Biogeographical Patterns of Herbivore Arthropods Associated with Chenopodium quinoa Grown along the Latitudinal Gradient of Chile
Abstract
:1. Introduction
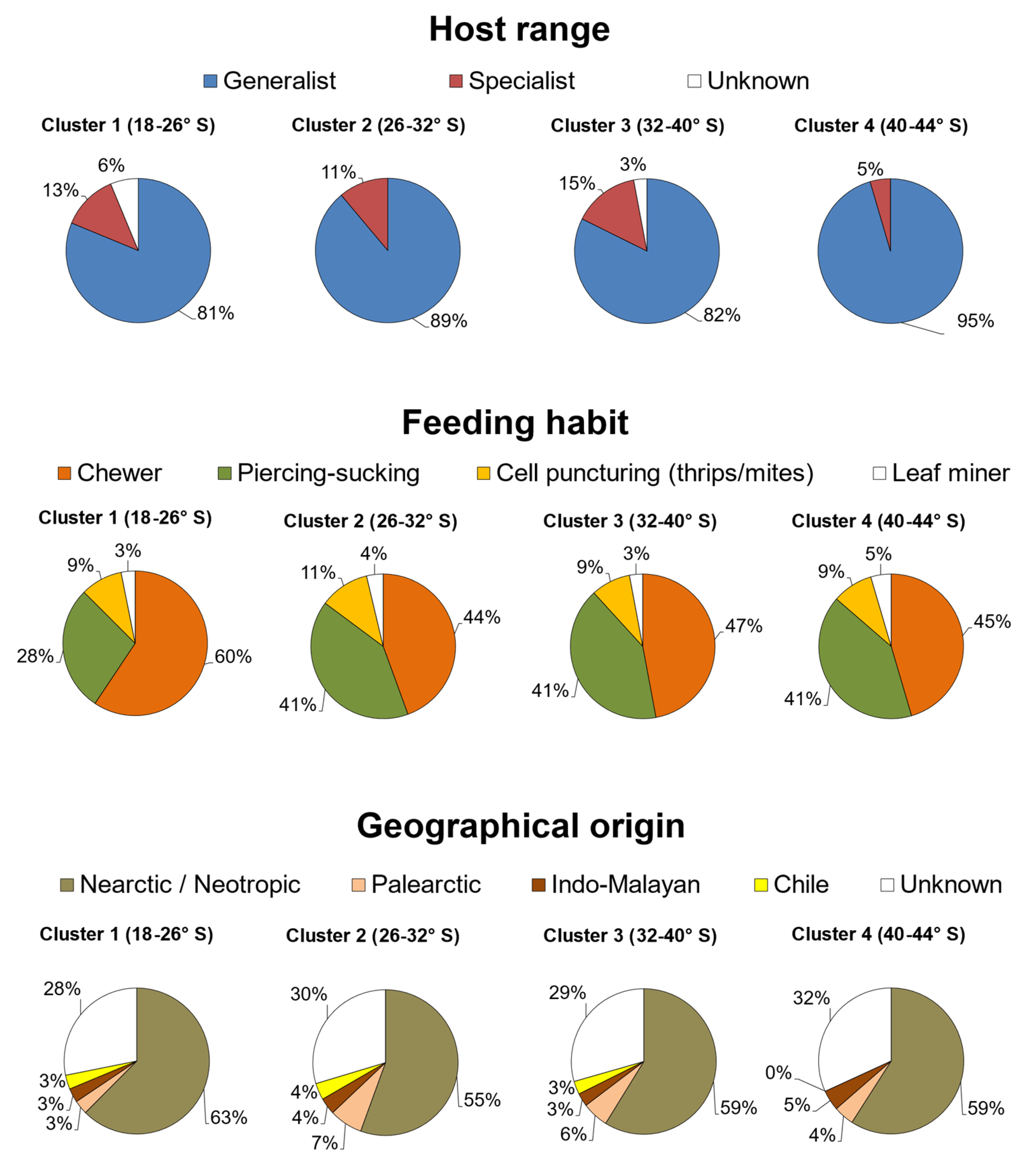
2. Results
3. Discussion
4. Materials and Methods
4.1. Field Sampling and Species Identification
- Tarapacá
- Ancovinto site with commercial plantations of salares ecotype (20 ha) (19°24′ S, 68°35′ W, 3720 m.a.s.l.). Inspected: 10 December 2016, 27 January 2017, 7 April 2017, and 29 January 2018.
