Enhancement of Photosynthetic Iron-Use Efficiency Is an Important Trait of Hordeum vulgare for Adaptation of Photosystems to Iron Deficiency
Abstract
1. Introduction
2. Results
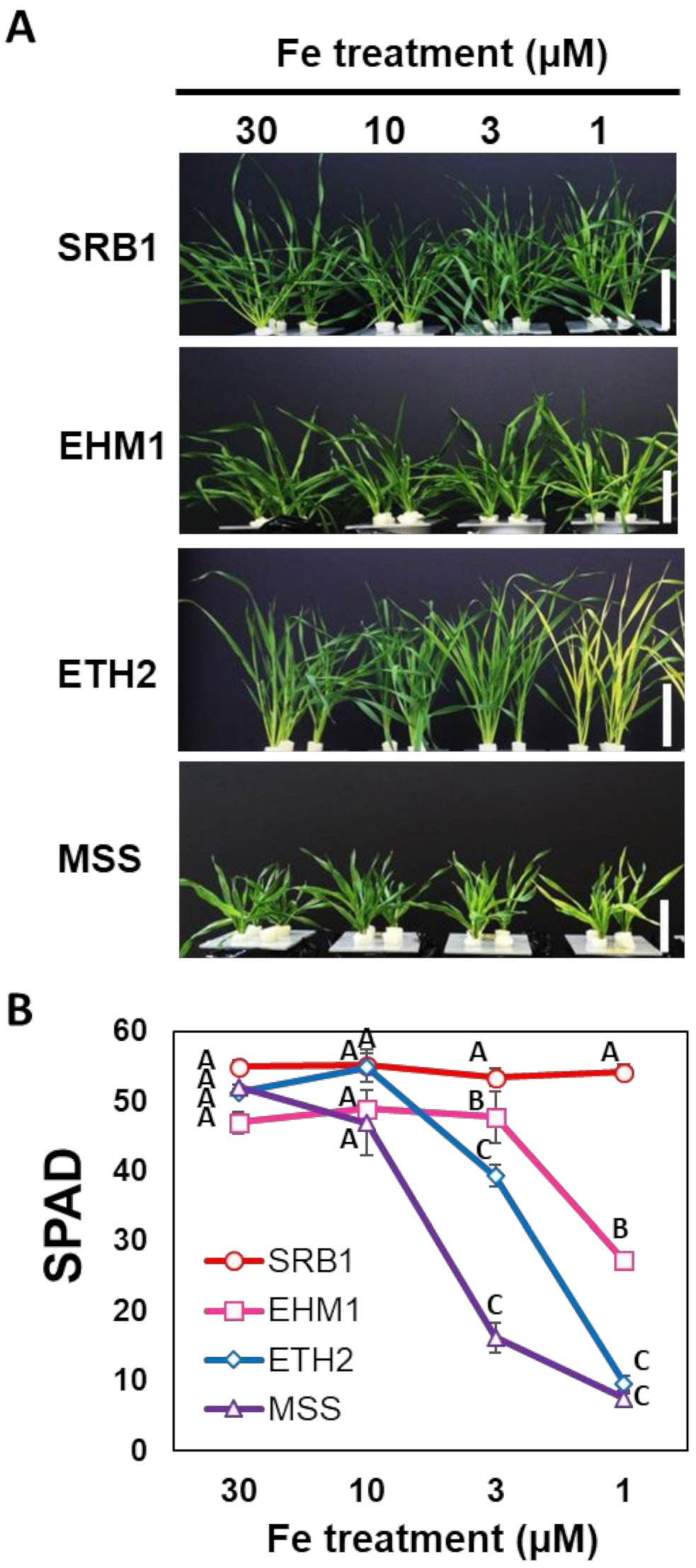
2.1. Fe-Deficiency-Tolerance within Barley Varieties Was Not Related to the Total Fe Content in Leaves
2.2. Thylakoid Fe Concentration Was Not Correlated with Photosynthesis under Fe-Deficient Conditions
2.3. Evaluation of ‘Photosynthetic Fe-Use Efficiency (PIUE)’
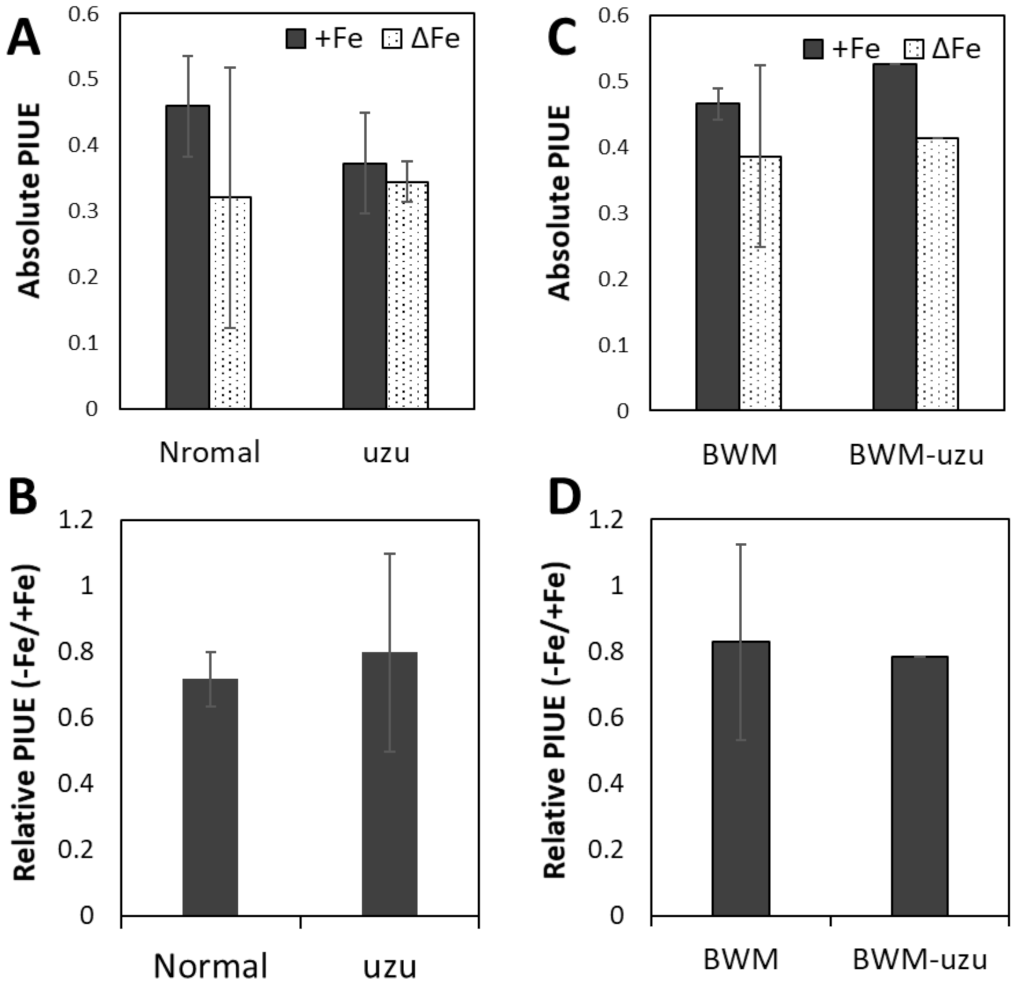
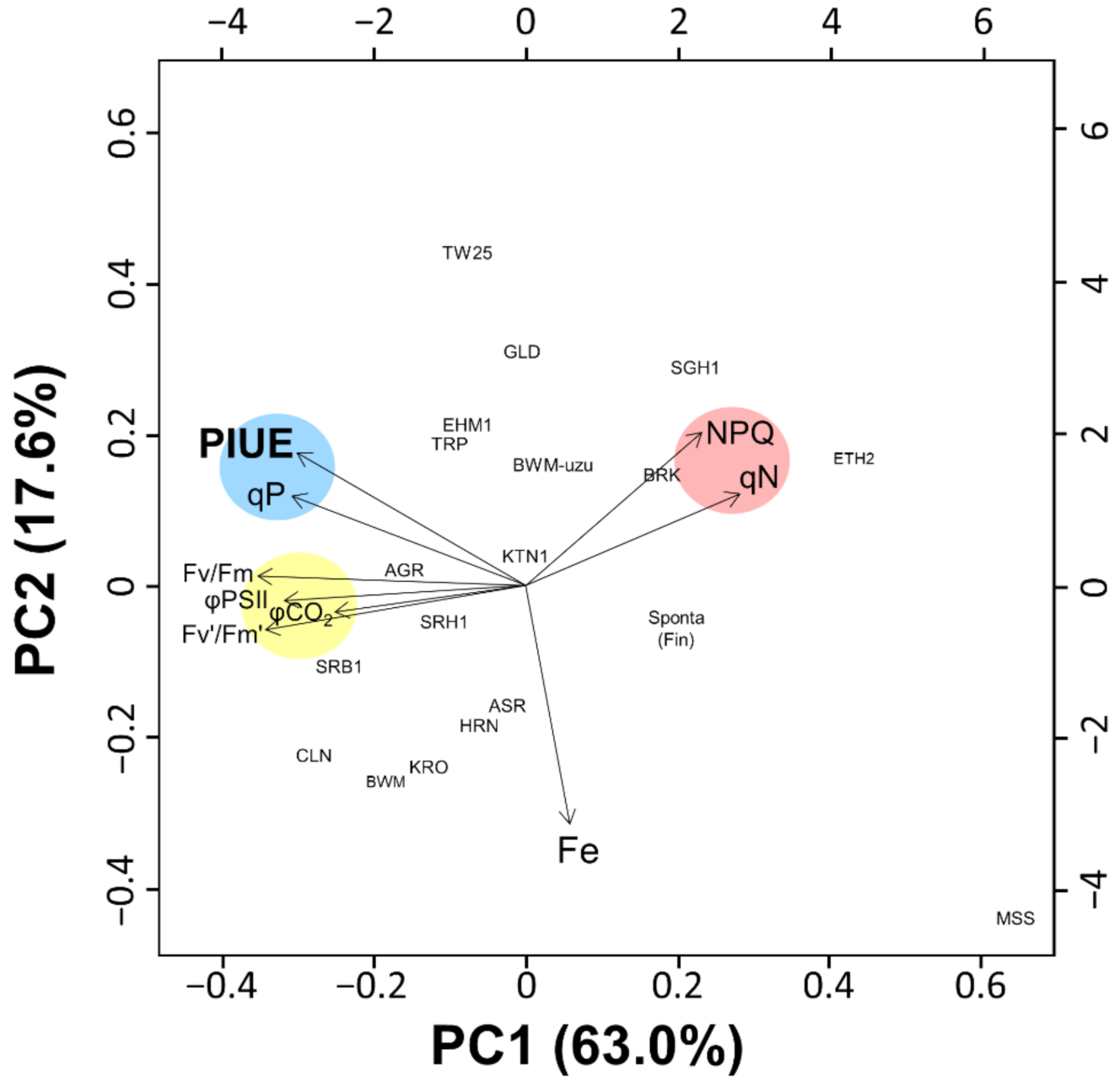
