Elevated Nitrogen Priming Induced Oxinitro-Responses and Water Deficit Tolerance in Rice
Abstract
1. Introduction
2. Results
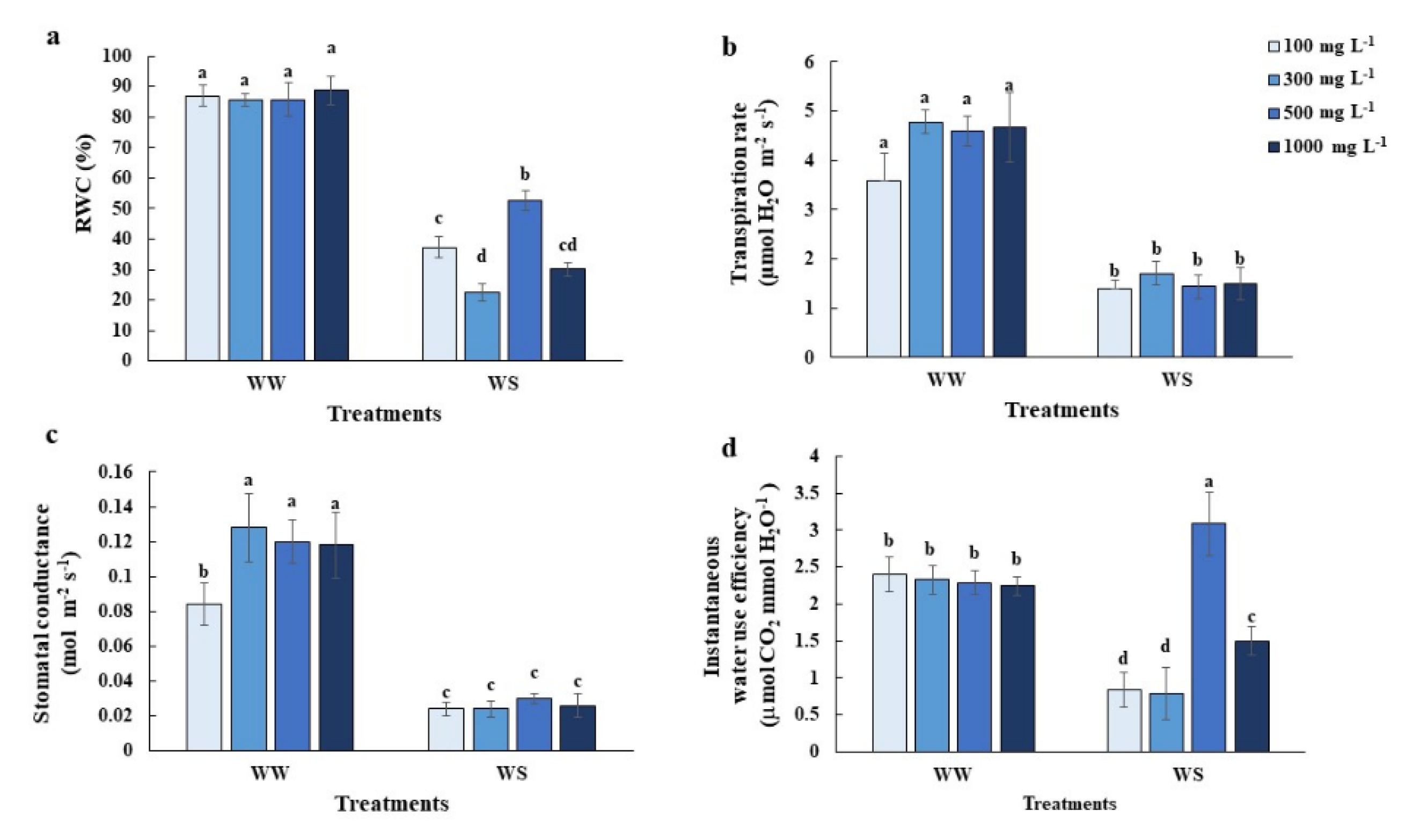
2.1. Elevated Nitrogen Priming Enhanced Photosynthesis and Leaf Growth under Water Deficit Conditions
2.2. Elevated Nitrogen Priming Promoted Relatively Higher Leaf Relative Water Content (RWC) under Water Deficit Conditions
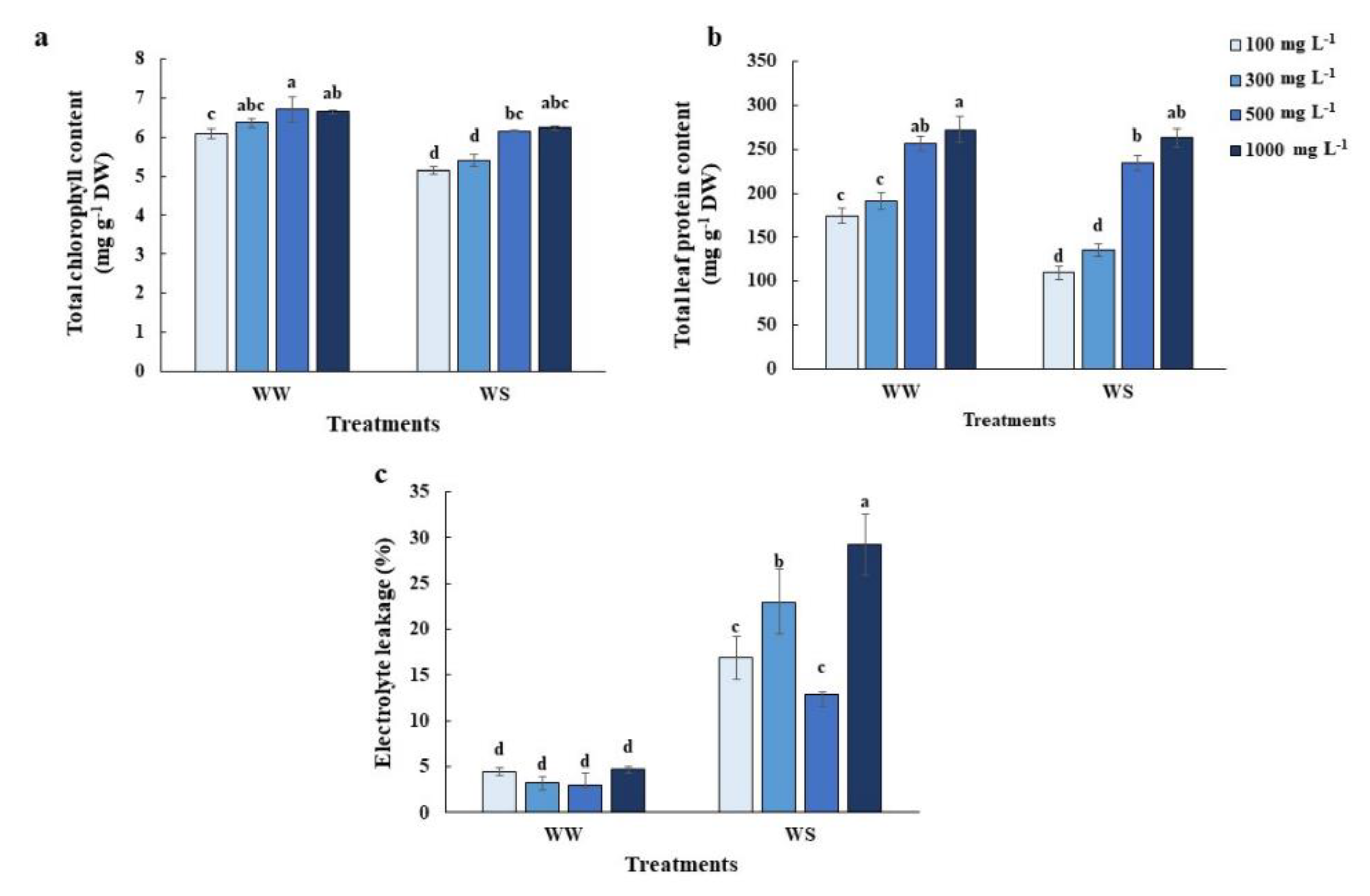
2.3. Elevated Nitrogen Priming Alleviated Chlorophyll and Protein Degradation as Well as Cell Damage under Water Deficit Conditions
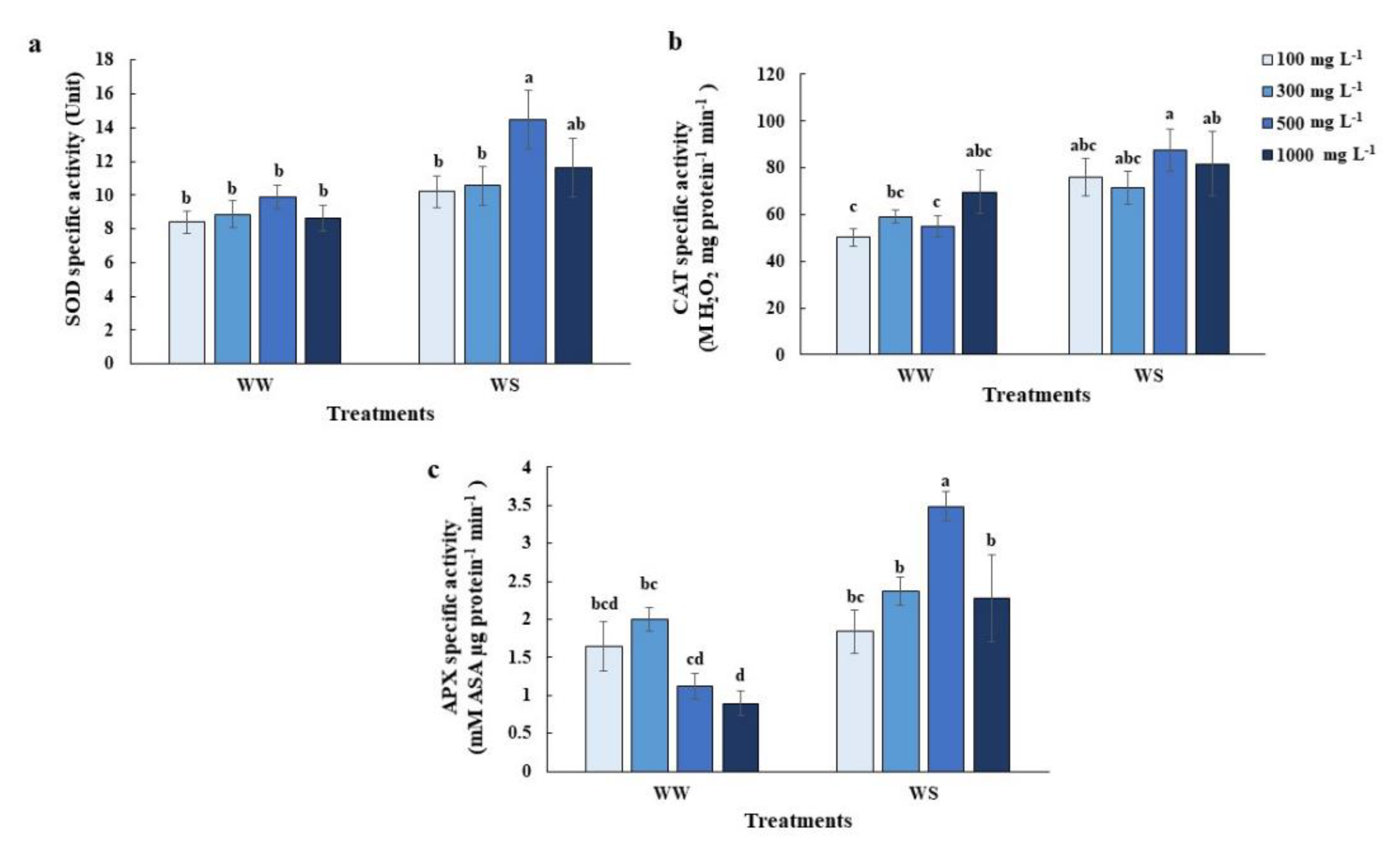
2.4. Elevated Nitrogen Priming Enhanced Antioxidant Defense Mechanisms under Water Deficit Conditions
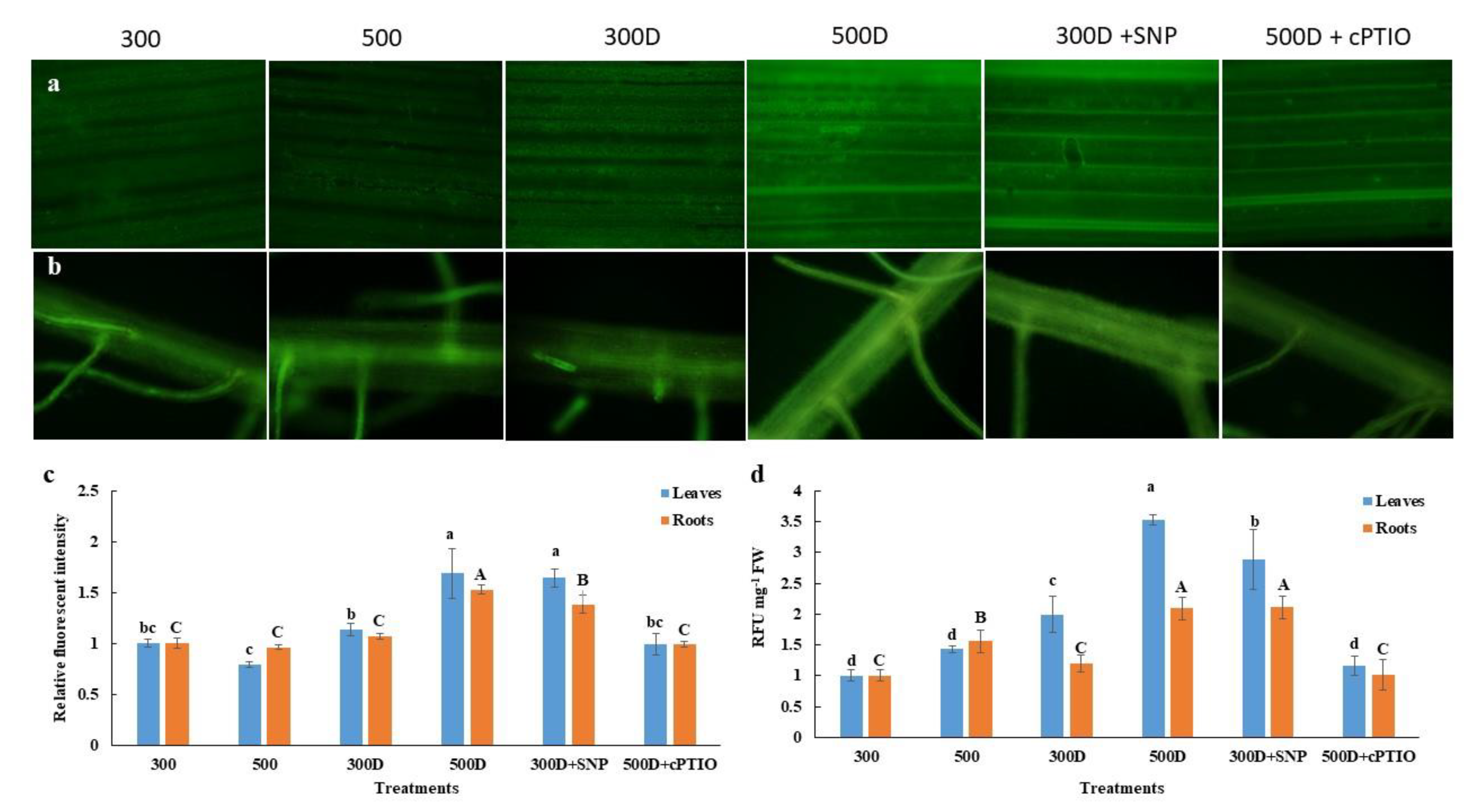
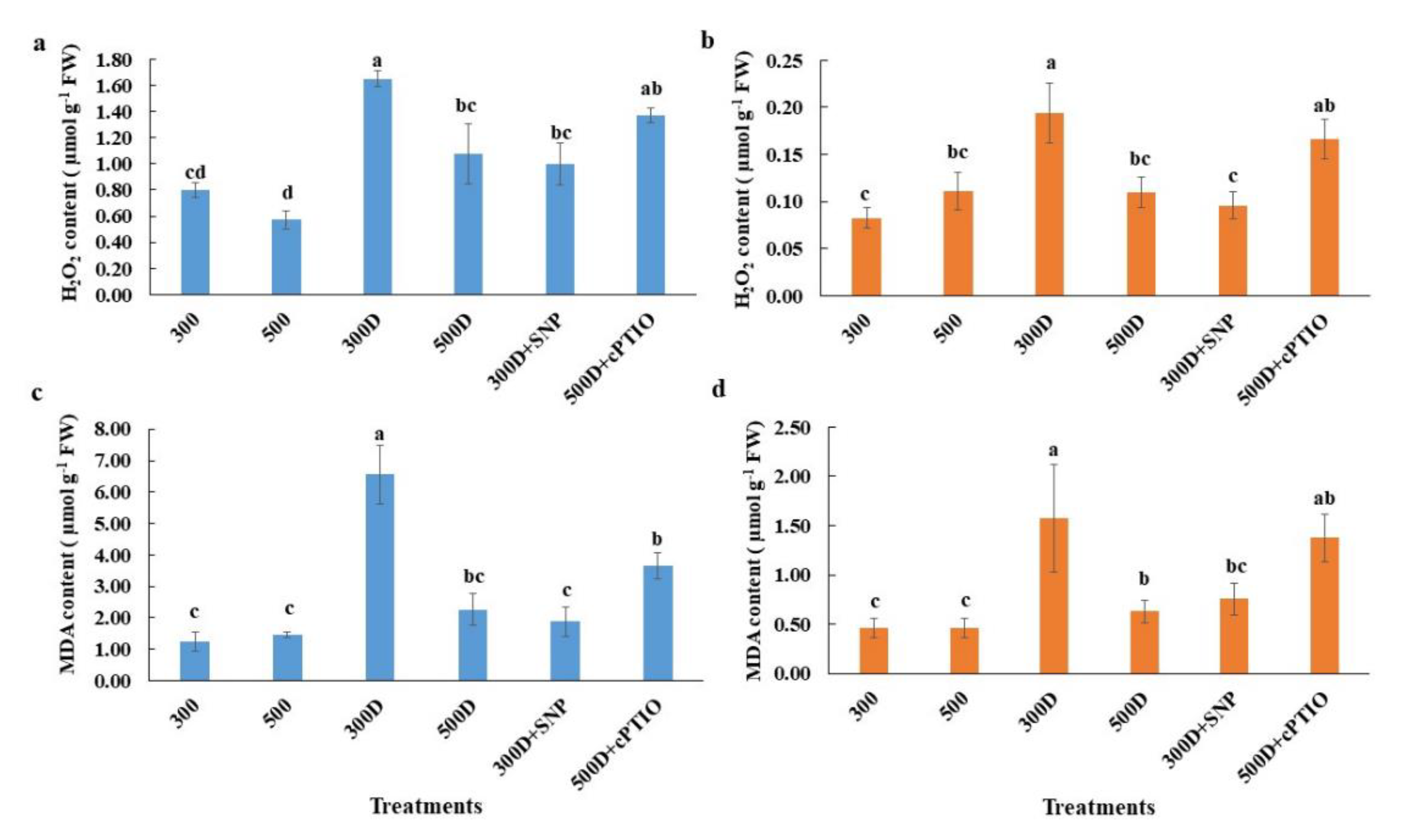
2.5. Nitric Oxide Accumulation in Elevated Nitrogen Primed-Plants Alleviated Reactive Oxygen Species (ROS) Accumulation under Water Deficit Conditions
3. Discussion
4. Materials and Methods
4.1. Plant Material and Greenhouse Growth Conditions
4.2. Gas-Exchange Measurements
4.3. Leaf Area
4.4. Relative Water Content (RWC) of Leaves
4.5. Electrolyte Leakage (EL)
4.6. Determining SOD, CAT and APX Activities
4.7. Protein Quantification
4.8. The RNS and ROS Experiment
4.9. NO Determination
4.10. Histochemical Detection of H2O2 and O2•−
4.11. H2O2 Quantification
4.12. Malondialdehyde (MDA) Measurements
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leng, G.; Hall, J. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci. Total Environ. 2019, 654, 811–821. [Google Scholar] [CrossRef]