- Metropolitana
- Pirque site. Research facility with experimental plantations (1 ha) of coastal ecotype (33°40′ S, 70°35′ W, 653 m.a.s.l.). Inspected: 22 December 2015, 14 January 2016, and 10 October 2018.
- Santiago site, research facility with demonstrative plantation of coastal ecotype (33°29′ S, 70°36′ W, 576 m.a.s.l.). Inspected on a monthly basis from November through April of 2016, 2017, and 2018.
- O’Higgins
- Cahuil site with commercial plantation of coastal ecotype (10 ha) (34°29′, 72°01′ W, 40 m.a.s.l.). Inspected: 12 October 2016, 12 December 2016, 12 January 2016, and 22 January 2017.
- Pailimo site with commercial plantation of coastal ecotype (5 ha) (34°15′ S, 71°47′ W, 242 m.a.s.l.). Inspected: 12 October 2016, 12 January 2016, and 22 January 2017.
- Los Lagos
- Ancud sites 1 and 2 with commercial plantations of coastal ecotypes (0.1 and 0.2 ha) (41°50′ S, 74°00′ W, 7 m.a.s.l., and 42°00′ S, 73°53′ W, 38 m.a.s.l.). Inspected: 16 December 2016, 13 January 2017, and 3 February 2017.
4.2. Total Number of Expected Species
4.3. Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuentes, F.; Bhargava, A. Morphological analysis of quinoa germplasm grown under lowland desert conditions. J. Agron. Crop Sci. 2011, 197, 124–134. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; Martinez, E.A.; López, J.; Marín, R.; Aranda, M.; Fuentes, F. Influence of contrasting environment on seed composition of two quinoa genotypes: Nutritional and functional properties. Chil. J. Agric. Res. 2013, 73, 108–116. [Google Scholar] [CrossRef]
- Fuentes, F.; Paredes-González, X. Nutraceutical Perspectives of Quinoa: Biological Properties and Functional Applications. In State of the Art Report on Quinoa around the World in 2013; Bazile, D., Bertero, H.D., Nieto, C., Eds.; FAO & CIRAD: Rome, Italy, 2015; pp. 286–299. [Google Scholar]
- Fuentes, F.F.; Martinez, E.A.; Hinrichsen, P.V.; Jellen, E.N.; Maughan, P.J. Assessment of genetic diversity patterns in Chilean quinoa (Chenopodium quinoa Willd.) germplasm using multiplex fluorescent microsatellite markers. Conserv. Genet. 2009, 10, 369–377. [Google Scholar] [CrossRef]
- Cruces, L.M.; Callohuari, Y.; Carrera, C. Quinua Manejo Integrado de Plagas. Estrategias en el Cultivo de la Quinua Para Fortalecer el Sistema Agroalimentario en las Zonas Andinas; Organización de las Naciones Unidas para la Alimentación y la Agricultura: Santiago, Chile, 2016; pp. 1–198. [Google Scholar]
- Fuentes, F.F.; Olguín, P.; Duarte, L.; Ojeda, M.; Figueroa, C.; Paredes-González, X.; Martínez, E.A. Potencial Competitivo de la Quinua Chilena; Publicaciones Fundación para la Innovación Agraria: Santiago, Chile, 2017; pp. 1–147. [Google Scholar]
- Rasmussen, C.; Lagnaoui, A.; Esbjerg, P. Advances in the knowledge of quinoa pests. Food Rev. Int. 2003, 19, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Saravia, R.; Plata, G.; Gandarillas, A. Plagas y Enfermedades del Cultivo de Quinua; Fundación PROINPA: Cochabamba, Bolivia, 2014; pp. 1–148. [Google Scholar]