2.4. Genetic Diversity of PIUE in Barley Is the Result of Selection on Alkaline Soils
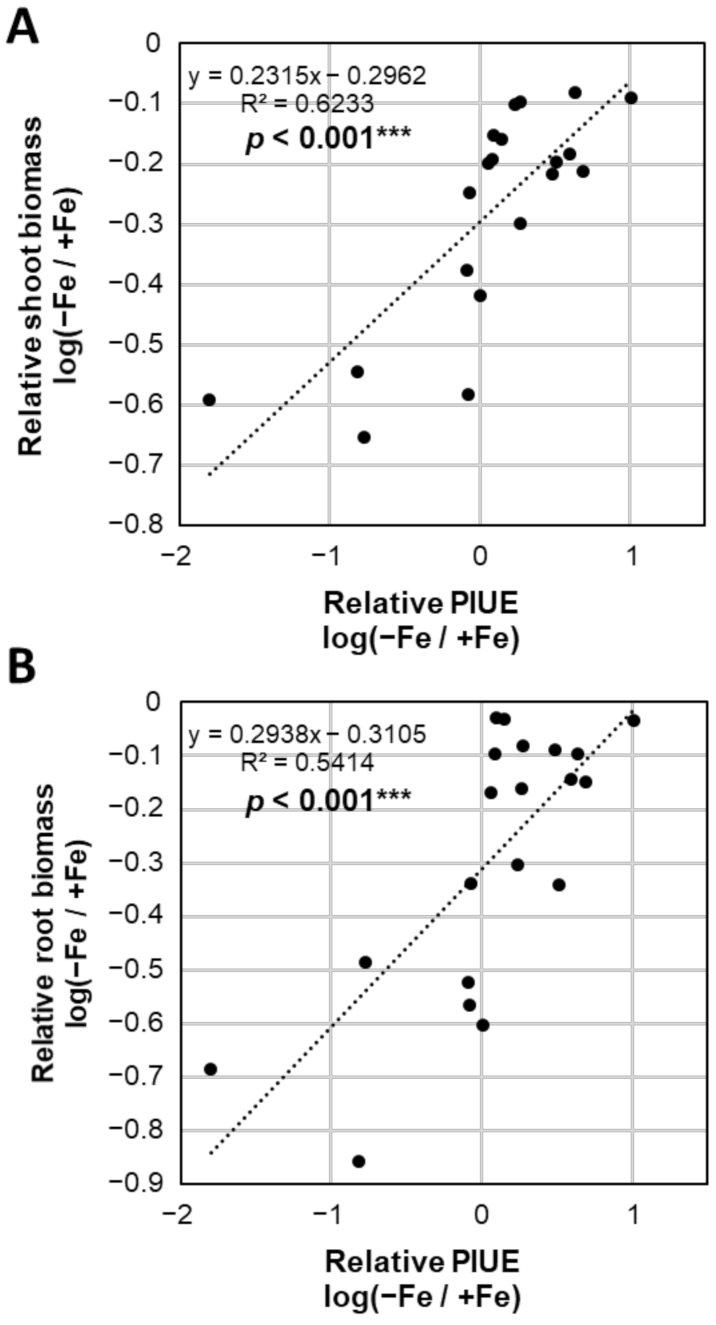
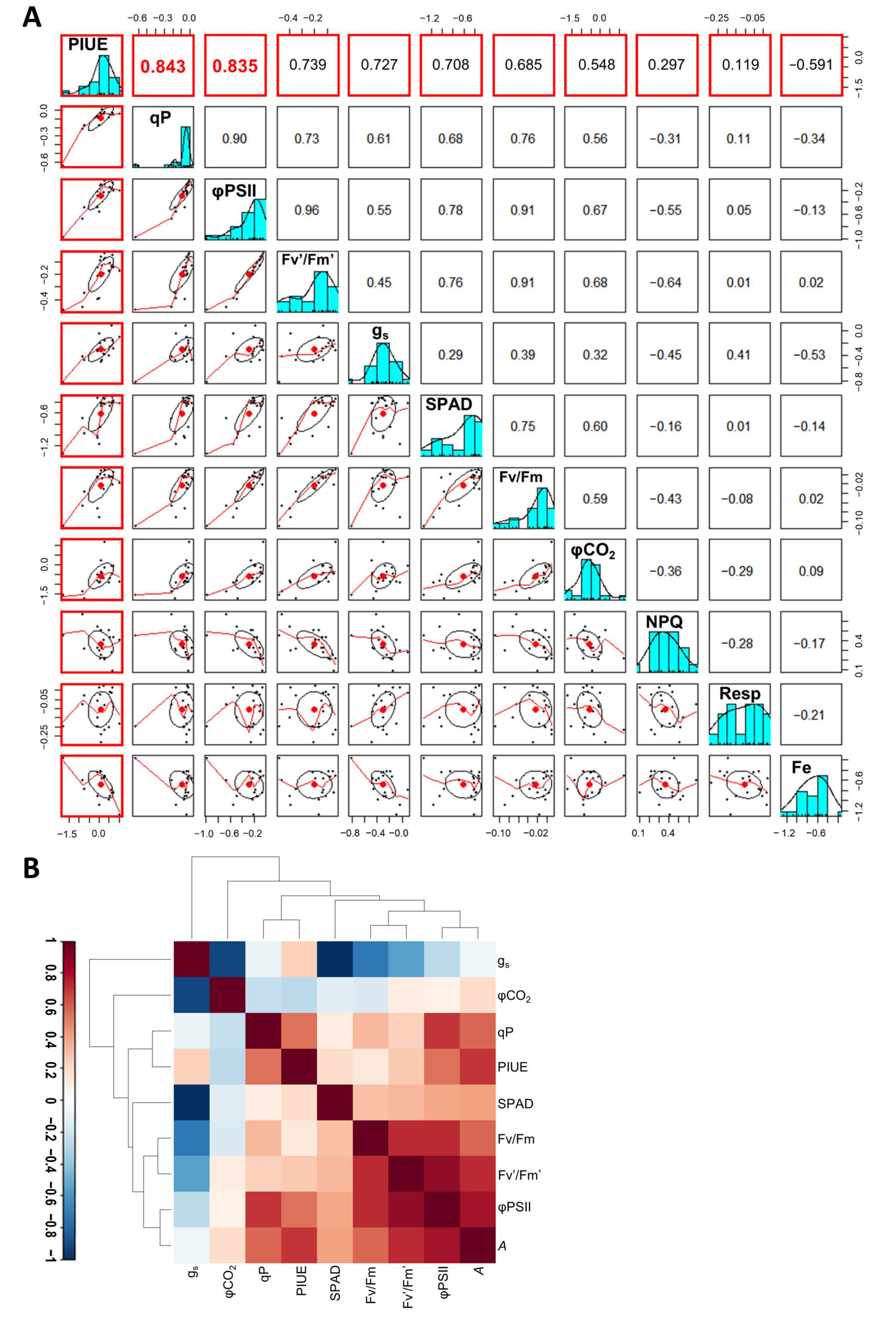
2.5. Photosynthetic Properties Associated with the Relative PIUE Value in Barley Varieties
3. Discussion
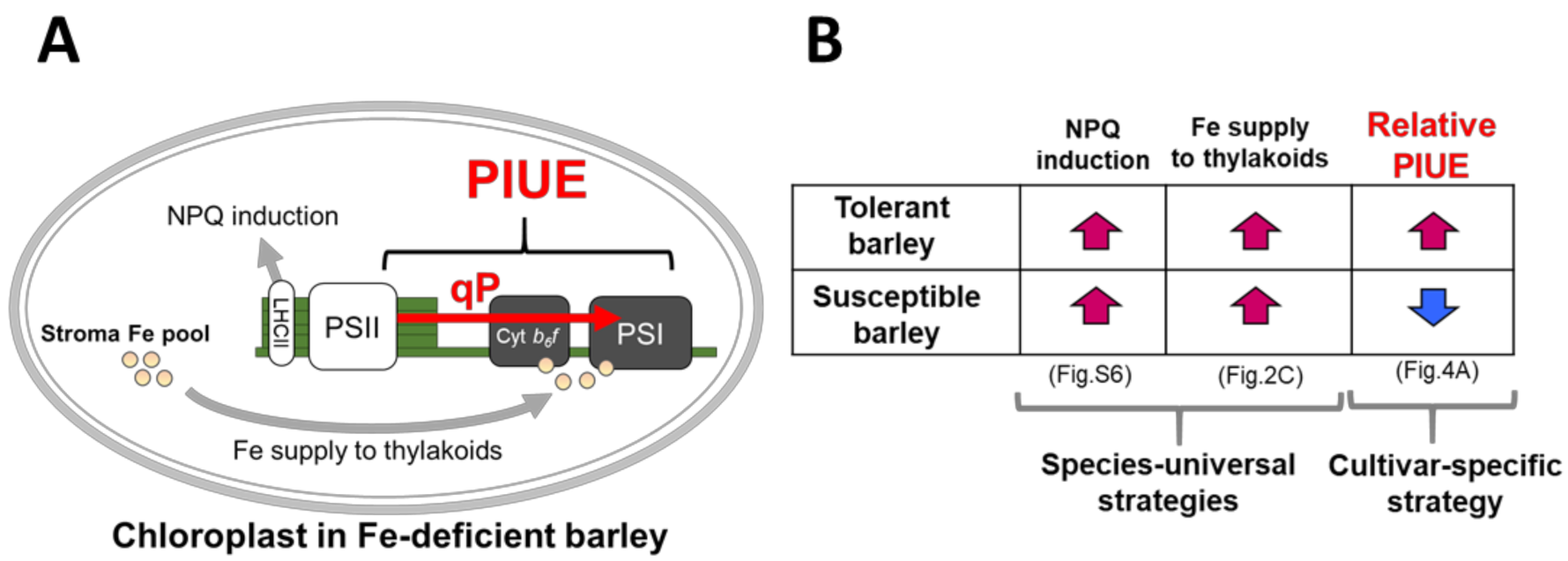
3.1. PIUE, as a New Indicator that Defines Photosynthetic Fe-Use Efficiency
3.2. Possible Mechanisms that Affect PIUE
3.3. Association of PIUE with Other Fe-Deficiency Acclimation Mechanisms Previously Found in Barley
3.4. New Insights on Barley Cultivation in Respect of Fe Nutrition