- Bodner, G.; Nakhforoosh, A.; Kaul, H.P. Management of crop water under drought: A review. Agron. Sustain. Dev. 2015, 35, 401–442. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U. Drought Resistance in Rice from Conventional to Molecular Breeding: A Review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; Groot, S.; De Soole, K.; Lan-gridge, P. Early Flowering as a Drought Escape Mechanism in Plants: How Can It Aid Wheat Production? Front. Plant Sci. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; del Mar Rubio-Wilhelmi, M.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2017, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Sade, N.; Umnajkitikorn, K.; Wilhelmi, M.d.M.R.; Wright, M.; Wang, S.; Blumwald, E. Delaying chloroplast turn-over increases water-deficit stress tolerance through the enhancement of nitrogen assimilation in rice. J. Exp. Bot. 2017, 69, 867–878. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.G.; Yun, B.W. Nitric oxide regulates plant re-sponses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Cai, W.; Liu, W.; Wang, W.; Fu, Z.; Han, T.; Lu, Y. Overexpression of Rat Neurons Nitric Oxide Synthase in Rice En-hances Drought and Salt Tolerance. PLoS ONE 2015, 10, e0131599. [Google Scholar] [CrossRef]
- Groß, F.; Durner, J.; Gaupels, F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 2013, 4, 1–15. [Google Scholar] [CrossRef]
