and Methods
4.1. Field Experiment Design
4.2. Seed Yield Components and N Use Efficiency
4.3. Elemental Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makino, A. Photosynthesis, Grain Yield, and Nitrogen Utilization in Rice and Wheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.L.; Kidwell, K.K.; McCracken, V.A.; Bolton, R.P.; Allen, M. Economically Optimal Wheat Yield, Protein and Nitrogen Use Component Responses to Varying N Supply and Genotype. Front. Plant Sci. 2020, 10, 1790. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Ramanantenasoa, M.M.J.; Génermont, S.; Gilliot, J.-M.; Bedos, C.; Makowski, D. Meta-modeling methods for estimating ammonia volatilization from nitrogen fertilizer and manure applications. J. Environ. Manag. 2019, 236, 195–205. [Google Scholar] [CrossRef]
- Krol, D.; Forrestal, P.; Wall, D.; Lanigan, G.; Sanz-Gomez, J.; Richards, K. Nitrogen fertilisers with urease inhibitors reduce nitrous oxide and ammonia losses, while retaining yield in temperate grassland. Sci. Total Environ. 2020, 725, 138329. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Rosa, E.A.S.; Saavedra, M.J. Effects of agriculture production systems on nitrate and nitrite accumulation on baby-leaf salads. Food Sci. Nutr. 2012, 1, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2005, 86, 10–17. [Google Scholar] [CrossRef]
- Hirel, B.; Tetu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant Nitrogen Assimilation and Use Efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.J. Genetic variation in traits for nitrogen use efficiency in wheat. J. Exp. Bot. 2017, 68, 2627–2632. [Google Scholar] [CrossRef]
- Habbib, H.; Hirel, B.; Verzeaux, J.; Roger, D.; Lacoux, J.; Lea, P.; Dubois, F.; Tétu, T. Investigating the Combined Effect of Tillage, Nitrogen Fertilization and Cover Crops on Nitrogen Use Efficiency in Winter Wheat. Agronomy 2017, 7, 66. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and Interpretation of Factors Which Contribute to Efficiency of Nitrogen Utilization1. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Recio, J.; Vallejo, A.; Le-Noë, J.; Garnier, J.; García-Marco, S.; Álvarez, J.M.; Sanz-Cobena, A. The effect of nitrification inhibitors on NH3 and N2O emissions in highly N fertilized irrigated Mediterranean cropping systems. Sci. Total Environ. 2018, 636, 427–436. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; González-López, J.; Vallejo, A.; Bedmar, E.J. Effect of urease and nitrification inhibitors on ammonia volatilization and abundance of N-cycling genes in an agricultural soil. J. Plant Nutr. Soil Sci. 2019, 183, 99–109. [Google Scholar] [CrossRef]
- Huf, M.T.; Olfs, H.-W. Effect of the nitrification inhibitor DMPP on nitrous oxide emissions and the stabilization of ammonium following the injection of dairy slurry and digestate in a soil-column experiment. J. Plant Nutr. Soil Sci. 2020, 183, 129–135. [Google Scholar] [CrossRef]
- Guardia, G.; Vallejo, A.; Cardenas, L.M.; Dixon, E.R.; García-Marco, S. Fate of 15 N-labelled ammonium nitrate with or without the new nitrification inhibitor DMPSA in an irrigated maize crop. Soil Biol. Biochem. 2018, 116, 193–202. [Google Scholar] [CrossRef]
- Artola, E.; Cruchaga, S.; Ariz, I.; Moran, J.F.; Garnica, M.; Houdusse, F.; Mina, J.M.G.; Irigoyen, I.; Lasa, B.; Aparicio-Tejo, P.M. Effect of N-(n-butyl) thiophosphoric triamide on urea metabolism and the assimilation of ammonium by Triticum aestivum L. Plant Growth Regul. 2011, 63, 73–79. [Google Scholar] [CrossRef]