- Cruces, L.; de la Peña, E.; De Clercq, P. Seasonal phenology of the major insect pests of quinoa (Chenopodium quinoa Willd.) and their natural enemies in a traditional zone and two new production zones of Peru. Agriculture 2020, 10, 644. [Google Scholar] [CrossRef]
- Artigas, J.N. Entomología Económica; Ediciones Universidad de Concepción: Concepción, Chile, 1994; Volumes I and II, pp. 1–1126 and 1–943. [Google Scholar]
- Lamborot, L.; Guerrero, M.A.; Araya, J.E. Lepidópteros asociados al cultivo de la quinoa (Chenopodium quinoa Willdenow) en la zona central de Chile. Bol. San. Veg. Plagas 1999, 25, 203–207. [Google Scholar]
- Klein-Koch, C.; Waterhouse, D.F. Distribución e importancia de los artrópodos asociados a la agricultura y silvicultura en Chile. ACIAR Monogr. 2000, 68, 1–234. [Google Scholar]
- Logarzo, G.A.; De León, J.H.; Triapitsyn, S.V.; González, R.H.; Virla, E.G. First report of a Proconiine Sharpshooter, Anacuerna centrolinea (Hemiptera: Cicadellidae), in Chile, with notes on its biology, host plants, and egg parasitoids. Ann. Entomol. Soc. Am. 2006, 99, 879–883. [Google Scholar] [CrossRef]
- Elgueta, M.; Camousseight, A.; Carbonell, C.S. Catálogo de Orthoptera (Insecta) de Chile; Publicación Ocasional Nº 54; Museo Nacional de Historia Natural: Santiago, Chile, 1999; pp. 1–60. [Google Scholar]
- Dughetti, A.C. Plagas de la Quinua y sus Enemigos Naturales en el Valle Inferior del Río Colorado, Buenos Aires, Argentina, 1st ed.; Ediciones INTA: Buenos Aires, Argentina, 2015; pp. 1–63. [Google Scholar]
- Gandarillas, A.; Saravia, R.; Plata, G.; Quispe, R.; Ortiz-Romero, R. Principales plagas y enfermedades de la quinua. In State of the Art Report on Quinoa around the World in 2013; Bazile, D., Bertero, H.D., Nieto, C., Eds.; FAO & CIRAD: Rome, Italy, 2015; pp. 192–215. [Google Scholar]
- Nieto-Nafría, J.M.; Fuentes-Contreras, E.; Castro Colmenero, M.; Aldea Piera, M.; Ortego, J.; Mier Durante, M.P. Catálogo de los áfidos (Hemiptera, Aphididae) de Chile, con plantas hospedadoras y distribuciones regional y provincial. Graellsia 2016, 72, e050. [Google Scholar] [CrossRef] [Green Version]
- Cranshaw, W.S.; Kondratieff, B.C.; Qian, T. Insect associated with Quinoa, Chenopodium quinoa, in Colorado. J. Kans. Entomol. Soc. 1990, 63, 195–199. [Google Scholar]
- Burckhardt, D. Generic key to Chilean jumping plant-lice (Homoptera: Psylloidea) with inclusion of potential exotic pests. Rev. Chil. Entomol. 1994, 21, 57–67. [Google Scholar]
- Faúndez, E.I.; Rocca, J. Descripción de un caso teratológico en Oncopeltus (Erythrischius) miles (Blanchard, 1852) (Heteroptera: Lygaeidae) con notas acerca de su distribución y biología. Arq. Entomol. 2016, 15, 39–43. [Google Scholar]
- Faúndez, E.I.; Carvajal, M.A. Sobre la relación entre Leptoglossus chilensis (Spinola, 1852) y Leptoglossus concaviusculus Berg, 1892 stat. rest. (Heteroptera: Coreidae), con nuevos datos acerca de su morfología. An. Inst. Patagon. 2016, 44, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Prado, E. Conocimiento actual de Hemiptera—Heteroptera de Chile con lista de especies. Bol. Mus. Nac. Hist. Nat. Chile 2008, 57, 31–75. [Google Scholar]
