Evaluation of Physiological and Morphological Traits for Improving Spring Wheat Adaptation to Terminal Heat Stress
Abstract
1. Introduction
2. Results
2.1. Crop Phenological Development
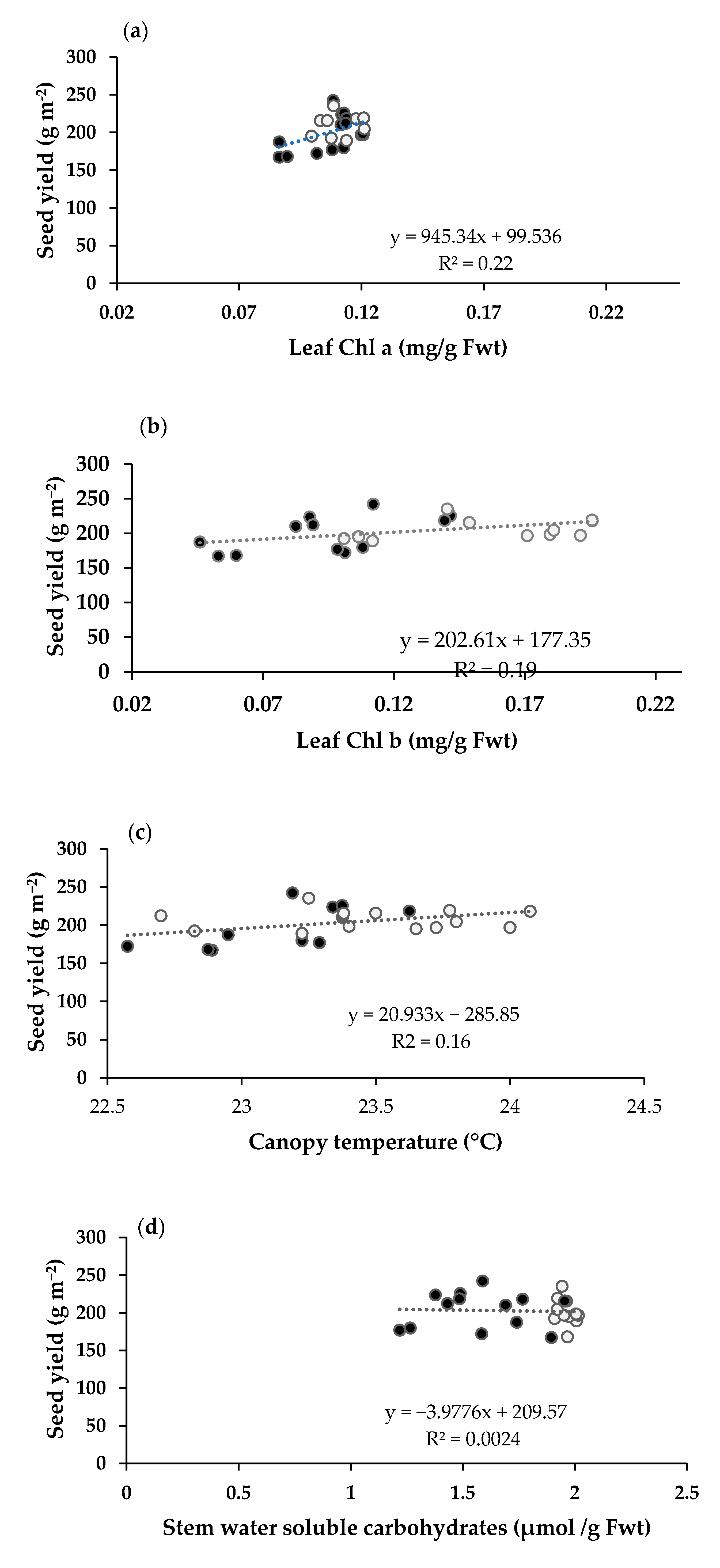
2.2. Physiological Traits
2.3. Gas Exchange Traits
2.4. Yield Related Traits
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Plant Material
4.3. Creation of Heat Stress Environment and Experimental Design
4.4. Climate and Weather Conditions
4.5. Crop Husbandry
4.6. Observations
4.6.1. Crop Phenology
4.6.2. Physiological Traits
4.6.3. Gas Exchange Traits
4.6.4. Yield Related Traits
4.6.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Asseng, S.; Ewert, F.; Martre, P.; Rotter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Braun, H.J.; Pfeiffer, W.H.; Pollmer, W.G. Environments for selecting widely adapted spring wheat. Crop Sci. 1992, 32, 1420–1427. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, R.P.; Crossa, J.; Huerta-Espinoab, J.; Sharmac, I.; Chatrathc, R.; Singhd, G.P.; Sohue, V.S.; Mavie, G.S.; Sukuru, V.S.P.; et al. Earliness in wheat: A key to adaptation under terminal and continual high temperature stress in South Asia. Field Crops Res. 2013, 151, 19–26. [Google Scholar] [CrossRef]
- Nawaz, A.; Farooq, M.; Nadeem, F.; Siddique, K.H.M.; Lal, R. Rice–wheat cropping systems in South Asia: Issues, options and opportunities. Crop Past. Sci. 2019, 70, 395–427. [Google Scholar] [CrossRef]
- Shah, M.A.; Farooq, M.; Hussain, M. Productivity and profitability of cotton–wheat system as influenced by relay intercropping of insect resistant transgenic cotton in bed planted wheat. Eur. J. Agron. 2016, 75, 33–41. [Google Scholar] [CrossRef]
- Joshi, A.K.; Mishra, B.; Chatrath, R.; Ferrara, G.O.; Singh, R.P. Wheat improvement in India: Present status, emerging challenges and future prospects. Euphytica 2007, 157, 431–446. [Google Scholar] [CrossRef]
- Al-Khatib, K.; Paulsen, G.M. Model of high temperature injury to wheat during grain development. Physiol. Plant. 1984, 61, 363–368. [Google Scholar] [CrossRef]
- Tashiro, T.; Wardlaw, I.F. The response to high temperature shock and humid-ity changes prior to and during the early stages of grain development in wheat. Aust. J. Plant Physiol. 1990, 17, 551–561. [Google Scholar]
- Wardlaw, I.; Wrigley, C. Heat tolerance in temperate cereals: An overview. Funct. Plant Biol. 1994, 21, 695–703. [Google Scholar] [CrossRef]
- Weigand, C.L.; Cueller, J.A. Duration of grain filling and kernel weight of wheat as affected by temperature. Crop Sci. 1981, 21, 95–101. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P. Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. J. Exp. Bot. 2012, 63, 3789–3798. [Google Scholar] [CrossRef] [PubMed]
