Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites
Abstract
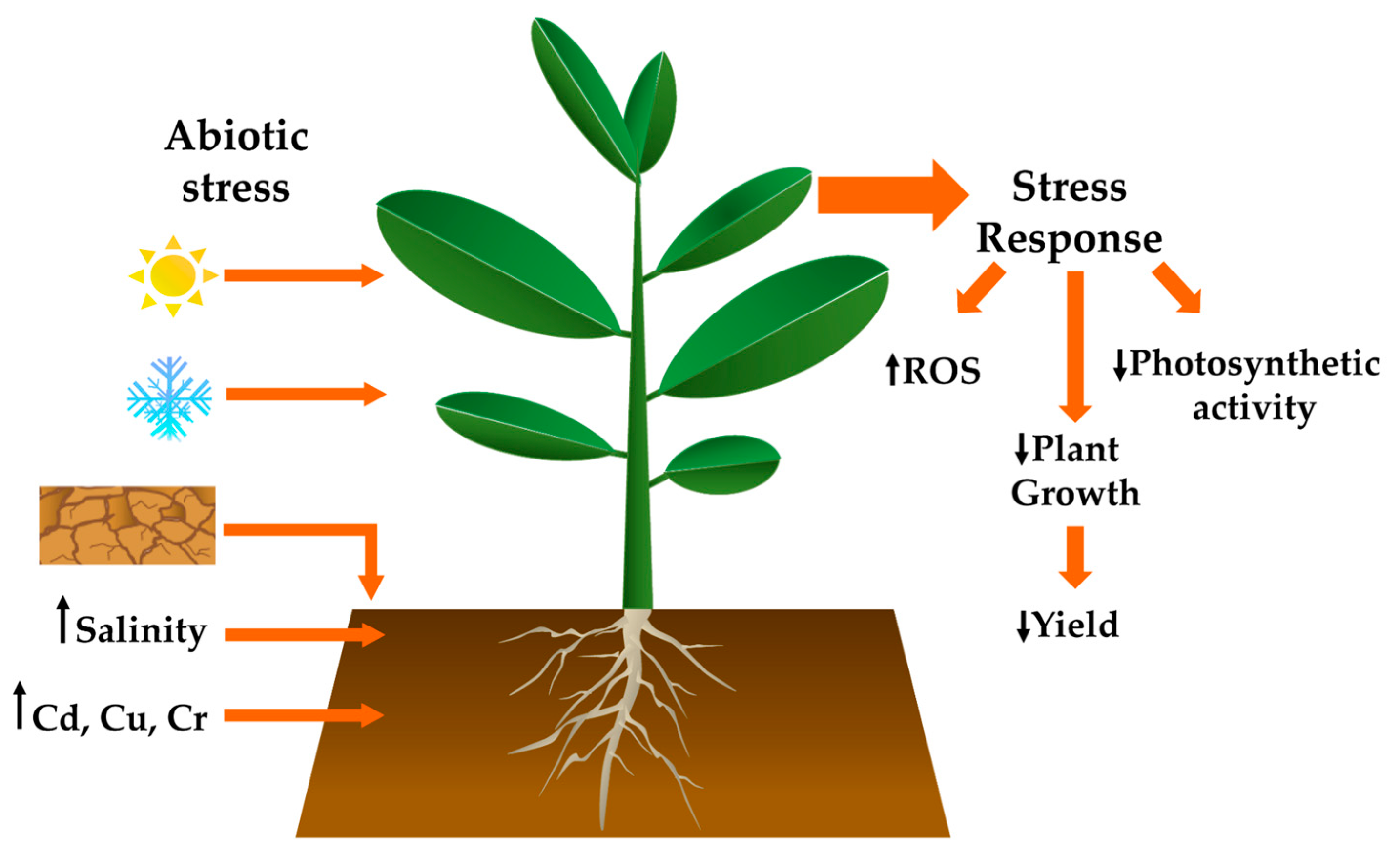
1. Introduction
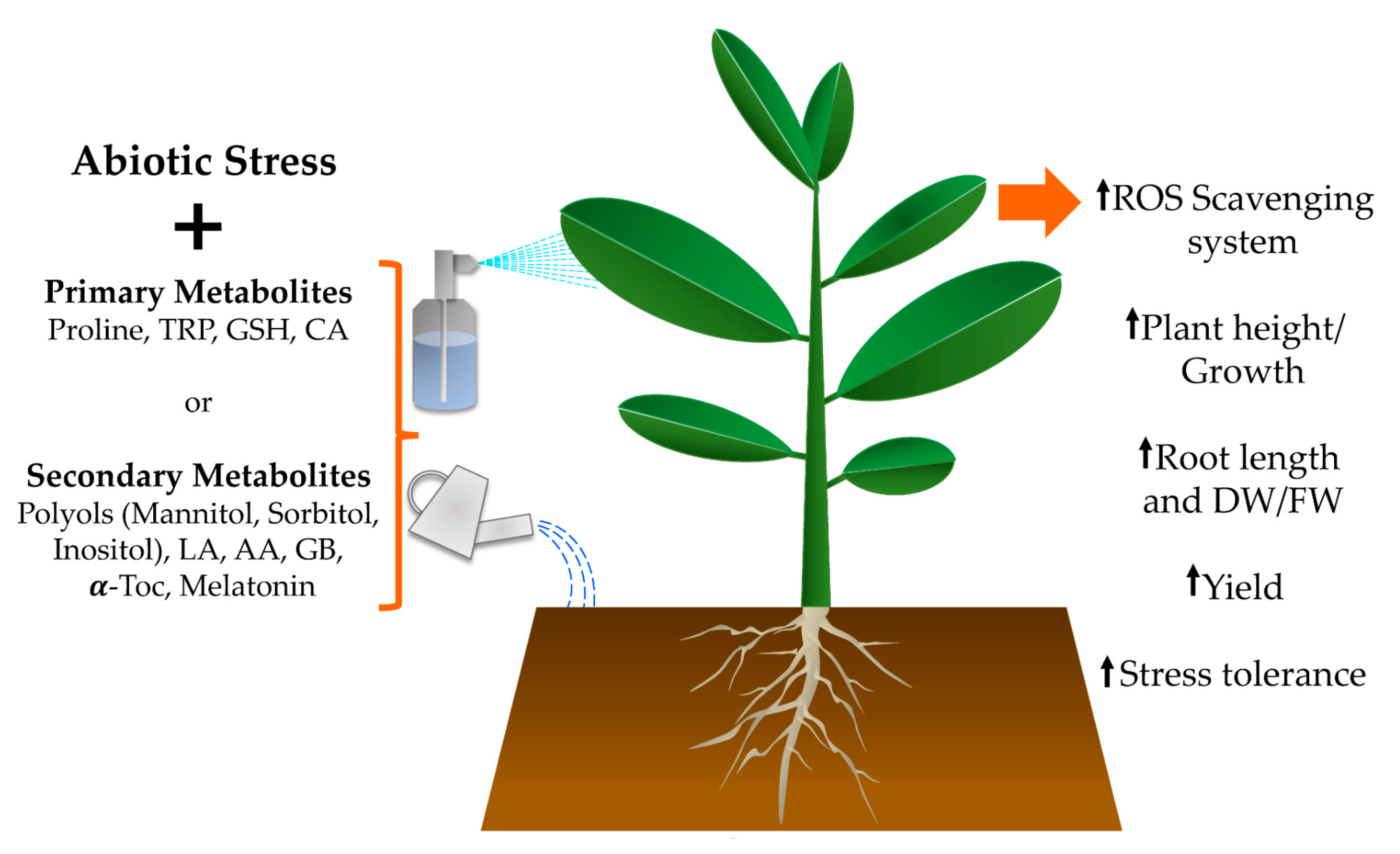
2. Primary Metabolites
2.1. Proline
2.2. L-Tryptophan (TRP)
2.3. Glutathione (GSH)
2.4. Citric Acid (CA)
3. Secondary Metabolites
3.1. Polyols
3.1.1. Mannitol
3.1.2. Sorbitol
3.1.3. Inositol
3.2. Lipoic Acid (LA)
3.3. Ascorbic Acid (AA)
3.4. Glycine Betaine (GB)
3.5. Alpha-Tocopherol (α-Toc)
3.6. Melatonin
4. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rockström, J.; Williams, J.; Daily, G.; Noble, A.; Matthews, N.; Gordon, L.; Wetterstrand, H.; DeClerck, F.; Shah, M.; Steduto, P.; et al. Sustainable intensification of agriculture for human prosperity and global sustainability. Ambio 2017, 46, 4–17. [Google Scholar] [CrossRef]
- Döös, B.R. Population growth and loss of arable land. Glob. Environ. Chang. 2002, 12, 303–311. [Google Scholar] [CrossRef]
- Hofmann, D.J.; Butler, J.H.; Tans, P.P. A new look at atmospheric carbon dioxide. Atmos. Environ. 2009, 43, 2084–2086. [Google Scholar] [CrossRef]
- U.S. Global Change Research Program. Global Climate Change Impacts in the United States: A State of Knowledge Report; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2009; ISBN 978-0-521-14407-0. [Google Scholar]
- Bot, A.J.; Nachtergaele, F.O.; Young, A. Land Resource Potential and Constraints at Regional and Country Levels; World Soil Resources Report; FAO: Rome, Italy, 2000; p. 122. [Google Scholar]
- Thornton, P.K.; Ericksen, P.J.; Herrero, M.; Challinor, A.J. Climate variability and vulnerability to climate change: A review. Glob. Chang. Biol. 2014, 20, 3313–3328. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B. Effects of Salinity on Yield and Component Characters in Canola (Brassica napus L.) Cultivars. Not. Sci. Biol. 2010, 2, 81–83. [Google Scholar] [CrossRef]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Manzoor, J.; Sharma, M.; Wani, K.A. Heavy metals in vegetables and their impact on the nutrient quality of vegetables: A review. J. Plant Nutr. 2018, 41, 1744–1763. [Google Scholar] [CrossRef]
- FAO. Status of the World’s Soil Resources: Main Report; FAO, ITPS: Rome, Italy, 2015; ISBN 978-92-5-109004-6. [Google Scholar]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance); European Parliament, Council of the European Union: Luxembourg, 2019; Volume 170, pp. 1–114.