- Elgueta, M.; Marvaldi, A.E. Lista sistemática de las especies de Curculionoidea (Insecta: Coleoptera) presentes en Chile, con su sinonímia. Bol. Mus. Nac. Hist. Nat. Chile 2006, 55, 113–153. [Google Scholar]
- Frías, D.; Henry, A.; Alviña, A.; Landry, J.F. Aspectos de la biología, taxonomía y control de las especies del género Coleophora (Lepidoptera: Coleophoridae) de distribución chilena. Acta Entomol. Chil. 1996, 20, 115–122. [Google Scholar]
- Guerrero, M.A.; Lamborot, L.; Araya, J.E. Observaciones biológicas de Achryra similalis (Guenèe) (Pyralidae) y otros lepidópteros en amaranto, Amaranthus cruentus L. (Amaranthaceae), en la Región Metropolitana de Chile. Bol. San. Veg. Plagas 2000, 26, 591–598. [Google Scholar]
- Cepeda, D.E. Contribution to the knowledge of Chilean Gelechiidae (Lepidoptera: Gelechioidea). Insecta Mundi 2017, 584, 1–8. [Google Scholar]
- Povolný, D. Gnorimoschemini of southern South America II: The genus Eurysacca (Lepidoptera, Gelechiidae). Steenstrupia 1986, 12, 1–47. [Google Scholar]
- Povolný, D. Eurysacca quinoae sp.n.—A new quinoa-feeding species of the tribe Gnorimoschemini (Lepidoptera, Gelechiidae) from Bolivia. Steenstrupia 1997, 22, 41–43. [Google Scholar]
- Angulo, A.O.; Weigert, G.T. Estados Inmaduros de Lepidópteros Nóctuidos de Importancia Económica en Chile y Claves Para su Determinación (Lepidoptera: Noctuidae); Publicación especial N°2; Sociedad de Biología de Concepción: Concepción, Chile, 1975; 153p. [Google Scholar]
- Hardwick, D.F. The corn earworm complex. Mem. Entomol. Soc. Can. 1965, 40, 1–247. [Google Scholar] [CrossRef]
- Kuschel, G. Los Curculionidae del extremo norte de Chile (Coleoptera. Curcul. Ap. 6°). Acta Zool. Lilloana 1949, 8, 5–54. [Google Scholar]
- Kuschel, G. New rank for varieties of Trichocyphus formosus. In Annotated Checklist of the Weevils (Curculionidae sensu lato) of South America (Coleoptera: Curculionoidea); Wibmer, G.J., O’Brien, N.C.W., Eds.; American Entomological Institute: Ann Arbor, MI, USA, 1986; Volume 39, p. 52. [Google Scholar]
- Lanteri, A.A. Estudio sistemático de los géneros Trichocyphus Heller y Mendozella Hustache (Coleoptera: Curculionidae). Bol. Soc. Biol. Concepción Chile 1989, 60, 139–147. [Google Scholar]
- Pogue, M.G. A review of the Copitarsia decolora (Guenée) (Lepidoptera: Noctuidae) species complex with the description of a new species from Chile and Argentina. Neotrop. Entomol. 2014, 43, 143–153. [Google Scholar] [CrossRef]
- Yábar, E.; Gianoli, E.; Echegaray, E.R. Insect pest and natural enemies in two varieties of quinoa (Chenopodium quinoa) at Cusco, Peru. J. Appl. Entomol. 2002, 126, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Quiroga, N.; Longone, V.; González, X.; Zamorano, A.; Pino, A.M.; Picciau, L.; Alma, A.; Paltrinieri, S.; Contaldo, N.; Bertaccini, A.; et al. Transmission of 16SrIII-J phytoplasmas by the leafhoppers Paratanus exitiousus and Bergallia valdiviana. Phytopathol. Mediter. 2019, 58, 231–237. [Google Scholar]
- Wibmer, G.J.; O’Brien, N.C.W. Annotated checklist of the weevils (Curculionidae sensu lato) of South America (Coleoptera: Curculionoidea). Mem. Am. Entomol. Inst. 1986, 39, 1–563. [Google Scholar]