- Talukder, S.K.; Babar, M.A.; Vijayalakshmi, K.; Poland, J.; Prasad, P.V.; Bowden, R.; Fritz, A. Mapping QTL for the traits associated with heat tolerance in wheat (Triticum aestivum L.). BMC Genet. 2014, 15, 97. [Google Scholar] [CrossRef]
- Pinto, R.S.; Reynolds, M.P. Common genetic basis for canopy temperature depression under heat and drought stress associated with optimized root distribution in bread wheat. Theor. Appl. Genet. 2015, 128, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Sinmena, B.; Mayer, J.; Golan, G.; Shpiler, L. Stem reserve mobilisation supports wheat-grain filling under heat stress. Funct. Plant Biol. 1994, 21, 771–781. [Google Scholar] [CrossRef]
- Kumari, M.; Singh, V.P.; Tripathi, R.; Joshi, A.K. Variation for staygreen trait and its association with canopy temperature depression and yield traits under terminal heat stress in wheat. In Wheat Production in Stressed Environments; Springer: Dordrecht, The Netherlands, 2007; pp. 357–363. [Google Scholar]
- Pinto, R.S.; Reynolds, M.P.; Mathews, K.L.; McIntyre, C.L.; Olivares-Villegas, J.-J.; Chapman, S.C. Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor. Appl. Genet. 2010, 121, 1001–1021. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.P.; Balota, M.; Delgado, M.I.B.; Amani, J.; Fischer, R.A. Physiological and morphological traits associated with spring wheat yield under hot irrigated conditions. Aust. J. Plant Physiol. 1994, 2, 717–730. [Google Scholar] [CrossRef]
- Kumari, M.; Pudake, R.N.; Singh, V.P.; Joshi, A.K. Association of stay green trait with canopy temperature depression and yield traits under terminal heat stress in wheat (Triticum aestivum L.). Euphytica 2013, 190, 87–97. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Tuberosa, R. Translational research impacting on crop productivity in drought-prone environments. Curr. Opin. Plant Biol. 2008, 11, 171–179. [Google Scholar] [CrossRef]
- Pinto, R.S.; Lopes, M.S.; Collins, N.C.; Reynolds, M.P. Modelling and genetic dissection of stay green under heat stress. Theor. Appl. Genet. 2016, 129, 2055–2074. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, P.; Kumar, U.; Grover, M.; Singh, A.K.; Singh, R.; Sengar, R.S. Molecular approaches for designing heat tolerant wheat. J. Plant Biochem. Biotechnol. 2013, 22, 359–371. [Google Scholar] [CrossRef]