- Kumar, K.; Gambhir, G.; Dass, A.; Tripathi, A.K.; Singh, A.; Jha, A.K.; Yadava, P.; Choudhary, M.; Rakshit, S. Genetically modified crops: Current status and future prospects. Planta 2020, 251, 91. [Google Scholar] [CrossRef] [PubMed]
- McDougall, P. The Cost and Time Involved in the Discovery, Development and Authorisation of a New Plant Biotechnology Derived Trait; Consultancy Study for Crop Life International by P McDougall: Pathhead, Midlothian, 2011; pp. 1–24. [Google Scholar]
- Waltz, E. Gene-edited CRISPR mushroom escapes US regulation. Nat. News 2016, 532, 293. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.R.; Dorn, K.M.; Smith, T.M.; Wolf, K.E.; Ewing, P.M.; Fernandez, A.L.; Runck, B.C.; Williams, A.; Lu, Y.; Kuzma, J. A cooperative governance network for crop genome editing. EMBO Rep. 2017, 18, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Ahmar, S.; Gill, R.A.; Jung, K.-H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Fawole, O.A.; Makunga, N.P.; Masondo, N.A.; Moyo, M.; Buthelezi, N.M.D.; Amoo, S.O.; Spíchal, L.; Doležal, K. Applications of Cytokinins in Horticultural Fruit Crops: Trends and Future Prospects. Biomolecules 2020, 10, 1222. [Google Scholar] [CrossRef]
- Javid, M.G.; Sorooshzadeh, A.; Moradi, F.; Modarres Sanavy, S.A.M.; Allahdadi, I. The Role of Phytohormones in Alleviating Salt Stress in Crop Plants. Aust. J. Crop Sci. 2011, 5, 726. [Google Scholar]
- Ribba, T.; Garrido-Vargas, F.; O’Brien, J.A. Auxin-mediated responses under salt stress: From developmental regulation to biotechnological applications. J. Exp. Bot. 2020, 71, 3843–3853. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Geilfus, C.-M. Controlled Environment Horticulture: Improving Quality of Vegetables and Medicinal Plants; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-23196-5. [Google Scholar]
- Wink, M. Evolution of Secondary Plant Metabolism. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–11. ISBN 978-0-470-01590-2. [Google Scholar]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.-Y.; Shaharuddin, N.A.; Ho, C.-L.; Mahmood, M. Exogenous proline significantly affects the plant growth and nitrogen assimilation enzymes activities in rice (Oryza sativa) under salt stress. Acta Physiol. Plant. 2016, 38, 151. [Google Scholar] [CrossRef]
- Tabssum, F.; Zaman, Q.; Chen, Y.; Riaz, U.; Ashraf, W.; Aslam, A.; Ehsan, N.; Nawaz, R.; Aziz, H.; Shah, S. Exogenous Application of Proline Improved Salt Tolerance in Rice through Modulation of Antioxidant Activities. Pak. J. Agric. Res. 2019, 32, 140–151. [Google Scholar] [CrossRef]
- Dawood, M.G.; Taie, H.A.A.; Nassar, R.M.A.; Abdelhamid, M.T.; Schmidhalter, U. The changes induced in the physiological, biochemical and anatomical characteristics of Vicia faba by the exogenous application of proline under seawater stress. South Afr. J. Bot. 2014, 93, 54–63. [Google Scholar] [CrossRef]
- Rady, M.M.; Taha, R.S.; Mahdi, A.H.A. Proline enhances growth, productivity and anatomy of two varieties of Lupinus termis L. grown under salt stress. South Afr. J. Bot. 2016, 102, 221–227. [Google Scholar] [CrossRef]
- Saradhi, P.P.; Mohanty, P. Involvement of proline in protecting thylakoid membranes against free radical-induced photodamage. J. Photochem. Photobiol. B 1997, 38, 253–257. [Google Scholar] [CrossRef]
- Singh, M.; Pratap Singh, V.; Dubey, G.; Mohan Prasad, S. Exogenous proline application ameliorates toxic effects of arsenate in Solanum melongena L. seedlings. Ecotoxicol. Environ. Saf. 2015, 117, 164–173. [Google Scholar] [CrossRef]
- Yu, X.-Z.; Lin, Y.-J.; Fan, W.-J.; Lu, M.-R. The role of exogenous proline in amelioration of lipid peroxidation in rice seedlings exposed to Cr(VI). Int. Biodeterior. Biodegrad. 2017, 123, 106–112. [Google Scholar] [CrossRef]