- Valoy, M.E.; Bruno, M.A.; Prado, F.E.; González, J.A. Insects associated to a quinoa crop in Amaicha del Valle, Tucumán, Argentina./Insectos asociados a un cultivo de quinoa en Amaicha del Valle, Tucumán, Argentina. Acta Zool. Lilloana 2011, 55, 16–22. [Google Scholar]
- Elgueta, M.; Arriagada, G. Estado actual del conocimiento de coleópteros de Chile (Insecta: Coleoptera). Rev. Chil. Entomol. 1989, 17, 5–60. [Google Scholar]
- Povolný, D. Gnorimoschemini of southern South America IV: The genus Symmetrischema (Lepidoptera, Gelechiidae). Steenstrupia 1989, 15, 57–104. [Google Scholar]
- Povolný, D. Gnorimoschemini of southern South America VI: Identification keys, checklist of Neotropical taxa and general considerations (Insecta, Lepidoptera, Gelechiidae). Steenstrupia 1994, 20, 1–42. [Google Scholar]
- Dioli, P.; Colamartino, A.D.; Negri, M.; Limonta, L. Hemiptera and coleoptera on Chenopodium quinoa. Redia 2016, 99, 139–141. [Google Scholar]
- Reguzzi, M.C.; Nicoli Aldini, R.; Vercesi, A.; Ganimede, C.; Tabaglio, V.; Mazzoni, E.; Dioli, P. Quinoa, quali insetti infestanti sono presenti al Nord Italia. L’Informatore Agrario 2019, 75, 59–61. [Google Scholar]
- Scaccini, D.; Furlan, L. Nysius cymoides (Hemiptera: Lygaeidae), a potential emerging pest: Overview of the information available to implement integrated pest management. Int. J. Pest Manag. 2021, 67, 73–88. [Google Scholar] [CrossRef]
- Göllner-Scheiding, U. Revision der Gattungen Liorhyssus STÅL, 1870 (Heteroptera, Rhopalidae). Dtsch. Entomol. Z. 1975, 23, 181–206. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Herbaceous Plants and Shrubs; John Wiley & Sons: Chichester, UK, 2006; pp. 1–1456. [Google Scholar]
- San Blas, G. Agrotis Ochsenheimer (Lepidoptera, Noctuidae): A systematic analysis of South American species. Zootaxa 2014, 3771, 1–64. [Google Scholar] [CrossRef]
- Wrzesinska, D.; Wawrzyniak, M.; Gesinski, K. Population of true bugs (Heteroptera) on the inflorescences of quinoa (Chenopodium quinoa Willd.). J. Plant Prot. Res. 2001, 41, 333–336. [Google Scholar]
- Schoonhoven, L.M.; van Loon, J.J.A.; Dicke, M. Herbivorous insects: Something for everyone. In Insect-Plant Biology, 2nd ed.; Oxford University Press Inc.: New York, NY, USA, 2005; pp. 5–28. [Google Scholar]
- San Blas, G. A morphological phylogeny of Agrotis Ochsenheimer (Lepidoptera, Noctuidae), with emphasis on the South American species. Zool. Scr. 2014, 44, 153–164. [Google Scholar] [CrossRef]

| Species | Distribution Range in Chile (° S) | Feeding Habit | Host Range | Geographical Origin | References |
|---|---|---|---|---|---|
| Orthoptera: Acrididae | |||||
| Dichroplus maculipennis (Blanchard, 1851) | 29–56 | Chewing | Generalist | Neotropic | [5,14,15] |
| Hemiptera: Cicadellidae | |||||
| Anacuerna centrolinea (Melichar, 1925) | 19–22 | Piercing–sucking | Generalist | Neotropic | [5,13] |
| Paratanus exitiosus Beamer, 1943 | 32–41 | Piercing–sucking | Generalist | Neotropic | [5,7,10,16] |
| Hemiptera: Aphididae | |||||