- Ortiz, R.; Sayre, K.D.; Govaerts, B.; Gupta, R.; Subbarao, G.; Ban, T.; Hodson, D.; Dixon, J.M.; Iva´n, O.M. Climate change: Can wheat beat the heat? Agric. Ecosys. Environ. 2008, 126, 46–58. [Google Scholar] [CrossRef]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Pask, A.; Joshi, A.K.; Manès, Y.; Sharma, I.; Chatrath, R.; Singh, G.P.; Sohu, V.S.; Mavi, G.S.; Sakuru, V.S.P.; Kalappanavar, I.K.; et al. A wheat phenotyping network to incorporate physiological traits for climate change in South Asia. Field Crops Res. 2014, 168, 156–167. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Foulkes, J.; Furbank, R.; Griffiths, S.; King, J.; Murchie, E.; Parry, M.; Slafer, G. Achieving yield gains in wheat. Plant Cell Environ. 2012, 35, 1799–1823. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.P.; Langridge, P. Physiological breeding. Curr. Opin. Plant Biol. 2016, 31, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Shabbir, G.; Ahmed, M.; Shah, K.N. Phenotyping for drought resistance in bread wheat using physiological and biochemical traits. Sci. Total Environ. 2020, 729, 139082. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Introduction to Modern Climate Change. Andrew E. Dessler: Cambridge University Press, 2011, 252 pp, ISBN-10: 0521173159. Sci. Total Environ. 2020, 734, 139397. [Google Scholar] [CrossRef]
- Ahmed, M.; Aslam, M.A.; Hassan, F.; Hayat, R.; Ahmad, S. Biochemical, Physiological and Agronomic Response of Wheat to Changing Climate of Rainfed Pakistan. Pak. J. Bot 2014, 51, 2. [Google Scholar]
- Ahmed, M.; Hassan, F.; Asif, M. Physiological response of bread wheat (‘Triticum aestivum’L.) to high temperature and moisture stresses. Aust. J. Crop Sci. 2012, 6, 749. [Google Scholar]
- Ahmed, M.; Hassan, F.; Aslam, M.A.; Akram, M.N.; Akmal, M. Regression model for the study of sole and cumulative effect of temperature and solar radiation on wheat yield. Afr. J. Biotechnol. 2011, 10, 9114–9121. [Google Scholar]
- Tariq, M.; Ahmed, M.; Iqbal, P.; Fatima, Z.; Ahmad, S. Crop Phenotyping. In Systems Modeling; Ahmed, M., Ed.; Springer Singapore: Singapore, 2020; pp. 45–60. [Google Scholar] [CrossRef]
- Liu, B.; Martre, P.; Ewert, F.; Porter, J.R.; Challinor, A.J.; Müller, C.; Ruane, A.C.; Waha, K.; Thorburn, P.J.; Aggarwal, P.K.; et al. Global wheat production with 1.5 and 2.0 °C above pre-industrial warming. Glob. Chang. Biol. 2019, 25, 1428–1444. [Google Scholar] [CrossRef]
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Babar, M.A.; et al. Climate change impact and adaptation for wheat protein. Glob. Chang. Biol. 2019, 25, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, M.; Ahmed, M.; Hussain, M.I. Rising Atmospheric Temperature Impact on Wheat and Thermotolerance Strategies. Plants 2021, 10, 43. [Google Scholar] [CrossRef]