- Noreen, S.; Akhter, M.S.; Yaamin, T.; Arfan, M. The ameliorative effects of exogenously applied proline on physiological and biochemical parameters of wheat (Triticum aestivum L.) crop under copper stress condition. J. Plant Interact. 2018, 13, 221–230. [Google Scholar] [CrossRef]
- Mustafa, A.; Imran, M.; Ashraf, M.; Mahmood, K. Perspectives of using L-Tryptophan for improving productivity of agricultural crops: A Review. Pedosphere 2018, 28, 16–34. [Google Scholar] [CrossRef]
- Jamil, M.; Kharal, M.A.; Ahmad, M.; Abbasi, G.H.; Nazli, F.; Hussain, A.; Akhtar, M.F.-Z. Inducing salinity tolerance in red pepper (Capsicum annuum L.) through exogenous application of proline and L-tryptophan. Soil Environ. 2018, 37, 160–168. [Google Scholar]
- Hozayn, M.; Abd-Elmonem, A.A.; Samaha, G.M. The physiological effect of pre-soaking with tryptophan on sugar beet (Beta vulgaris L.) productivity under different levels of salinity stresses. Bull. Natl. Res. Cent. 2020, 44, 65. [Google Scholar] [CrossRef]
- Jamil, M.; Ahamd, M.; Anwar, F.; Zahir, Z.; Ali Kharal, M.; Nazli, F. Inducing drought tolerance in wheat through combined use of L-tryptophan and pseudomonas fluorescens. Pak. J. Agric. Sci. 2018, 55, 331–337. [Google Scholar] [CrossRef]
- Farooq, H.; Asghar, H.N.; Khan, M.Y.; Saleem, M.; Zahir, Z.A. Auxin-mediated growth of rice in cadmium-contaminated soil. Turk. J. Agric. For. 2015, 39, 272–276. [Google Scholar] [CrossRef]
- Hanci, F.; Çingi, M.; Akinci, H. Influence of L-Tryptophan and Melatonin on Germination of Onion and Leek Seeds at Different Temperatures. Türkiye Tarımsal Araştırmalar Derg. 2019, 6, 214–221. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Alharby, H.F.; Fujita, M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol. Plant. 2015, 59, 745–756. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, Z.; Zhang, J.; Chen, X.; Cui, J.; Xu, W.; Liu, H. Exogenous glutathione alleviates salt-induced oxidative stress in tomato seedlings by regulating glutathione metabolism, redox status, and the antioxidant system. Sci. Hortic. 2017, 220, 90–101. [Google Scholar] [CrossRef]
- Akram, S.; Siddiqui, M.N.; Hussain, B.M.N.; Al Bari, M.A.; Mostofa, M.G.; Hossain, M.A.; Tran, L.-S.P. Exogenous glutathione modulates salinity tolerance of soybean [Glycine max (L.) Merrill] at reproductive stage. J. Plant Growth Regul. 2017, 36, 877–888. [Google Scholar] [CrossRef]
- Khan, M.; Daud, M.K.; Basharat, A.; Khan, M.J.; Azizullah, A.; Muhammad, N.; Muhammad, N.; ur Rehman, Z.; Zhu, S.J. Alleviation of lead-induced physiological, metabolic, and ultramorphological changes in leaves of upland cotton through glutathione. Environ. Sci. Pollut. Res. 2016, 23, 8431–8440. [Google Scholar] [CrossRef]
- Kim, Y.-O.; Bae, H.-J.; Cho, E.; Kang, H. Exogenous Glutathione Enhances Mercury Tolerance by Inhibiting Mercury Entry into Plant Cells. Front. Plant Sci. 2017, 8, 683. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ. Exp. Bot. 2015, 112, 44–54. [Google Scholar] [CrossRef]
- Kumar, P.; Dubey, K.K. Citric acid cycle regulation: Back bone for secondary metabolite production. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–181. ISBN 978-0-444-63504-4. [Google Scholar]
- Soccol, C.R.; Vandenberghe, L.P.S.; Rodrigues, C.; Pandey, A. New perspectives for citric acid production and application. Food Technol. Biotechnol. 2006, 44, 141–149. [Google Scholar]
- Pérez-Labrada, F.; Benavides-Mendoza, A.; Valdez-Aguilar, L.A.; Robledo-Torres, V. Citric acid in the nutrient solution increases the mineral absorption in potted tomato grown in calcareous soil. Pak. J. Bot. 2016, 48, 67–74. [Google Scholar]
- El-Hawary, M.M.; Nashed, M.E. Effect of Foliar Application by some Antioxidants on Growth and Productivity of Maize under Saline Soil Conditions. J. Plant Prod. 2019, 10, 93–99. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Hayat, S. Supplementation of Salicylic Acid and Citric Acid for Alleviation of Cadmium Toxicity to Brassica juncea. J. Plant Growth Regul. 2020, 39, 641–655. [Google Scholar] [CrossRef]