- Zanin, L.; Venuti, S.; Tomasi, N.; Zamboni, A.; Francisco, R.M.D.B.; Varanini, Z.; Pinton, R. Short-Term Treatment with the Urease Inhibitor N-(n-Butyl) Thiophosphoric Triamide (NBPT) Alters Urea Assimilation and Modulates Transcriptional Profiles of Genes Involved in Primary and Secondary Metabolism in Maize Seedlings. Front. Plant Sci. 2016, 7, 845. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; El-Nakhel, C.; Bonini, P.; D’Amelia, L.; Dell’Aversana, E.; Pannico, A.; Giordano, M.; Sifola, M.I.; Kyriacou, M.C.; et al. Biostimulant Application with a Tropical Plant Extract Enhances Corchorus olitorius Adaptation to Sub-Optimal Nutrient Regimens by Improving Physiological Parameters. Agronomy 2019, 9, 249. [Google Scholar] [CrossRef]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous Application of Amino Acids Improves the Growth and Yield of Lettuce by Enhancing Photosynthetic Assimilation and Nutrient Availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-Based Biostimulants Influence the Agronomical, Physiological, and Qualitative Responses of Baby Rocket Leaves under Diverse Nitrogen Conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Carillo, P.; Colla, G.; Fiorentino, N.; Sabatino, L.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cirillo, V.; Shabani, E.; et al. Appraisal of Combined Applications of Trichoderma virens and a Biopolymer-Based Biostimulant on Lettuce Agronomical, Physiological, and Qualitative Properties under Variable N Regimes. Agronomy 2020, 10, 196. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Brisson, N.; Gate, P.; Gouache, D.; Charmet, G.; Oury, F.-X.; Huard, F. Why are wheat yields stagnating in Europe? A comprehensive data analysis for France. Field Crop. Res. 2010, 119, 201–212. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 26 February 2021).
- Sootahar, M.K.; Zeng, X.; Wang, Y.; Su, S.; Soothar, P.; Bai, L.; Kumar, M.; Zhang, Y.; Mustafa, A.; Ye, N. The Short-Term Effects of Mineral- and Plant-Derived Fulvic Acids on Some Selected Soil Properties: Improvement in the Growth, Yield, and Mineral Nutritional Status of Wheat (Triticum aestivum L.) under Soils of Contrasting Textures. Plants 2020, 9, 205. [Google Scholar] [CrossRef]
- Wang, S.; Tian, X.; Liu, Q. The Effectiveness of Foliar Applications of Zinc and Biostimulants to Increase Zinc Concentration and Bioavailability of Wheat Grain. Agronomy 2020, 10, 178. [Google Scholar] [CrossRef]
- Yadav, R.; Ror, P.; Rathore, P.; Ramakrishna, W. Bacteria from native soil in combination with arbuscular mycorrhizal fungi augment wheat yield and biofortification. Plant Physiol. Biochem. 2020, 150, 222–233. [Google Scholar] [CrossRef]
- Karimzadeh, J.; Alikhani, H.A.; Etesami, H.; Pourbabaei, A.A. Improved Phosphorus Uptake by Wheat Plant (Triticum aestivum L.) with Rhizosphere Fluorescent Pseudomonads Strains Under Water-Deficit Stress. J. Plant Growth Regul. 2020, 1–17. [Google Scholar] [CrossRef]
- Kowalska, J.; Tyburski, J.; Jakubowska, M.; Krzymińska, J. Effect of Different Forms of Silicon on Growth of Spring Wheat Cultivated in Organic Farming System. Silicon 2021, 13, 211–217. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the New Plant Growth Biostimulants Based on Amino Acids on Yield and Grain Quality of Winter Wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Glaes, J.; Spaepen, S.; Bodson, B.; Du Jardin, P.; Delaplace, P. Biostimulant effects ofBacillusstrains on wheat fromin vitrotowards field conditions are modulated by nitrogen supply. J. Plant Nutr. Soil Sci. 2019, 182, 325–334. [Google Scholar] [CrossRef]
- Laurent, E.-A.; Ahmed, N.; Durieu, C.; Grieu, P.; Lamaze, T. Marine and fungal biostimulants improve grain yield, nitrogen absorption and allocation in durum wheat plants. J. Agric. Sci. 2020, 158, 279–287. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Maignan, V.; Bernay, B.; Géliot, P.; Avice, J.-C. Biostimulant Effects of Glutacetine® and Its Derived Formulations Mixed With N Fertilizer on Post-heading N Uptake and Remobilization, Seed Yield, and Grain Quality in Winter Wheat. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The World Health Report 2002—Reducing Risks, Promoting Healthy Life. 2002. Available online: https://www.who.int/whr/2002/en/ (accessed on 15 December 2020).