3.5. The Origin of the Trait of PIUE Increase
3.6. C4 Photosynthesis Is Disadvantageous in PIUE Increase under Fe Deficient Conditions
3.7. The Future Application of PIUE
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Isolation of Thylakoid Membranes
4.3. Measurement of Chlorophyll and Fe Content in Leaves
4.4. Measurement of Photosynthesis
4.5. Calculation of PIUE
4.6. Statistical Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Methods A1: Isolation of Thylakoid Membranes
- 250 mM HEPES-KOH (pH 7.6), stored at 4 °C
- Antioxidant solution, stored at −20 °C
- 20 mM ascorbate
- 20 mM EDTA-2Na
- 10 × HM buffer, stored at 4 °C
- 100 mM HEPES-KOH (pH 7.6)
- 50 mM MgCl2·6H2O
- 10 × EDTA washing buffer, stored at 4 °C 100 mM HEPES-KOH (pH 7.6)
- 50 mM EDTA-2Na
- Blending medium
- 0.35 M sucrose
- 25 mM HEPES-KOH (pH 7.6)
- 2 mM ascorbate
- 2 mM EDTA-2Na
- HM buffer
- 10 mM HEPES-KOH (pH 7.6)
- 5 mM MgCl2
- EDTA washing buffer
- 10 mM HEPES-KOH (pH 7.6)
- 5 mM EDTA-2Na
- (1)
- Leaf strips (2.5 g) were homogenized twice for 5 s each with 25 mL blending medium in 50 mL plastic tubes using a Physcotron homogenizer (MICROTEC Co., Ltd., Japan) set at 29,000 rpm.
- (2)
- Homogenate was filtered with three-layered Miracloth (EMD Millipore Corp., Bedford, MA, USA).
- (3)
- Crude chloroplasts were collected as pellets by centrifugation at 2500× g at 4 °C for 10 min.
- (4)
- Green pellets were suspended in 10 mL of HM buffer and then incubated for 10 min.
- (5)
- Extracted thylakoid membranes were centrifuged at 9000× g at 4 °C for 5 min.
- (6)
- Thylakoid pellets were re-suspended in EDTA washing buffer, and then centrifuged at 9000× g at 4 °C for 5 min. The precipitate was resuspended in HM buffer and stored at −80 °C as a thylakoid fraction.