- Mallhi, Z.I.; Rizwan, M.; Mansha, A.; Ali, Q.; Asim, S.; Ali, S.; Hussain, A.; Alrokayan, S.H.; Khan, H.A.; Alam, P.; et al. Citric acid enhances plant growth, photosynthesis, and phytoextraction of lead by alleviating the oxidative stress in Castor beans. Plants 2019, 8, 525. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Farid, M.; Shakoor, M.B.; Gill, R.A.; Najeeb, U.; Iqbal, N.; Ahmad, R. Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol. Environ. Saf. 2015, 120, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ben Massoud, M.; Sakouhi, L.; Chaoui, A. Effect of plant growth regulators, calcium and citric acid on copper toxicity in pea seedlings. J. Plant Nutr. 2019, 42, 1230–1242. [Google Scholar] [CrossRef]
- Parveen, A.; Saleem, M.H.; Kamran, M.; Haider, M.Z.; Chen, J.-T.; Malik, Z.; Rana, M.S.; Hassan, A.; Hur, G.; Javed, M.T.; et al. Effect of citric acid on growth, ecophysiology, chloroplast ultrastructure, and phytoremediation potential of jute (Corchorus capsularis L.) seedlings exposed to copper stress. Biomolecules 2020, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.R.; Mukta, R.H.; Islam, M.A.; Huda, A.K.M.N. Insight into citric acid-induced chromium detoxification in rice (Oryza sativa L.). Int. J. Phytoremediation 2019, 21, 1234–1240. [Google Scholar] [CrossRef]
- Maqbool, A.; Ali, S.; Rizwan, M.; Ishaque, W.; Rasool, N.; Rehman, M.Z.u.; Bashir, A.; Abid, M.; Wu, L. Management of tannery wastewater for improving growth attributes and reducing chromium uptake in spinach through citric acid application. Environ. Sci. Pollut. Res. 2018, 25, 10848–10856. [Google Scholar] [CrossRef]
- Munnik, T.; Vermeer, J.E.M. Osmotic stress-induced phosphoinositide and inositol phosphate signalling in plants. Plant Cell Environ. 2010, 33, 655–669. [Google Scholar] [CrossRef]
- Chatterjee, J.; Majumder, A.L. Salt-induced abnormalities on root tip mitotic cells of Allium cepa: Prevention by inositol pretreatment. Protoplasma 2010, 245, 165–172. [Google Scholar] [CrossRef]
- Gerke, J. Phytate (Inositol Hexakisphosphate) in soil and phosphate acquisition from inositol phosphates by higher plants. A Review. Plants 2015, 4, 253–266. [Google Scholar] [CrossRef]
- Kaya, C.; Sonmez, O.; Aydemir, S.; Ashraf, M.; Dikilitas, M. Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.). J. Plant Interact. 2013, 8, 234–241. [Google Scholar] [CrossRef]
- Ramadan, M.E.; Shalaby, O.A. Effect of salicylic acid and mannitol on white cabbage plants under saline conditions. J. Plant Prod. 2018, 9, 397–402. [Google Scholar] [CrossRef]
- Habiba, U.; Ali, S.; Rizwan, M.; Ibrahim, M.; Hussain, A.; Shahid, M.R.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Alleviative role of exogenously applied mannitol in maize cultivars differing in chromium stress tolerance. Environ. Sci. Pollut. Res. 2019, 26, 5111–5121. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Ali, S.; Iqbal, M.; Aslam Bharwana, S.; Siddiqi, Z.; Farid, M.; Ali, Q.; Saeed, R.; Rizwan, M. Mannitol alleviates chromium toxicity in wheat plants in relation to growth, yield, stimulation of anti-oxidative enzymes, oxidative stress and Cr uptake in sand and soil media. Ecotoxicol. Environ. Saf. 2015, 122, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Margaritopoulou, T.; Papadakis, I.E.; Araniti, F. Boron toxicity in higher plants: An update. Planta 2019, 250, 1011–1032. [Google Scholar] [CrossRef]
- Coskun, Y.; Olgunsoy, P.; Karatas, N.; Bulut, F.; Yarar, F. Mannitol application alleviates boron toxicity in wheat seedlings. Commun. Soil Sci. Plant Anal. 2014, 45, 944–952. [Google Scholar] [CrossRef]
- Issa, D.B.; Alturki, S.M.; Sajyan, T.K.; Sassine, Y.N. Sorbitol and lithovit-guano25 mitigates the adverse effects of salinity on eggplant grown in pot experiment. Agron. Res. 2020, 18, 113–126. [Google Scholar] [CrossRef]