- Ciccolini, V.; Pellegrino, E.; Coccina, A.; Fiaschi, A.I.; Cerretani, D.; Sgherri, C.; Quartacci, M.F.; Ercoli, L. Biofortification with Iron and Zinc Improves Nutritional and Nutraceutical Properties of Common Wheat Flour and Bread. J. Agric. Food Chem. 2017, 65, 5443–5452. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef]
- Oury, F.-X.; Leenhardt, F.; Rémésy, C.; Chanliaud, E.; Duperrier, B.; Balfourier, F.; Charmet, G. Genetic variability and stability of grain magnesium, zinc and iron concentrations in bread wheat. Eur. J. Agron. 2006, 25, 177–185. [Google Scholar] [CrossRef]
- Cu, S.T.; Guild, G.; Nicolson, A.; Velu, G.; Singh, R.; Stangoulis, J. Genetic dissection of zinc, iron, copper, manganese and phosphorus in wheat (Triticum aestivum L.) grain and rachis at two developmental stages. Plant Sci. 2020, 291, 110338. [Google Scholar] [CrossRef]
- Shewry, P.; Lafiandra, D.; Bedő, Z. Improving the nutritional quality and health benefits of wheat. Qual. Assur. Saf. Crop. Foods 2012, 4, 136. [Google Scholar] [CrossRef]
- Bharti, K.; Pandey, N.; Shankhdhar, D.; Pc, S.; Sc, S. Improving nutritional quality of wheat through soil and foliar zinc application. Plant Soil Environ. 2013, 59, 348–352. [Google Scholar] [CrossRef]
- Vanoni, M.A.; Dossena, L.; Heuvel, R.H.H.V.D.; Curti, B. Structure–function studies on the complex iron–sulfur flavoprotein glutamate synthase: The key enzyme of ammonia assimilation. Photosynth. Res. 2005, 83, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Thor, K. Calcium—Nutrient and Messenger. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Navazio, L.; Formentin, E.; Cendron, L.; Szabò, I. Chloroplast Calcium Signaling in the Spotlight. Front. Plant Sci. 2020, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.; Tabbita, F.; Cantu, D.; Buffalo, V.; Avni, R.; Vazquez-Gross, H.; Zhao, R.; Conley, C.J.; Distelfeld, A.; Dubcovksy, J. Regulation of Zn and Fe transporters by the GPC1gene during early wheat monocarpic senescence. BMC Plant Biol. 2014, 14, 1–23. [Google Scholar] [CrossRef]
- Connorton, J.M.; Jones, E.R.; Rodríguez-Ramiro, I.; Fairweather-Tait, S.; Uauy, C.; Balk, J. Wheat Vacuolar Iron Transporter TaVIT2 Transports Fe and Mn and Is Effective for Biofortification. Plant Physiol. 2017, 174, 2434–2444. [Google Scholar] [CrossRef]
- Evens, N.P.; Buchner, P.; Williams, L.E.; Hawkesford, M.J. The role of ZIP transporters and group F bZIP transcription factors in the Zn-deficiency response of wheat (Triticum aestivum). Plant J. 2017, 92, 291–304. [Google Scholar] [CrossRef]
- Waters, B.M.; Uauy, C.; Dubcovsky, J.; Grusak, M.A. Wheat (Triticum aestivum) NAM proteins regulate the translocation of iron, zinc, and nitrogen compounds from vegetative tissues to grain. J. Exp. Bot. 2009, 60, 4263–4274. [Google Scholar] [CrossRef] [PubMed]
- Maillard, A.; DiquéLou, S.; Billard, V.; Laîné, P.; Egarnica, M.; Eprudent, M.; Garcia-Mina, J.-M.; Eyvin, J.-C.; Eourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Riefolo, C.; Nicastro, G.; De Simone, V.; Di Gesù, A.M.; Beleggia, R.; Platani, C.; Cattivelli, L.; De Vita, P. Phytate and mineral elements concentration in a collection of Italian durum wheat cultivars. Field Crop. Res. 2009, 111, 235–242. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Biofortification and bioavailability of Zn, Fe and Se in wheat: Present status and future prospects. Theor. Appl. Genet. 2021, 134, 1–35. [Google Scholar] [CrossRef]