References
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Amunts, A.; Nelson, N. Plant Photosystem I Design in the Light of Evolution. Structure 2009, 17, 637–650. [Google Scholar] [CrossRef]
- Hantzis, L.J.; Kroh, G.E.; Jahn, C.E.; Cantrell, M.; Peers, G.; Pilon, M.; Ravet, K. A Program for Iron Economy during Deficiency Targets Specific Fe Proteins. Plant Physiol. 2018, 176, 596–610. [Google Scholar] [CrossRef]
- Michel, K.P.; Pistorius, E.K. Adaptation of the Photosynthetic Electron Transport Chain in Cyanobacteria to Iron Deficiency: The Function of IdiA and IsiA. Physiologia Plantarum; Wiley: Hoboken, NJ, USA, 2004; Volume 120, pp. 36–50. [Google Scholar]
- Kroh, G.E.; Pilon, M. Regulation of Iron Homeostasis and Use in Chloroplasts. Int. J. Mol. Sci. 2020, 21, 3395. [Google Scholar] [CrossRef]
- Loeppert, R.H. Reactions of iron and carbonates in calcareous soils. J. Plant Nutr. 1986, 9, 195–214. [Google Scholar] [CrossRef]
- Lindsay, W.L. Chemical Equilibria in Soils; Wiley Interscience: New York, NY, USA, 1979. [Google Scholar]
- Marschner, H.; Romheld, V.; Kissel, M. Different strategies in higher plants in mobilization and uptake of iron. J. Plant Nutr. 1986, 9, 695–713. [Google Scholar] [CrossRef]
- Bashir, K.; Nishizawa, N.K. Deoxymugineic Acid Synthase. Plant Signal. Behav. 2006, 1, 290–292. [Google Scholar] [CrossRef]
- Takagi, S.; Kamei, S.; Yu, M. Efficiency of iron extraction from soil by mugineic acid family phytosiderophores. J. Plant Nutr. 1988, 11, 643–651. [Google Scholar] [CrossRef]
- Higuchi, K.; Watanabe, S.; Takahashi, M.; Kawasaki, S.; Nakanishi, H.; Nishizawa, N.K.; Mori, S. Nicotianamine synthase gene expression differs in barley and rice under Fe-deficient conditions. Plant J. 2001, 25, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Higuchi, K.; Yoshida, M.; Tadano, T. Comparison of Iron Availability in Leaves of Barley and Rice. Soil Sci. Plant Nutr. 2005, 46, 1035–1042. [Google Scholar] [CrossRef]
- Saito, A.; Iino, T.; Sonoike, K.; Miwa, E.; Higuchi, K. Remodeling of the Major Light-Harvesting Antenna Protein of PSII Protects the Young Leaves of Barley (Hordeum vulgare L.) from Photoinhibition under Prolonged Iron Deficiency. Plant Cell Physiol. 2010, 51, 2013–2030. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Higuchi, K.; Sasaki, H.; Suzuki, T.; Maruyama, T.; Yoshiba, M.; Tadano, T. Contribution of iron associated with high-molecular-weight substances to the maintenance of the SPAD value of young leaves of barley under iron-deficient conditions. Soil Sci. Plant Nutr. 2007, 53, 612–620. [Google Scholar] [CrossRef]
- Higuchi, K.; Saito, A.; Mikami, Y.; Miwa, E. Modulation of macronutrient metabolism in barley leaves under iron-deficient condition. Soil Sci. Plant Nutr. 2011, 57, 233–247. [Google Scholar] [CrossRef]
- Higuchi, K.; Iwase, J.; Tsukiori, Y.; Nakura, D.; Kobayashi, N.; Ohashi, H.; Saito, A.; Miwa, E. Early senescence of the oldest leaves of Fe-deficient barley plants may contribute to phytosiderophore release from the roots. Physiol. Plant. 2014, 151, 313–322. [Google Scholar] [CrossRef]
- Mikami, Y.; Saito, A.; Miwa, E.; Higuchi, K. Allocation of Fe and ferric chelate reductase activities in mesophyll cells of barley and sorghum under Fe-deficient conditions. Plant Physiol. Biochem. 2011, 49, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Shimizu, M.; Nakamura, H.; Maeno, S.; Katase, R.; Miwa, E.; Higuchi, K.; Sonoike, K. Fe deficiency induces phosphorylation and translocation of Lhcb1 in barley thylakoid membranes. FEBS Lett. 2014, 588, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Pourkheirandish, M.; Hensel, G.; Kilian, B.; Senthil, N.; Chen, G.; Sameri, M.; Azhaguvel, P.; Sakuma, S.; Dhanagond, S.; Sharma, R.; et al. Evolution of the Grain Dispersal System in Barley. Cell 2015, 162, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Yokosho, K.; Yamaji, N.; Saisho, D.; Yamane, M.; Takahashi, H.; Sato, K.; Nakazono, M.; Ma, J.F. Acquisition of aluminium tolerance by modification of a single gene in barley. Nat. Commun. 2012, 3, 713. [Google Scholar] [CrossRef]
- Xia, Y.; Li, R.; Bai, G.; Siddique, K.H.M.; Varshney, R.K.; Baum, M.; Yan, G.; Guo, P. Genetic variations of HvP5CS1 and their association with drought tolerance related traits in barley (Hordeum vulgare L.). Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Hemshrot, A.; Poets, A.M.; Tyagi, P.; Lei, L.; Carter, C.K.; Hirsch, C.N.; Li, L.; Brown-Guedira, G.; Morrell, P.L.; Muehlbauer, G.J.; et al. Development of a Multiparent Population for Genetic Mapping and Allele Discovery in Six-Row Barley. Genetics 2019, 213, 595–613. [Google Scholar] [CrossRef]