- Theerakulpisut, P.; Gunnula, W. Exogenous sorbitol and trehalose mitigated salt stress damage in Salt- sensitive but not Salt-tolerant rice seedlings. Asian J. Crop Sci. 2012, 4, 165–170. [Google Scholar] [CrossRef]
- Kokitkar, S.S.; Chavan, L.R.; Bhagat, S.A. Alleviation of adverse effects of salt stress in triticum aestivum by foliar application of compatible solutes. IJRBAT 2017, V, 272–276. [Google Scholar]
- Hu, L.; Zhou, K.; Li, Y.; Chen, X.; Liu, B.; Li, C.; Gong, X.; Ma, F. Exogenous myo-inositol alleviates salinity-induced stress in Malus hupehensis Rehd. Plant Physiol. Biochem. 2018, 133, 116–126. [Google Scholar] [CrossRef]
- Yildizli, A.; Çevik, S.; Ünyayar, S. Effects of exogenous myo-inositol on leaf water status and oxidative stress of Capsicum annuum under drought stress. Acta Physiol. Plant. 2018, 40, 122. [Google Scholar] [CrossRef]
- Araya-Flores, J.; Miranda, S.; Covarrubias, M.P.; Stange, C.; Handford, M. Solanum lycopersicum (tomato) possesses mitochondrial and plastidial lipoyl synthases capable of increasing lipoylation levels when expressed in bacteria. Plant Physiol. Biochem. 2020, 151, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, M.; Akçalı, N.; Terzi, H. Proteomic and biochemical responses of canola (Brassica napus L.) exposed to salinity stress and exogenous lipoic acid. J. Plant Physiol. 2015, 179, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Elrys, A.S.; Abdo, A.I.E.; Abdel-Hamed, E.M.W.; Desoky, E.-S.M. Integrative application of licorice root extract or lipoic acid with fulvic acid improves wheat production and defenses under salt stress conditions. Ecotoxicol. Environ. Saf. 2020, 190, 110144. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, A.; Altuntaş, C.; Demiralay, M.; Cinemre, S.; Terzi, R. Exogenous alpha lipoic acid can stimulate photosystem II activity and the gene expressions of carbon fixation and chlorophyll metabolism enzymes in maize seedlings under drought. J. Plant Physiol. 2019, 232, 65–73. [Google Scholar] [CrossRef]
- Turk, H.; Erdal, S.; Karayel, U.; Dumlupinar, R. Attenuation of lead toxicity by promotion of tolerance mechanism in wheat roots by lipoic acid. Cereal Res. Commun. 2018, 46, 424–435. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Huang, R. Regulation of ascorbic acid synthesis in plants. Plant Signal. Behav. 2013, 8, e24536. [Google Scholar] [CrossRef]
- Hossain, M.A.; Munné-Bosch, S.; Burritt, D.J.; Diaz-Vivancos, P.; Fujita, M.; Lorence, A. Ascorbic Acid in Plant Growth, Development and Stress Tolerance. Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-74056-0. [Google Scholar]
- Bilska, K.; Wojciechowska, N.; Alipour, S.; Kalemba, E.M. Ascorbic Acid—The Little-Known Antioxidant in Woody Plants. Antioxidants 2019, 8, 645. [Google Scholar] [CrossRef]
- Aziz, A.; Akram, N.A.; Ashraf, M. Influence of natural and synthetic vitamin C (Ascorbic acid) on primary and secondary metabolites and associated metabolism in quinoa (Chenopodium quinoa Willd.) plants under water deficit regimes. Plant Physiol. Biochem. 2018, 123, 192–203. [Google Scholar] [CrossRef]
- Penella, C.; Calatayud, Á.; Melgar, J.C. Ascorbic acid alleviates water stress in young peach trees and improves their performance after rewatering. Front. Plant Sci. 2017, 8, 1627. [Google Scholar] [CrossRef]
- Hafez, E.M.; Gharib, H.S. Effect of exogenous application of ascorbic acid on physiological and biochemical characteristics of wheat under water stress. Int. J. Plant Prod. 2016, 10, 579–596. [Google Scholar]
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Nasir Khan, M.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A.; Al-Rabiah, H.; Mateen, M. Ascorbic acid improves the tolerance of wheat plants to lead toxicity. J. Plant Interact. 2018, 13, 409–419. [Google Scholar] [CrossRef]