- Khan, A.; Ahmad, M.; Shah, M.K.N.; Ahmed, M. Genetic manifestation of physio-morphic and yield related traits conferring thermotolerance in wheat. Pak. J. Bot. 2020, 52, 1545–1552. [Google Scholar] [CrossRef]
- Ahmad, S.; Abbas, G.; Ahmed, M.; Fatima, Z.; Anjum, M.A.; Rasul, G.; Khan, M.A.; Hoogenboom, G. Climate warming and management impact on the change of phenology of the rice-wheat cropping system in Punjab, Pakistan. Field Crops Res. 2019, 230, 46–61. [Google Scholar] [CrossRef]
- Ahmed, M.; Fayyazul, H. Response of Spring Wheat (Triticum aestivum L.) Quality Traits and Yield to Sowing Date. PLoS ONE 2015, 10, e0126097. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Farooq, S. Growth and physiological responses of wheat cultivars under various planting windows. JAPS J. Anim. Plant Sci. 2013, 23, 1407–1414. [Google Scholar]
- Wheeler, T.R.; Hong, T.D.; Ellis, R.H.; Batts, G.R.; Morrison, J.I.L.; Hadley, P. The duration and rate of grain growth, and harvest index, of wheat (Triticum aestivum L.) in response to temperature and carbon dioxide. J. Exp. Bot. 1996, 47, 623–630. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Jeuffroy, M.H. Effects of nitrogen and radiation on dry matter and nitrogen accumulation in the spike of winter wheat. Field Crops Res. 2004, 87, 221–233. [Google Scholar] [CrossRef]
- Anjum, F.; Wahid, A.; Javed, F.; Arshad, M. Influence of foliar applied thiourea on flag leaf gas exchange and yield parameters of bread wheat (Triticum aestivum L.) cultivars under salinity and heat stresses. Int. J. Agric. Biol. 2008, 10, 619–626. [Google Scholar]
- Singha, P.; Bhowmick, J.; Chaudhury, B.K. Effect of temperature on yield and yield components of fourteen wheat (Triticum aestivum L.) genotypes. Environ. Ecol. 2006, 24, 550–554. [Google Scholar]
- Shirdelmoghanloo, H.; Taylor, J.D.; Lohraseb, I.; Rabie, H.; Brien, C.; Timmins, A.; Martin, P.; Mather, D.E.; Emebiri, L.; Collins, N.C. A QTL on the short arm of wheat (Triticum aestivum L.) chromosome 3B affects the stability of grain weight in plants exposed to a brief heat shock early in grain filling. BMC Plant Biol. 2016, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.P.; Pierre, C.S.; Saad, A.S.I.; Vargas, M.; Condon, A.G. Evaluating potential genetic gains in wheat associated with stress-adaptive trait expression in elite genetic resources under drought and heat stress. Crop Sci. 2007, 47, S-172–S-189. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; van Herwaarden, A.F.; Jenkins, C.; Weiss, M.; Lewis, D.; Ruuska, S.; Tabe, L.; Fettell, N.A.; Richards, R.A. Quantitative trait loci for water-soluble carbohydrates and associations with agronomic traits in wheat. Aust. J. Agric. Res. 2008, 59, 891–905. [Google Scholar] [CrossRef]