- Ahmad, P.; Latef, A.A.A.; Hashem, A.; Abd Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regu-lating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Li, C.; Li, T.; Zhang, D.; Jiang, L.; Shao, Y. Exogenous nitric oxide effect on fructan accumulation and FBEs expression in chilling-sensitive and chilling-resistant wheat. Environ. Exp. Bot. 2013, 86, 2–8. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Rehman, H. Exogenously Applied Nitric Oxide Enhances the Drought Tolerance in Fine Grain Aromatic Rice (Oryza sativa L.). J. Agron. Crop Sci. 2009, 195, 254–261. [Google Scholar] [CrossRef]
- Wildt, J.; Kley, D.; Rockel, A.; Rockel, P.; Segschneide, H.J. Emission of NO from several higher plant species. J. Geophys. Res. 1997, 102, 5919–5927. [Google Scholar] [CrossRef]
- Tejada-jimenez, M.; Llamas, A.; Galv, A.; Fern, E. Photosynthetic Eukaryotes. Plants 2019, 8, 56. [Google Scholar] [CrossRef]
- Sun, H.; Li, J.; Song, W.; Tao, J.; Huang, S.; Chen, S.; Hou, M.; Xu, G.; Zhang, Y. Nitric oxide generated by nitrate re-ductase increases nitrogen uptake capacity by inducing lateral root formation and inorganic nitrogen uptake under par-tial nitrate nutrition in rice. J. Exp. Bot. 2015, 66, 2449–2459. [Google Scholar] [CrossRef]
- Cao, X.; Zhong, C.; Sajid, H.; Zhu, L. Effects of watering regime and nitrogen application rate on the photosynthetic parameters, physiological characteristics, and agronomic traits of rice. Acta Physiol. Plant. 2017, 39, 1–12. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Alejandro, L.; Mur, J.; Shen, Q.; Guo, S. Unravelling the Roles of Nitrogen Nutrition in Plant Disease Defences. Int. J. Mol. Sci. 2020, 21, 572. [Google Scholar] [CrossRef] [PubMed]