- Hikosaka, K. Interspecific difference in the photosynthesis?nitrogen relationship: Patterns, physiological causes, and ecological importance. J. Plant Res. 2004, 117, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F.; Pearcy, R.W. The Nitrogen Use Efficiency of C3 and C4 Plants. Plant Physiol. 1987, 84, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Mae, T.; Ohira, K. Differences between wheat and rice in the enzymic properties of ribulose-1,5-bisphosphate carboxylase/oxygenase and the relationship to photosynthetic gas exchange. Planta 1988, 174, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Boogaard, R.V.D.; Kostadinova, S.; Veneklaas, E.; Lambers, H. Association of water use efficiency and nitrogen use efficiency with photosynthetic characteristics of two wheat cultivars. J. Exp. Bot. 1995, 46, 1429–1438. [Google Scholar] [CrossRef]
- Guo, R.; Sun, S.; Liu, B. Difference in leaf water use efficiency/photosynthetic nitrogen use efficiency of Bt-cotton and its conventional peer. Sci. Rep. 2016, 6, 33539. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Predicting long-term dynamics of soil salinity and sodicity on a global scale. Proc. Natl. Acad. Sci. USA 2020, 117, 33017–33027. [Google Scholar] [CrossRef]
- FAO/IIASA/ISRIC/ISS-CAS/JRC. Harmonized World Soil Database (Version 1.2); FAO: Rome, Italy; IIASA: Laxenburg, Austria, 2012. [Google Scholar]
- Zivcak, M.; Brestic, M.; Kalaji, H.M.; Govindjee, G. Photosynthetic responses of sun- and shade-grown barley leaves to high light: Is the lower PSII connectivity in shade leaves associated with protection against excess of light? Photosynth. Res. 2014, 119, 339–354. [Google Scholar] [CrossRef]
- Amann, K.; Lezhneva, L.; Wanner, G.; Herrmann, R.G.; Meurer, J. ACCUMULATION OF PHOTOSYSTEM ONE1, a Member of a Novel Gene Family, Is Required for Accumulation of [4Fe-4S] Cluster–Containing Chloroplast Complexes and Antenna Proteins. Plant Cell 2004, 16, 3084–3097. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Yuasa, K.; Shikata, T.; Kitatsuji, S.; Yamasaki, Y.; Nishiyama, Y. Extracellular secretion of superoxide is regulated by photosynthetic electron transport in the noxious red-tide-forming raphidophyte Chattonella antiqua. J. Photochem. Photobiol. B Biol. 2020, 205, 111839. [Google Scholar] [CrossRef]
- Lezhneva, L.; Amann, K.; Meurer, J. The universally conserved HCF101 protein is involved in assembly of [4Fe-4S]-cluster-containing complexes in Arabidopsis thaliana chloroplasts. Plant J. 2003, 37, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Tasaka, M.; Shikanai, T. PPR motifs of the nucleus-encoded factor, PGR3, function in the selective and distinct steps of chloroplast gene expression inArabidopsis. Plant J. 2004, 38, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Terashima, I. Elucidation of Photoprotective Mechanisms of PSI Against Fluctuating Light photoinhibition. Plant Cell Physiol. 2016, 57, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Makino, A.; Shikanai, T. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci. Rep. 2016, 6, 20147. [Google Scholar] [CrossRef]
- Baxter, I.R.; Vitek, O.; Lahner, B.; Muthukumar, B.; Borghi, M.; Morrissey, J.; Guerinot, M.L.; Salt, D.E. The leaf ionome as a multivariable system to detect a plant’s physiological status. Proc. Natl. Acad. Sci. USA 2008, 105, 12081–12086. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Díaz, M.; Alberdi, M.; Ivanov, A.G.; Krol, M.; Hüner, N.P.A. Excess manganese differentially inhibits photosystem I versus II inArabidopsis thaliana. J. Exp. Bot. 2013, 64, 343–354. [Google Scholar] [CrossRef]
- Eroglu, S.; Meier, B.; von Wirén, N.; Peiter, E. The Vacuolar Manganese Transporter MTP8 Determines Tolerance to Iron Deficiency-Induced Chlorosis in Arabidopsis. Plant Physiol. 2016, 170, 1030–1045. [Google Scholar] [CrossRef]
- Russell, J.; Mascher, M.; Dawson, I.K.; Kyriakidis, S.; Calixto, C.; Freund, F.; Bayer, M.; Milne, I.; Marshall-Griffiths, T.; Heinen, S.; et al. Exome sequencing of geographically diverse barley landraces and wild relatives gives insights into environmental adaptation. Nat. Genet. 2016, 48, 1024–1030. [Google Scholar] [CrossRef]
- Higuchi, K.; Ono, K.; Araki, S.; Nakamura, S.; Uesugi, T.; Makishima, T.; Ikari, A.; Hanaoka, T.; Sue, M. Elongation of barley roots in high-pH nutrient solution is supported by both cell proliferation and differentiation in the root apex. Plant Cell Environ. 2017, 40, 1609–1617. [Google Scholar] [CrossRef]