- Paltridge, N.G.; Milham, P.J.; Ortiz-Monasterio, J.I.; Velu, G.; Yasmin, Z.; Palmer, L.J.; Guild, G.E.; Stangoulis, J.C.R. Energy-dispersive X-ray fluorescence spectrometry as a tool for zinc, iron and selenium analysis in whole grain wheat. Plant Soil 2012, 361, 261–269. [Google Scholar] [CrossRef]
- Khokhar, J.S.; Sareen, S.; Tyagi, B.S.; Singh, G.; Wilson, L.; King, I.P.; Young, S.D.; Broadley, M.R. Variation in grain Zn concentration, and the grain ionome, in field-grown Indian wheat. PLoS ONE 2018, 13, e0192026. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Al-Hashmi, K.S.; Nadeem, F.; Ullah, A. Characterizing bread wheat genotypes of Pakistani origin for grain zinc biofortification potential. J. Sci. Food Agric. 2018, 98, 4824–4836. [Google Scholar] [CrossRef]
- Ramzani, P.M.A.; Khalid, M.; Naveed, M.; Ahmad, R.; Shahid, M. Iron biofortification of wheat grains through integrated use of organic and chemical fertilizers in pH affected calcareous soil. Plant Physiol. Biochem. 2016, 104, 284–293. [Google Scholar] [CrossRef]
- Qiao, K.; Wang, F.; Liang, S.; Wang, H.; Hu, Z.; Chai, T. New Biofortification Tool: Wheat TaCNR5 Enhances Zinc and Manganese Tolerance and Increases Zinc and Manganese Accumulation in Rice Grains. J. Agric. Food Chem. 2019, 67, 9877–9884. [Google Scholar] [CrossRef]
- Power, J.F.; Alessi, J. Tiller development and yield of standard and semidwarf spring wheat varieties as affected by nitrogen fertilizer. J. Agric. Sci. 1978, 90, 97–108. [Google Scholar] [CrossRef]
- Wang, X.T.; Below, F.E. Tillering, nutrient accumulation, and yield of winter wheat as influenced by nitrogen form1. J. Plant Nutr. 1995, 18, 1177–1189. [Google Scholar] [CrossRef]
- Slafer, G.A.; Elia, M.; Savin, R.; García, G.A.; Terrile, I.I.; Ferrante, A.; Miralles, D.J.; González, F.G. Fruiting efficiency: An alternative trait to further rise wheat yield. Food Energy Secur. 2015, 4, 92–109. [Google Scholar] [CrossRef]
- Pretini, N.; Terrile, I.I.; Gazaba, L.N.; Donaire, G.M.; Arisnabarreta, S.; Vanzetti, L.S.; González, F.G. A comprehensive study of spike fruiting efficiency in wheat. Crop. Sci. 2020, 60, 1541–1555. [Google Scholar] [CrossRef]
- Ganesh, S. Aftab Hussain Bio Stimulant Activity of Protein Hydrolysate: Influence on Plant Growth and Yield. J. Plant Sci. Res. 2015, 2, 125. [Google Scholar]
- Hussain, A.; Ahmad, M.; Mumtaz, M.Z.; Ali, S.; Sarfraz, R.; Naveed, M.; Jamil, M.; Damalas, C.A. Integrated Application of Organic Amendments with Alcaligenes sp. AZ9 Improves Nutrient Uptake and Yield of Maize (Zea mays). J. Plant Growth Regul. 2020, 39, 1277–1292. [Google Scholar] [CrossRef]
- Haddad, C.; Arkoun, M.; Jamois, F.; Schwarzenberg, A.; Yvin, J.-C.; Etienne, P.; Laîné, P. Silicon Promotes Growth of Brassica napus L. and Delays Leaf Senescence Induced by Nitrogen Starvation. Front. Plant Sci. 2018, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Laîné, P.; Haddad, C.; Arkoun, M.; Yvin, J.-C.; Etienne, P. Silicon Promotes Agronomic Performance in Brassica napus Cultivated under Field Conditions with Two Nitrogen Fertilizer Inputs. Plants 2019, 8, 137. [Google Scholar] [CrossRef]