- Sarwar, M.; Saleem, M.F.; Ullah, N.; Rizwan, M.; Ali, S.; Shahid, M.R.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Exogenously applied growth regulators protect the cotton crop from heat-induced injury by modulating plant defense mechanism. Sci. Rep. 2018, 8, 17086. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Saleem, M.F.; Shahid, M.; Awais, M.; Khan, H.Z.; Ahmed, K. Ascorbic acid triggered physiochemical transformations at different phenological stages of heat-stressed Bt cotton. J. Agron. Crop Sci. 2017, 203, 323–331. [Google Scholar] [CrossRef]
- Elkelish, A.; Qari, S.H.; Mazrou, Y.S.A.; Abdelaal, K.A.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; Batiha, G.E.-S.; El-Esawi, M.A.; El Nahhas, N. Exogenous Ascorbic acid induced chilling tolerance in tomato plants through modulating metabolism, osmolytes, antioxidants, and transcriptional regulation of catalase and heat shock proteins. Plants 2020, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Lo’ay, A.A.; EL-Khateeb, A.Y. Antioxidant enzyme activities and exogenous ascorbic acid treatment of ‘Williams’ banana during long-term cold storage stress. Sci. Hortic. 2018, 234, 210–219. [Google Scholar] [CrossRef]
- Min, K.; Chen, K.; Arora, R. A metabolomics study of ascorbic acid-induced in spinach (Spinacia oleracea L. ) Plant Direct 2020, 4, 1–13. [Google Scholar]
- Giri, J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Ivanov, A.G.; Zaman, M.; Pharis, R.P.; Hurry, V.; Hüner, N.P.A. Interaction of Glycine Betaine and Plant Hormones: Protection of the Photosynthetic Apparatus during Abiotic Stress. In Photosynthesis: Structures, Mechanisms, and Applications; Hou, H.J.M., Najafpour, M.M., Moore, G.F., Allakhverdiev, S.I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 185–202. ISBN 978-3-319-48873-8. [Google Scholar]
- Huang, S.; Zuo, T.; Ni, W. Important roles of glycinebetaine in stabilizing the structure and function of the photosystem II complex under abiotic stresses. Planta 2020, 251, 36. [Google Scholar] [CrossRef]
- Alasvandyari, F.; Mahdavi, B. Effect of glycine betaine and salinity on photosynthetic pigments and ion concentration of safflower. Desert 2018, 23, 265–271. [Google Scholar]
- Shams, M.; Yildirim, E.; Ekinci, M.; Turan, M.; Dursun, A.; Parlakova, F.; Kul, R. Exogenously applied glycine betaine regulates some chemical characteristics and antioxidative defence systems in lettuce under salt stress. Hortic. Environ. Biotechnol. 2016, 57, 225–231. [Google Scholar] [CrossRef]
- Osman, H.S. Enhancing antioxidant–yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Ann. Agric. Sci. 2015, 60, 389–402. [Google Scholar] [CrossRef]
- Gupta, N.; Thind, S. Improving photosynthetic performance of bread wheat under field drought stress by foliar applied glycine betaine. J. Agric. Sci. Tech. 2015, 17, 75–86. [Google Scholar]
- Sajyan, T.K.; Allaw, W.; Shaban, N.; Sassine, Y.N. Effect of exogenous application of glycine betaine on tomato plants subjected to salt stress. Acta Hortic. 2019, 1253, 41–48. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, S.; Yuan, M.; Song, H.; Wang, T.; Zhang, W.; Zhang, Z. Effect of glycine betaine on chilling injury in relation to energy metabolism in papaya fruit during cold storage. Food Sci. Nutr. 2019, 7, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-Mageed, T.A.; Howladar, S.M.; Rady, M.M. Foliar-applied alpha-tocopherol enhances salt-tolerance in onion plants by improving antioxidant defense system. Aust. J. Crop Sci. 2016, 10, 1030. [Google Scholar] [CrossRef]
- Lalarukh, I. Alpha-tocopherol induced modulations in morpho-physiological attributes of sunflower (Helianthus annuus) grown under saline environment. Int. J. Agric. Biol. 2018, 20, 661–668. [Google Scholar] [CrossRef]
- Lalarukh, I.; Shahbaz, M. Response of antioxidants and lipid peroxidation to exogenous application of alpha-tocopherol in sunflower (Helianthus annuus L.) under salt stress. Pak. J. Bot. 2020, 52, 75–83. [Google Scholar] [CrossRef]