- AARI. Wheat: An Overview; Ayub Agriculture Research Institute: Faisalabad, Pakistan, 2018; pp. 1–13. [Google Scholar]
- Nawaz, A.; Farooq, M.; Lal, R.; Rehman, A.; Rehman, H. Comparison of conventional and conservation rice-wheat systems in Punjab, Pakistan. Soil Till. Res. 2017, 169, 35–43. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper, enzyme in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what they can tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef]



| Wheat Genotypes | Days to Heading | Grain Filling Period (Days) | ||||
| 2012–2013 | 2013–2014 | |||||
| Planting Time | Means Genotypes | Planting Time | Means Genotypes | |||
| Normal | Late Sown | Normal | Late Sown | |||
| Millet-11 | 92.00 g | 94.50 f | 93.25 D | 35.67 a | 18.50 e | 27.08 AB |
| Punjab-11 | 103.00 c | 100.17 e | 101.58 C | 32.50 b | 23.00 d | 27.75 A |
| V-07096 | 104.83 b | 101.50 d | 103.17 B | 30.17 c | 22.67 d | 26.41 B |
| V-10110 | 107.00 a | 103.33 c | 105.17 A | 29.50 c | 22.67 d | 26.08 B |
| Means SD | 101.71 A | 99.87 B | 31.96 A | 21.71 B | ||
| HSD | SD = 0.36; G = 0.76, SD × G = 1.30 | SD = 1.08.; G = 1.00, SD × G = 1.72 | ||||
| Wheat Genotypes | Days to Booting | |||||
| 2012–2013 | 2013–2014 | |||||
| Planting Time | Means Genotypes | Planting Time | Means Genotypes | |||
| Normal | Late Sown | Normal | Late Sown | |||
| Millet-11 | 77.67 f | 80.67 e | 79.17 C | 78.67 f | 81.33 e | 80.00 C |
| Punjab-11 | 94.33 b | 83.33 d | 88.83 B | 95.67 b | 86.33 cd | 91.00 B |
| V-07096 | 95.67 ab | 85.33 c | 90.50 A | 98.33 a | 86.67 c | 92.50 A |
| V-10110 | 96.67 a | 85.67 c | 91.17 A | 99.33 a | 84.67 d | 92.00 AB |
| Means SD | 91.08 A | 83.75 B | 93.00 A | 84.75 B | ||
| HSD | SD = 0.71; G = 1.07, SD × G = 1.84 | SD = 0.62; G = 1.09, SD × G = 1.88 | ||||
| Wheat Genotypes | Days to Anthesis | |||||
| 2012–2013 | 2013–2014 | |||||
| Normal | Late Sown | Means Genotypes | Normal | Late Sown | Means Genotypes | |
| Millet-11 | 96.67 f | 103.67 e | 100.17 D | 99.33 d | 100.67 d | 100.00 D |
| Punjab-11 | 107.33 cd | 106.67 d | 107.00 C | 111.33 b | 108.33 c | 109.83 C |
| V-07096 | 110.33 b | 108.00 bc | 109.17 B | 114.33 a | 109.33 bc | 111.83 B |
| V-10110 | 112.00 a | 108.67 bc | 110.33 A | 115.33 a | 111.33 b | 113.33 A |
| Means SD | 106.58 | 106.75 | 110.08 A | 107.42 B | ||
| HSD | SD = n.s.; G = 0.76, SD × G = 1.30 | SD = 0.36; G = 1.31, SD × G = 2.26 | ||||
| Wheat Genotypes | Days to Maturity | |||||
| 2012–2013 | 2013–2014 | |||||