- Peris-Felipo, F.J.; Benavent-Gil, Y.; Hernández-Apaolaza, L. Silicon beneficial effects on yield, fruit quality and shelf-life of strawberries grown in different culture substrates under different iron status. Plant Physiol. Biochem. 2020, 152, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein Hydrolysate Stimulates Growth in Tomato Coupled With N-Dependent Gene Expression Involved in N Assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef]
- Kong, L.; Xie, Y.; Hu, L.; Feng, B.; Li, S. Remobilization of vegetative nitrogen to developing grain in wheat (Triticum aestivum L.). Field Crop. Res. 2016, 196, 134–144. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Khourchi, S.; Ibnyasser, A.; Ghoulam, C.; Rchiad, Z.; Zeroual, Y.; Lyamlouli, K.; Bargaz, A. Phosphate Solubilizing Rhizobacteria Could Have a Stronger Influence on Wheat Root Traits and Aboveground Physiology Than Rhizosphere P Solubilization. Front. Plant Sci. 2020, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Del Coco, L.; Laddomada, B.; Migoni, D.; Mita, G.; Simeone, R.; Fanizzi, F.P. Variability and Site Dependence of Grain Mineral Contents in Tetraploid Wheats. Sustainability 2019, 11, 736. [Google Scholar] [CrossRef]
- Likar, M.; Vogel-Mikuš, K.; Potisek, M.; Hančević, K.; Radić, T.; Nečemer, M.; Regvar, M. Importance of soil and vineyard management in the determination of grapevine mineral composition. Sci. Total Environ. 2015, 505, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-los-Reyes, C.; Amorós Ortíz-Villajos, J.A.; García Navarro, F.J.; Bravo Martín-Consuegra, S.; Jiménez Ballesta, R. Grapevine Leaf Uptake of Mineral Elements Influenced by Sugar Foam Amendment of an Acidic Soil. Vitis 2013, 52, 157–164. [Google Scholar]
- Krochmal-Marczak, B.; Sawicka, B. Relationship between Physiacal and Chemical Properties of Soil and Iron, Manganese and Zinc Content in a Grain of Winter Wheat. Pol. J. Environ. Stud. 2008, 17, 278–283. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Butphu, S.; Rasche, F.; Cadisch, G.; Kaewpradit, W. Eucalyptus biochar application enhances Ca uptake of upland rice, soil available P, exchangeable K, yield, and N use efficiency of sugarcane in a crop rotation system. J. Plant Nutr. Soil Sci. 2019, 183, 58–68. [Google Scholar] [CrossRef]
- Safar-Noori, M.; Dong, Q.; Saneoka, H. Improvement of Grain Yield, Nutritional and Antinutritional Quality, and Seed Physiological Performance of Wheat by NPK Fertilization. J. Agric. Sci. Technol. 2018, 20, 1467–1477. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nozoye, T.; Nishizawa, N.K. Iron transport and its regulation in plants. Free. Radic. Biol. Med. 2019, 133, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lancashire, P.D.; Bleiholder, H.; Van Den Boom, T.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Comité Français D’étude et de Développement de la Fertilisation Raisonnée (COMIFER). Calcul de la Fertilisation Azotée; Acta: Paris, France, 2000; ISBN 978-2-910393-09-0. [Google Scholar]