- Gentili, R.; Ambrosini, R.; Montagnani, C.; Caronni, S.; Citterio, S. Effect of Soil pH on the Growth, Reproductive Investment and Pollen Allergenicity of Ambrosia artemisiifolia L. Front. Plant Sci. 2018, 9, 1335. [Google Scholar] [CrossRef]
- Taylor, S.H.; Ripley, B.S.; Woodward, F.I.; Osborne, C.P. Drought limitation of photosynthesis differs between C3 and C4 grass species in a comparative experiment. Plant Cell Environ. 2010, 34, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Vogan, P.J.; Sage, R.F. Water-use efficiency and nitrogen-use efficiency of C3-C4 intermediate species of Flaveria Juss. (Asteraceae). Plant Cell Environ. 2011, 34, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Majeran, W.; van Wijk, K.J. Cell-type-specific differentiation of chloroplasts in C4 plants. Trends Plant Sci. 2009, 14, 100–109. [Google Scholar] [CrossRef]
- De Miguel, M.; Cabezas, J.A.; de María, N.; Sánchez-Gómez, D.; Guevara, M.Á.; Vélez, D.; Sáez-Laguna, E.; Díaz, L.M.; Mancha, J.A.; Barbero, M.-C.; et al. Genetic control of functional traits related to photosynthesis and water use efficiency in Pinus pinaster Ait. drought response: Integration of genome annotation, allele association and QTL detection for candidate gene identification. BMC Genom. 2014, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lü, H.; Yang, Y.; Li, H.; Liu, Q.; Zhang, J.; Yin, J.; Chu, S.; Zhang, X.; Yu, K.; Lv, L.; et al. Genome-Wide Association Studies of Photosynthetic Traits Related to Phosphorus Efficiency in Soybean. Front. Plant Sci. 2018, 9, 1226. [Google Scholar] [CrossRef] [PubMed]
- Cuello, J.; Quiles, M.J.; Albacete, M.E.; Sabater, B. Properties of a Large Complex with NADH Dehydrogenase Activity from Barley Thylakoids. Plant Cell Physiol. 1995, 36, 265–271. [Google Scholar] [CrossRef]
- Caffarri, S.; Croce, R.; Breton, J.; Bassi, R. The Major Antenna Complex of Photosystem II Has a Xanthophyll Binding Site Not Involved in Light Harvesting. J. Biol. Chem. 2001, 276, 35924–35933. [Google Scholar] [CrossRef]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. et Biophys. Acta (BBA) Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Shaaf, S.; Bretani, G.; Biswas, A.; Fontana, I.M.; Rossini, L. Genetics of barley tiller and leaf development. J. Integr. Plant Biol. 2019, 61, 226–256. [Google Scholar] [CrossRef]
- Makiko, C.; Honda, I.; Zeniya, H.; Yoneyama, K.; Saisho, D.; Takeda, K.; Takatsuto, S.; Hoshino, T.; Watanabe, Y. A Semidwarf Phenotype of Barley uzu Results from a Nucleotide Substitution in the Gene Encoding a Putative Brassinosteroid Receptor. Plant Physiol. 2003, 133, 1209–1219. [Google Scholar] [CrossRef]
- Saisho, D.; Tanno, K.-I.; Chono, M.; Honda, I.; Kitano, H.; Takeda, K. Spontaneous Brassinolide-insensitive Barley Mutants ‘uzu’ Adapted to East Asia. Breed. Sci. 2004, 54, 409–416. [Google Scholar] [CrossRef]
- Gooding, M.; Addisu, M.; Uppal, R.K.; Snape, J.W.; Jones, H. Effect of wheat dwarfing genes on nitrogen-use efficiency. J. Agric. Sci. 2012, 150, 3–22. [Google Scholar] [CrossRef]
- Wang, B.; Li, G.; Zhang, W.-H. Brassinosteroids are involved in Fe homeostasis in rice (Oryza sativa L.). J. Exp. Bot. 2015, 66, 2749–2761. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, A.; Shinjo, S.; Ito, D.; Doi, Y.; Sato, A.; Wakabayashi, Y.; Honda, J.; Arai, Y.; Maeda, T.; Ohyama, T.; et al. Enhancement of Photosynthetic Iron-Use Efficiency Is an Important Trait of Hordeum vulgare for Adaptation of Photosystems to Iron Deficiency. Plants 2021, 10, 234. https://doi.org/10.3390/plants10020234
Saito A, Shinjo S, Ito D, Doi Y, Sato A, Wakabayashi Y, Honda J, Arai Y, Maeda T, Ohyama T, et al. Enhancement of Photosynthetic Iron-Use Efficiency Is an Important Trait of Hordeum vulgare for Adaptation of Photosystems to Iron Deficiency. Plants. 2021; 10(2):234. https://doi.org/10.3390/plants10020234
Chicago/Turabian StyleSaito, Akihiro, Shotaro Shinjo, Daiki Ito, Yuko Doi, Akira Sato, Yuna Wakabayashi, Juma Honda, Yuka Arai, Tsubasa Maeda, Takuji Ohyama, and et al. 2021. "Enhancement of Photosynthetic Iron-Use Efficiency Is an Important Trait of Hordeum vulgare for Adaptation of Photosystems to Iron Deficiency" Plants 10, no. 2: 234. https://doi.org/10.3390/plants10020234
APA StyleSaito, A., Shinjo, S., Ito, D., Doi, Y., Sato, A., Wakabayashi, Y., Honda, J., Arai, Y., Maeda, T., Ohyama, T., & Higuchi, K. (2021). Enhancement of Photosynthetic Iron-Use Efficiency Is an Important Trait of Hordeum vulgare for Adaptation of Photosystems to Iron Deficiency. Plants, 10(2), 234. https://doi.org/10.3390/plants10020234