| Normal | Late Sown | Means Genotypes | Normal | Late Sown | Means | |
| Genotypes | ||||||
| Millet-11 | 132.67 b | 122.33 c | 127.50 B | 134.67 b | 119.00 d | 126.83 D |
| Punjab-11 | 140.33 a | 130.33 b | 135.33 A | 143.33 a | 130.67 c | 137.00 C |
| V-07096 | 140.67 a | 130.33 b | 135.50 A | 144.33 a | 132.33 b | 138.33 B |
| V-10110 | 141.67 a | 130.67 b | 136.17 A | 144.67 a | 134.67 b | 139.67 A |
| Means SD | 138.83 A | 128.42 B | 141.75 A | 129.17 B | ||
| HSD | SD = 1.43; G = 0.99, SD × G = 1.71 | SD = 0.95; G = 1.10, SD × G = 1.91 | ||||
| Wheat Genotypes | Chl a (mg/g Fwt) | |||||
| 2012–2013 | 2013–2014 | |||||
| Normal | Late Sown | Means | Normal | Late Sown | Means | |
| Genotypes | Genotypes | |||||
| Millet-11 | 0.10 d | 0.12 a | 0.11 | 0.08 e | 0.09 d | 0.08 C |
| Punjab-11 | 0.10 cd | 0.11 b | 0.10 | 0.12 ab | 0.12 ab | 0.12 A |
| V-07096 | 0.11 c | 0.12 ab | 0.11 | 0.11 c | 0.09 d | 0.10 B |
| V-10110 | 0.11 c | 0.11 b | 0.11 | 0.11 bc | 0.13 a | 0.12 A |
| Means SD | 0.10 B | 0.12 A | 0.10 | 0.10 | ||
| HSD | SD = 0.006; G = n.s., SD × G = 0.006 | SD = n.s.; G = 0.004, SD × G = 0.006 | ||||
| Wheat Genotypes | Chl b (mg/g Fwt) | |||||
| 2012–2013 | 2013–2014 | |||||
| Normal | Late Sown | Means | Normal | Late Sown | Means | |
| Genotypes | Genotypes | |||||
| Millet-11 | 0.07 | 0.15 | 0.11 D | 0.03 e | 0.03 e | 0.03 D |
| Punjab-11 | 0.09 | 0.19 | 0.14 B | 0.08 d | 0.19 a | 0.13 A |
| V-07096 | 0.10 | 0.19 | 0.15 A | 0.09 c | 0.09 c | 0.09 C |
| V-10110 | 0.08 | 0.17 | 0.12 C | 0.08 d | 0.17 b | 0.12 B |
| Means SD | 0.08 B | 0.18 A | 0.07 B | 0.12 A | ||
| HSD | SD = 0.006; G = 0.004, SD × G = n.s. | SD = 0.006; G = 0.004, SD × G = 0.006 | ||||
| Wheat Genotypes | Canopy Temperature (°C) | |||||
| 2012–2013 | 2013–2014 | |||||
| Normal | Late Sown | Means | Normal | Late Sown | Means | |
| Genotypes | Genotypes | |||||
| Millet-11 | 22.53 | 21.32 | 21.92 B | 23.27 | 25.15 | 24.21 AB |
| Punjab-11 | 22.67 | 22.36 | 22.52 A | 23.92 | 25.53 | 24.73 A |
| V-07096 | 22.93 | 22.17 | 22.56 A | 23.45 | 24.57 | 24.01 B |
| V-10110 | 22.82 | 22.1 | 22.46 AB | 23.31 | 25.18 | 24.25 AB |
| Means SD | 22.74 A | 21.99 B | 23.49 B | 25.11 A | ||
| HSD | SD = 0.50; G = 0.56, SD × G = n.s. | SD = 0.88; G = 0.55, SD × G = n.s. | ||||
| Wheat Genotypes | Water Soluble Carbohydrates (μmol /g Fwt) | |||||
| Average over two seasons | ||||||
| Normal | Late Sown | Means | ||||
| Genotypes | ||||||
| Millet-11 | 1.90 b | 2.37 a | 2.14 | |||
| Punjab-11 | 1.34 c | 1.99 ab | 1.66 | |||
| V-07096 | 1.29 c | 2.04 ab | 1.67 | |||
| V-10110 | 1.05 d | 2.04 ab | 1.55 | |||
| Means SD | 1.40 | 2.11 | ||||