- Cohan, J.-P.; Le Souder, C.; Guicherd, C.; Lorgeou, J.; Du Cheyron, P.; Bonnefoy, M.; Decarrier, A.; Piraux, F.; Laurent, F. Combining breeding traits and agronomic indicators to characterize the impact of cultivar on the nitrogen use efficiency of bread wheat. Field Crop. Res. 2019, 242, 107588. [Google Scholar] [CrossRef]
| Grain Yield and Its Components | N-Related Traits | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield (t/ha) | Spikes/m² | Grains/Spike | Grains/m² | TGW | Specific Weight | %N in Grain | %N in Straw | Total Grain N | NUE | %N in the Flag Leaf at Heading | %N in the Flag Leaf during Seed Development | Δ%N in the Flag Leaf | |||
| Global ANOVA | Site (S) | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎ | ⁎⁎⁎ | |
| Fertilizer (F) | ⁎⁎⁎ | ⁎⁎ | |||||||||||||
| Treatment (T) | <0.08 | <0.07 | ⁎ | ⁎ | <0.06 | <0.08 | |||||||||
| S × F | |||||||||||||||
| S × T | ⁎ | ||||||||||||||
| F × T | |||||||||||||||
| S × F × T | |||||||||||||||
| ANOVA Urea | Site | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | |||
| Treatment | ⁎ | ⁎ | ⁎ | ||||||||||||
| S × T | <0.08 | ||||||||||||||
| ANOVA UAN | Site | ⁎⁎ | ⁎⁎ | ⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | <0.08 | ⁎⁎⁎ | |
| Treatment | ⁎ | ||||||||||||||
| S × T | |||||||||||||||
| Grain Ionome | Straw Ionome | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Fe | K | Mg | Mn | P | S | Zn | Ca | Fe | K | Mg | S | |||
| Global ANOVA | Site (S) | ⁎⁎⁎ | <0.06 | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎ | ⁎⁎⁎ | ⁎ | ⁎⁎⁎ | ⁎⁎ | |||||
| Fertilizer (F) | ⁎ | ⁎ | |||||||||||||
| Treatment (T) | <0.06 | ||||||||||||||
| S × F | ⁎ | <0.08 | ⁎ | ||||||||||||
| S × T | ⁎ | ||||||||||||||
| F × T | |||||||||||||||
| S × F × T | <0.08 | ⁎⁎ | ⁎ | ||||||||||||
| ANOVA Urea | Site | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎ | ⁎⁎⁎ | ⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎ | |||||
| Treatment | ⁎ | <0.07 | |||||||||||||
| S × T | <0.06 | ||||||||||||||
| ANOVA UAN | Site | ⁎⁎⁎ | ⁎⁎⁎ | <0.06 | ⁎⁎⁎ | ⁎⁎⁎ | <0.06 | ||||||||
| Treatment | |||||||||||||||
| S × T | <0.08 | <0.06 | ⁎ | ||||||||||||
| P | K | S | Mg | Ca | Fe | Zn | Mn | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Urea | Control | 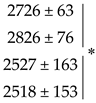 |  | 1112 ± 10 | 1154 ± 45 | 616 ± 19.2 | 34.0 ± 5.6 | 37.7 ± 1.7 | 30.6 ± 2.8 |
| Glutacetine® | 1105 ± 29 | 1278 ± 99 | 608 ± 10.1 | 26.8 ± 8.1 | 36.7 ± 1.4 | 38.7 ± 3.7 | ||||
| UAN | Control | 1083 ± 8 | 1273 ± 61 | 546 ± 24.0 | 29.3 ± 4.4 | 35.3 ± 4.2 | 34.9 ± 2.5 | |||
| Glutacetine® | 1080 ± 37 | 1187 ± 12 † | 608 ± 36.1 | 30.9 ± 1.4 | 38.6 ± 3.0 | 33.3 ± 1.8 | ||||
| Site 2 | Urea | Control | 2667 ± 18 | 4957 ± 114 | 1274 ± 19 | 1227 ± 76 | 677 ± 20.9 | 33.8 ± 2.9 | 34.8 ± 3.8 | 30.8 ± 1.6 |
| Glutacetine® | 2541 ± 34 ⁎ | 4899 ± 59 | 1240 ± 16 | 1118 ± 58 | 677 ± 14.5 | 30.8 ± 1.9 | 33.8 ± 2.7 | 31.7 ± 1.6 | ||
| UAN | Control | 2659 ± 60 | 5337 ± 381 | 1259 ± 7 | 1264 ± 82 | 708 ± 22.1 | 32.2 ± 3.1 | 34.8 ± 1.7 | 31.3 ± 3.6 | |
| Glutacetine® | 2621 ± 143 | 5054 ± 313 | 1264 ± 2 | 1186 ± 29 | 681 ± 27.1 | 34.2 ± 2.5 | 32.8 ± 4.4 | 31.5 ± 2.1 | ||