- Ali, Q.; Ali, S.; Iqbal, N.; Javed, M.; Rizwan, M.; Khaliq, R.; Shahid, S.; Parveen, R.; Alamri, S.; Alyemeni, M.; et al. Alpha-tocopherol fertigation confers growth physio-biochemical and qualitative yield enhancement in field grown water deficit wheat (Triticum aestivum L.). Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Sadiq, M.; Akram, N.A.; Ashraf, M. Impact of exogenously applied tocopherol on some key physio-biochemical and yield attributes in mungbean [Vigna radiata (L.) Wilczek] under limited irrigation regimes. Acta Physiol. Plant. 2018, 40, 131. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Hardeland, R.; Madrid, J.A.; Tan, D.-X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling: Melatonin and peripheral oscillators. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Khan, A.; Numan, M.; Khan, A.L.; Lee, I.-J.; Imran, M.; Asaf, S.; Al-Harrasi, A. Melatonin: Awakening the defense mechanisms during plant oxidative stress. Plants 2020, 9, 407. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Al-Khaishany, M.Y.; Khan, M.N.; Al-Amri, A.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A. Exogenous melatonin counteracts nacl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.; Buck, G.A.; Fu, C.; Ren, S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 2014, 9, e93462. [Google Scholar] [CrossRef]
- Fan, J.; Hu, Z.; Xie, Y.; Chan, Z.; Chen, K.; Amombo, E.; Chen, L.; Fu, J. Alleviation of cold damage to photosystem II and metabolisms by melatonin in Bermudagrass. Front. Plant Sci. 2015, 6, 925. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.-X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chen, K.; Wei, Y.; He, C. Fundamental issues of melatonin-mediated stress signaling in plants. Front. Plant Sci. 2016, 7, 1124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Yang, S.J.; Chen, Y.Y. Effects of melatonin on photosynthetic performance and antioxidants in melon during cold and recovery. Biol. Plant. 2017, 61, 571–578. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Shi, X.-C.; Wang, R.; Wang, H.-L.; Liu, F.; Laborda, P. Melatonin in fruit production and postharvest preservation: A review. Food Chem. 2020, 320, 126642. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, Z. Improving growth and yield of salt-stressed cowpea plants by exogenous application of Ascobin. Life Sci. J. 2014, 11, 43–51. [Google Scholar]
- Allahveran, A.; Farokhzad, A.; Asghari, M.; Sarkhosh, A. Foliar application of ascorbic and citric acids enhanced ‘Red Spur’ apple fruit quality, bioactive compounds and antioxidant activity. Physiol. Mol. Biol. Plants 2018, 24, 433–440. [Google Scholar] [CrossRef]
- Rodriguez-Furlán, C.; Miranda, G.; Reggiardo, M.; Hicks, G.R.; Norambuena, L. High throughput selection of novel plant growth regulators: Assessing the translatability of small bioactive molecules from Arabidopsis to crops. Plant Sci. 2016, 245, 50–60. [Google Scholar] [CrossRef]
- Fozard, S.; Forde, B.G. Novel micro-phenotyping approach to chemical genetic screening for increased plant tolerance to abiotic stress. In Plant Chemical Genomics; Fauser, F., Jonikas, M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1795, pp. 9–25. ISBN 978-1-4939-7873-1. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. https://doi.org/10.3390/plants10020186
Godoy F, Olivos-Hernández K, Stange C, Handford M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants. 2021; 10(2):186. https://doi.org/10.3390/plants10020186
Chicago/Turabian StyleGodoy, Francisca, Karina Olivos-Hernández, Claudia Stange, and Michael Handford. 2021. "Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites" Plants 10, no. 2: 186. https://doi.org/10.3390/plants10020186
APA StyleGodoy, F., Olivos-Hernández, K., Stange, C., & Handford, M. (2021). Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants, 10(2), 186. https://doi.org/10.3390/plants10020186