| HSD | SD = n.s.; G = n.s., SD × G = 0.16 | |||||
| Wheat Genotypes | Total Productive Tillers Per Plant | |||||
| 2012–2013 | 2013–2014 | |||||
| Planting Time | Planting Time | |||||
| Normal | Late Sown | Means Genotypes | Normal | Late Sown | Means Genotypes | |
| Millet-11 | 5.03 | 6.33 | 5.68 | 4.50 | 5.50 | 5.00 |
| Punjab-11 | 5.73 | 6.07 | 5.90 | 5.67 | 6.00 | 5.78 |
| V-07096 | 4.87 | 6.07 | 5.47 | 4.67 | 5.83 | 5.19 |
| V-10110 | 5.13 | 6.20 | 5.67 | 5.44 | 6.67 | 6.06 |
| Means SD | 5.19 B | 6.17A | 5.01 B | 6.00 A | ||
| HSD | SD = 0.63; G = n.s. SD × G = n.s. | NS = 0.91, G = n,s, SD × G = n.s. | ||||
| Wheat Genotypes | Spike Length (cm) | Grains Per Spike | ||||
| Average over two seasons | Average over two seasons | |||||
| Normal | Late Sown | Means Genotypes | Normal | Late Sown | Means Genotypes | |
| Millet-11 | 10.93 b | 9.46 c | 10.19 B | 42.77 | 37.93 | 40.35 B |
| Punjab-11 | 10.93 b | 10.02 bc | 10.48 B | 41.40 | 32.97 | 37.18 B |
| V-07096 | 11.91 a | 10.49 b | 11.20 A | 48.87 | 44.20 | 46.53 A |
| V-10110 | 12.48 a | 10.20 b | 11.34 A | 41.87 | 41.70 | 41.78 AB |
| Means SD | 11.57 A | 10.04 B | 43.72 | 39.20 | ||
| HSD | SD = 0.61; G = 0.42, SD × G = 0.72 | SD = n.s.; G = 4.79, SD × G = n.s. | ||||
| Wheat Genotypes | Thousand grain weight (g) | |||||
| 2012–2013 | 2013–2014 | |||||
| Normal | Late Sown | Means Genotypes | Normal | Late Sown | Means Genotypes | |
| Millet-11 | 40.67 | 39.00 | 39.83 B | 43.17 bc | 42.00 c | 42.58 B |
| Punjab-11 | 39.83 | 41.20 | 40.51 B | 48.83 ab | 43.33 bc | 46.08 A |
| V-07096 | 47.67 | 47.90 | 47.78 A | 49.50 a | 39.83 c | 44.67 AB |
| V-10110 | 46.00 | 48.23 | 47.11 A | 51.50 a | 43.17 bc | 47.33 A |
| Means SD | 43.54 | 44.08 | 48.25 A | 42.08 B | ||
| HSD | SD = n.s.; G = 3.73, SD × G = n.s. | SD = 4.40; G = 3.43, SD × G = 5.99 | ||||
| Wheat Genotypes | Biological Yield (g m−2) | |||||
| 2012–2013 | 2013–2014 | |||||
| Normal | Late Sown | Means Genotypes | Normal | Late Sown | Means Genotypes | |
| Millet-11 | 1212.20 | 1049.70 | 1130.90 B | 1110.50 b | 988.00 b | 1049.30 B |
| Punjab-11 | 1448.80 | 1216.70 | 1332.80 A | 1472.90 a | 1053.80 a | 1263.40 A |
| V-07096 | 1577.20 | 1247.00 | 1412.10 A | 1462.00 a | 1106.30 b | 1284.20 A |
| V-10110 | 1238.30 | 1137.80 | 1188.10 B | 1129.00 b | 973.80 b | 1051.40 B |
| Means SD | 1369.10 A | 1162.80 B | 1293.60 A | 1030.50 B | ||
| HSD | SD = 79.01; G = 122.18, SD × G = n.s. | SD = 107.88; G = 83.03; SD × G = 143.1 | ||||
| Wheat Genotypes | Grain Yield (g m−2) | |||||
| 2012–2013 | 2013–2014 | |||||
| Normal | Late Sown | Means Genotypes | Normal | Late Sown | Means Genotypes | |