| Site 3 | Urea | Control | 2532 ± 100 | 5971 ± 326 | 1285 ± 30 | 1147 ± 49 | 829 ± 41.5 | 34.8 ± 3.9 | 40.8 ± 3.1 | 35.7 ± 3.5 |
| Glutacetine® | 2595 ± 24 | 5858 ± 167 | 1316 ± 24 | 1192 ± 58 | 803 ± 37.8 | 37.3 ± 0.2 | 40.8 ± 3.4 | 32.0 ± 2.0 | ||
| UAN | Control | 2369 ± 62 | 5650 ± 143 | 1267 ± 13 | 1216 ± 124 | 792 ± 9.4 | 39.7 ± 2.3 | 42.3 ± 2.2 | 30.8 ± 1.6 | |
| Glutacetine® | 2560 ± 184 | 5603 ± 465 | 1239 ± 10 | 1287 ± 83 | 778 ± 51.4 | 31.4 ± 1.9 ⁎ | 39.1 ± 2.1 | 36.6 ± 1.3 ⁎ |
| K | Mg | S | Ca | Fe | |||
|---|---|---|---|---|---|---|---|
| Site 1 | Urea | Control | 10945 ± 632 | 937 ± 58.9 | 866 ± 24.3 | 2639 ± 53 | 152 ± 3.6 |
| Glutacetine® | 11231 ± 315 | 895 ± 52.6 | 852 ± 33.3 | 2726 ± 106 | 135 ± 9.0 | ||
| UAN | Control | 11008 ± 461 | 826 ± 113.1 | 897 ± 72.4 | 2664 ± 86 | 144 ± 18.1 | |
| Glutacetine® | 10062 ± 406 | 835 ± 123.6 | 918 ± 27.4 | 2638 ± 34 | 196 ± 21.9 | ||
| Site 2 | Urea | Control | 14796 ± 480 | 829 ± 48.6 | 964 ± 25.5 | 3487 ± 125 | 111 ± 14.9 |
| Glutacetine® | 15630 ± 1155 | 711 ± 141.9 | 1004 ± 41.0 | 3236 ± 201 | 78 ± 6.0 | ||
| UAN | Control | 16132 ± 1990 | 714 ± 39.5 | 949 ± 72.0 | 3269 ± 189 | 132 ± 55.8 | |
| Glutacetine® | 16289 ± 1405 | 764 ± 76.7 | 971 ± 35.3 | 3394 ± 93 | 162 ± 71.1 | ||
| Site 3 | Urea | Control | 4692 ± 785 | 686 ± 149.6 | 1008 ± 75.9 | 4845 ± 320 | 131 ± 14.7 |
| Glutacetine® | 6946 ± 1168 | 858 ± 82.2 | 977 ± 102.0 | 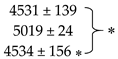 | 119 ± 8.9 | ||
| UAN | Control | 5862 ± 965 | 975 ± 111.3 | 1050 ± 93.7 | 116 ± 9.2 | ||
| Glutacetine® | 5299 ± 431 | 791 ± 135.2 | 1034 ± 38.1 | 98 ± 1.5 |
| Glutamic acid (%DW) | 3.6 | |
| Organic acids (%DW) | 7.4 | |
| Total Soluble Sugars (%DW) | 10 | |
| Elements (%DW) | Cl | 19.8 |
| Ca | 15.6 | |
| C | 6.26 | |
| N | 0.76 | |
| K | 0.31 | |
| Na | 0.31 | |
| Mo | 0.15 | |
| Elements (mg kg−1 DW) | S | 300 |
| B | 30 | |
| Mg | 19 | |
| Si | 14 | |
| P | 9.5 | |
| Cu | 2.6 | |
| Ni | 1.8 | |
| Co | 0.6 | |
| Zn | 0.3 | |
| Se | 0.08 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maignan, V.; Géliot, P.; Avice, J.-C. Glutacetine® Biostimulant Applied on Wheat under Contrasting Field Conditions Improves Grain Number Leading to Better Yield, Upgrades N-Related Traits and Changes Grain Ionome. Plants 2021, 10, 456. https://doi.org/10.3390/plants10030456
Maignan V, Géliot P, Avice J-C. Glutacetine® Biostimulant Applied on Wheat under Contrasting Field Conditions Improves Grain Number Leading to Better Yield, Upgrades N-Related Traits and Changes Grain Ionome. Plants. 2021; 10(3):456. https://doi.org/10.3390/plants10030456
Chicago/Turabian StyleMaignan, Victor, Patrick Géliot, and Jean-Christophe Avice. 2021. "Glutacetine® Biostimulant Applied on Wheat under Contrasting Field Conditions Improves Grain Number Leading to Better Yield, Upgrades N-Related Traits and Changes Grain Ionome" Plants 10, no. 3: 456. https://doi.org/10.3390/plants10030456
APA StyleMaignan, V., Géliot, P., & Avice, J.-C. (2021). Glutacetine® Biostimulant Applied on Wheat under Contrasting Field Conditions Improves Grain Number Leading to Better Yield, Upgrades N-Related Traits and Changes Grain Ionome. Plants, 10(3), 456. https://doi.org/10.3390/plants10030456