- Menge, D.M.; Onyango, J.C.; Yamauchi, A.; Kano-Nakata, M.; Asanuma, S.; Thi, T.T.; Inukai, Y.; Kikuta, M.; Makihara, D. Effect of nitrogen application on the expression of drought-induced root plasticity of upland NERICA rice. Plant Prod. Sci. 2019, 22, 180–191. [Google Scholar] [CrossRef]
- Sedri, M.H.; Amini, A.; Golchin, A. Evaluation of Nitrogen Effects on Yield and Drought Tolerance of Rainfed Wheat using Drought Stress Indices. J. Crop Sci. Biotechnol. 2019, 22, 235–242. [Google Scholar] [CrossRef]
- Tran, T.T.; Kano-Nakata, M.; Takeda, M.; Menge, D.; Mitsuya, S.; Inukai, Y.; Yamauchi, A. Nitrogen application enhanced the expression of developmental plasticity of root systems triggered by mild drought stress in rice. Plant Soil 2014, 378, 139–152. [Google Scholar] [CrossRef]
- Li, S.; Zhou, L.; Daniel, S.D.A.; Guochang, D.; Sun, M.; Wu, S.; Lin, S. Nitrogen supply enhances the physiological resistance of Chinese fir plantlets under polyethylene glycol (PEG)-induced drought stress. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.H.; Watanabe, K.; Takaragawa, H.; Nakabaru, M. Photosynthetic response and nitrogen use efficiency of sugarcane under drought stress conditions with different nitrogen application levels. Plant Prod. Sci. 2017, 1008, 1–11. [Google Scholar] [CrossRef]
- Haefele, S.M.; Kato, Y.; Singh, S. Field Crops Research Climate ready rice: Augmenting drought tolerance with best management practices. Crop. Res. 2016, 190, 60–69. [Google Scholar] [CrossRef]