| Millet-11 | 199.18 b | 228.67 ab | 213.93 B | 149.37 c | 155.93 c | 152.65 B |
| Punjab-11 | 251.87 a | 239.50 ab | 245.69 A | 198.92 a | 183.33 ab | 191.13 A |
| V-07096 | 245.97 a | 253.72 a | 249.84 A | 194.60 a | 190.74 ab | 192.67 A |
| V-10110 | 195.28 b | 255.58 a | 225.43 AB | 163.24 bc | 144.32 c | 153.78 B |
| Means SD | 223.08 B | 244.37 A | 176.53 | 168.58 | ||
| HSD | SD = 13.72.; G = 26.67, SD × G = 45.97 | SD = n.s.; G = 12.55, SD × G = 21.63 | ||||
| Wheat Genotypes | Plant Height (cm) | |||||
| 2012–2013 | 2013–2014 | |||||
| Normal | Late sown | Means Genotypes | Normal | Late sown | Means Genotypes | |
| Millet-11 | 105.88 bc | 90.13 e | 98.01 B | 95.07 | 88.47 | 91.77 B |
| Punjab-11 | 106.93 b | 95.78 de | 101.35 B | 106.10 | 95.73 | 100.92 A |
| V-07096 | 115.67 a | 104.83 bc | 110.25 A | 103.60 | 98.90 | 101.25 A |
| V-10110 | 119.50 a | 100.19 cd | 109.84 A | 101.40 | 91.50 | 96.45 AB |
| Means SD | 111.99 A | 97.73 B | 101.54 A | 93.65 B | ||
| HSD | SD = 6.37; G = 3.48, SD × G = 6.69 | SD = 0.02.; G = n.s., SD × G = n.s. | ||||
| Pearson’s Correlation (rp) for Thousand Grain Weight and Grain Yield | ||||
|---|---|---|---|---|
| Thousand Grain Weight | Grain Yield | |||
| 1st Year | 2nd Year | 1st Year | 2nd Year | |
| Days to heading | 0.61 (0.0016) | 0.58 (0.0034) | 0.23 (0.2764) | 0.37 (0.0792) |
| Days to maturity | 0.27 (0.1981) | 0.81 (>0.0001) | −0.12 (0.5871) | 0.39 (0.0604) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, H.u.; Tariq, A.; Ashraf, I.; Ahmed, M.; Muscolo, A.; Basra, S.M.A.; Reynolds, M. Evaluation of Physiological and Morphological Traits for Improving Spring Wheat Adaptation to Terminal Heat Stress. Plants 2021, 10, 455. https://doi.org/10.3390/plants10030455
Rehman Hu, Tariq A, Ashraf I, Ahmed M, Muscolo A, Basra SMA, Reynolds M. Evaluation of Physiological and Morphological Traits for Improving Spring Wheat Adaptation to Terminal Heat Stress. Plants. 2021; 10(3):455. https://doi.org/10.3390/plants10030455
Chicago/Turabian StyleRehman, Hafeez ur, Absaar Tariq, Imran Ashraf, Mukhtar Ahmed, Adele Muscolo, Shahzad M. A. Basra, and Matthew Reynolds. 2021. "Evaluation of Physiological and Morphological Traits for Improving Spring Wheat Adaptation to Terminal Heat Stress" Plants 10, no. 3: 455. https://doi.org/10.3390/plants10030455
APA StyleRehman, H. u., Tariq, A., Ashraf, I., Ahmed, M., Muscolo, A., Basra, S. M. A., & Reynolds, M. (2021). Evaluation of Physiological and Morphological Traits for Improving Spring Wheat Adaptation to Terminal Heat Stress. Plants, 10(3), 455. https://doi.org/10.3390/plants10030455