- Zhong, S.; Xu, Y.; Meng, B.; Loik, M.E.; Ma, J.Y.; Sun, W. Nitrogen addition increases the sensitivity of photosynthesis to drought and re-watering differentially in C3 versus C4 grass species. Front. Plant Sci. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Abid, M.; Tian, Z.; Ata-ul-karim, S.T.; Cui, Y.; Liu, Y. Nitrogen Nutrition Improves the Potential of Wheat (Triticum aestivum L.) to Alleviate the Effects of Drought Stress during Vegetative Growth Periods. Front Plant Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Luo, Q.; Sun, C.; Hu, H.; Wang, F.; Tian, Z.; Jiang, D.; Cao, W.; Dai, T. Low nitrogen priming enhances photo-synthesis adaptation to water-deficit stress in winter wheat (Triticum aestivum L.) seedlings. Front. Plant Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Heckathorn, S.A.; DeLucia, E.H.; Zielinski, R.E. The contribution of drought-related decreases in foliar nitrogen concentration to decreases in photosynthetic capacity during and after drought in prairie grasses. Physiol. Plant. 1997, 101, 173–182. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Xiong, B.; Cao, B.; Deng, X. Carbon/nitrogen imbalance associated with drought-induced leaf senescence in sorghum bicolor. PLoS ONE 2015, 10, e0137026. [Google Scholar] [CrossRef]
- Kong, L.; Xie, Y.; Hu, L.; Si, J.; Wang, Z. Excessive nitrogen application dampens antioxidant capacity and grain filling in wheat as revealed by metabolic and physiological analyses. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Iqbal, A.; Dong, Q.; Wang, X.; Gui, H.; Zhang, H.; Zhang, X.; Song, M. High Nitrogen Enhances Drought Tolerance in Cotton through Antioxidant Enzymatic Activities, Nitrogen Metabolism and Osmotic Adjustment Asif. Plants 2020, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Liu, Y.; Dong, H.; Teng, K.; Han, L.; Zhang, X. Effects of cytokinin and nitrogen on drought tolerance of creeping bentgrass. PLoS ONE 2016, 11, e0154005. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596. [Google Scholar] [CrossRef]
- He, H.; Oo, T.L.; Huang, W.; He, L.; Gu, M. Nitric oxide acts as an antioxidant and inhibits programmed cell death induced by aluminum in the root tips of peanut (Arachis hypogaea L.). Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Guo, Z.; Ou, W.; Lu, S.; Zhong, Q. Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol. Biochem. 2006, 44, 828–836. [Google Scholar] [CrossRef]
- Shehab, G.G.; Ahmed, O.K.; El-beltagi, H.S. Effects of Various Chemical Agents for Alleviation of Drought Stress in Rice Plants (Oryza sativa L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 139–148. [Google Scholar]
- Fukudome, M.; Watanabe, E.; Osuki, K.I.; Uchi, N.; Uchiumi, T. Ectopic or over-expression of class 1 phytoglobin genes confers flooding tolerance to the root nodules of lotus japonicus by scavenging nitric oxide. Antioxidants 2019, 8, 206. [Google Scholar] [CrossRef]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Chamizo-ampudia, A.; Sanz-luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate Reductase Regulates Plant Nitric Oxide Homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Rubbo, H.; Radi, R.; Anselmi, D.K.; Marion, B.; Stephen, B.; John, E.; Jason, P. Nitric oxide reaction with lipid peroxyl radicals spares alpha-tocopherol during lipid peroxidation. J. Biol. Chem. 2000, 275, 10812–10818. [Google Scholar] [CrossRef]
- Jain, P.; Singh, N.; Kaur, H. Mechanisms of nitric oxide crosstalk with reactive oxygen species scavenging enzymes during abiotic stress tolerance in plants. Free Radic Res. 2015, 50, 291–303. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxi-fication system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Umnajkitikorn, K.; Faiyue, B.; Saengnil, K. Enhancing Antioxidant Properties of Germinated Thai rice (Oryza sativa L.) cv. Kum Doi Saket with Salinity. Rice Res. Open Access 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Vaidyanathan, H.; Sivakumar, P.; Chakrabarty, R.; Thomas, G. Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.)—Differential response in salt-tolerant and sensitive varieties. Plant Sci. 2003, 165, 1411–1418. [Google Scholar] [CrossRef]
- Sunohara, Y.; Matsumoto, H. Oxidative injury induced by the herbicide quinclorac on Echinochloa oryzicola Vasing and the involvement of antioxidative ability in its highly selective action in grass species. Plant Sci. 2004, 167, 597–606. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the prin-ciple of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fukudome, M.; Calvo-Begueria, L.; Kado, T.; Osuki, K.I.; Rubio, M.C.; Murakami, E.I.; Nagata, M.; Kucho, K.I.; Sandal, N.; Stougaard, J.; et al. Hemoglobin LjGlb1-1 is involved in nodulation and regulates the level of nitric oxide in the Lotus japonicus-Mesorhizobium loti symbiosis. J. Exp. Bot. 2016, 67, 5275–5283. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.; Corpas, F.J.; Borsani, O.; Barroso, J.B.; Monza, J. Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicus. Plant Sci. 2013, 201–202, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://imagej.nih.gov/ij/docs/examples/stained-sections/index.html (accessed on 16 February 2021).
- Brumbarova, T.; Le, C.T.T.; Bauer, P. Hydrogen Peroxide Measurement in Arabidopsis Root Tissue Using Amplex Red. Bio-protocol 2016, 6, 1–11. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umnajkitikorn, K.; Fukudome, M.; Uchiumi, T.; Teaumroong, N. Elevated Nitrogen Priming Induced Oxinitro-Responses and Water Deficit Tolerance in Rice. Plants 2021, 10, 381. https://doi.org/10.3390/plants10020381
Umnajkitikorn K, Fukudome M, Uchiumi T, Teaumroong N. Elevated Nitrogen Priming Induced Oxinitro-Responses and Water Deficit Tolerance in Rice. Plants. 2021; 10(2):381. https://doi.org/10.3390/plants10020381
Chicago/Turabian StyleUmnajkitikorn, Kamolchanok, Mitsutaka Fukudome, Toshiki Uchiumi, and Neung Teaumroong. 2021. "Elevated Nitrogen Priming Induced Oxinitro-Responses and Water Deficit Tolerance in Rice" Plants 10, no. 2: 381. https://doi.org/10.3390/plants10020381
APA StyleUmnajkitikorn, K., Fukudome, M., Uchiumi, T., & Teaumroong, N. (2021). Elevated Nitrogen Priming Induced Oxinitro-Responses and Water Deficit Tolerance in Rice. Plants, 10(2), 381. https://doi.org/10.3390/plants10020381