| Aphis craccivora Koch, 1854 | 18–44 a,b,c | Piercing–sucking | Generalist | Palearctic | [5,7,10,15,16] |
| Aphis gossypii Glover, 1877 | 18–44 | Piercing–sucking | Generalist | Unknown | [5,7,12,16,17] |
| Macrosiphum euphorbiae (Thomas, 1878) | 18–56 a,b,c,d | Piercing–sucking | Generalist | Neotropic—Nearctic | [5,7,10,16,17,18] |
| Myzus persicae (Sulzer, 1776) | 18–56 b | Piercing–sucking | Generalist | Indo-Malayan | [5,7,10,16,17] |
| Smynthurodes betae Westwood, 1849 | 18–22; 32–56 | Piercing–sucking | Generalist | Unknown | [10,17] |
| Hemiptera: Triozidae | |||||
| Heterotrioza chenopodii (Reuter, 1876) (=Trioza chenopodii Reuter) | 29–34 | Piercing–sucking | Specialist | Palearctic | [5,15,19] |
| Hemiptera: Pentatomidae | |||||
| Nezara viridula (Linnaeus, 1758) | 18–56 | Piercing–sucking | Generalist | Unknown | [5,10,15] |
| Hemiptera: Lygaeidae | |||||
| Nysius simulans (Stål, 1859) | 29–38 | Piercing–sucking | Generalist | Neotropic | [10] |
| Oncopeltus miles (Blanchard, 1852) | 19–40 | Piercing–sucking | Specialist | Chile | [10,20] |
| Hemiptera: Coreidae | |||||
| Leptoglossus chilensis (Spinola, 1852) | 26–44 | Piercing–sucking | Generalist | Neotropic | [5,15,21] |
| Hemiptera: Rhopalidae | |||||
| Liorhyssus hyalinus (Fabricius, 1794) | 18–38 | Piercing–sucking | Generalist | Unknown | [10] |
| Liorhyssus lineatoventris (Spinola, 1852) | 30–40 b | Piercing–sucking | Generalist | Neotropic | [22] |
| Hemiptera: Miridae | |||||
| Orthotylus flavosparsus (Sahlberg, 1841) | 33–34 b | Piercing–sucking | Generalist | Unknown | [5,15,22] |
| Thysanoptera: Thripidae | |||||
| Frankliniella occidentalis (Pergande, 1895) | 18–44 b,c | Cell puncturing | Generalist | Nearctic | [12,18] |
| Thrips tabaci Lindeman, 1889 | 18–40 | Cell puncturing | Generalist | Unknown | [7,10,16,18] |
| Diptera: Agromyzidae | |||||
| Liriomyza huidobrensis Blanchard, 1926 | 18–49 a,b,c | Leaf mining | Generalist | Neotropic | [5,7,10,16] |
| Coleoptera: Chrysomelidae | |||||
| Epitrix sp. | 32–34 b | Chewing | Unknown | Neotropic—Nearctic | [5,7,10,12,16] |
| Coleoptera: Curculionidae | |||||
| Trichocyphus rubricollis (Blanchard, 1847) | 18–26 a | Chewing | Unknown | Neotropic | [23] |
| Coleoptera: Meloidae | |||||
| Pseudomeloe sp. | 19–22 a | Chewing | Unknown | Neotropic | [7] |
| Lepidoptera: Coleophoridae | |||||
| Coleophora versurella Zeller, 1849 | 32–40 | Chewing | Specialist | Neotropic—Nearctic | [11,24,25] |
| Lepidoptera: Crambidae | |||||
| Achyra similalis (Guenée, 1854) (=Loxostege similalis (Guenée)) | 18–40 b | Chewing | Specialist | Neotropic—Nearctic | [10,11,25] |
| Spoladea recurvalis (Fabricius, 1794) | 18–19 | Chewing | Specialist | Neotropic | [5,7,10] |
| Lepidoptera: Gelechiidae | |||||
| Eurysacca media Povolný, 1986 | 32–34 | Chewing | Specialist | Neotropic | [11,26,27] |
| Eurysacca quinoae Povolný, 1997 | 19 a | Chewing | Specialist | Neotropic | [28] |
| Lepidoptera: Noctuidae | |||||
| Agrotis experta (Walker, 1869) (=Feltia experta (Walker)) | 18–26 | Chewing | Generalist | Neotropic | [5,7,10,16] |
| Agrotis ipsilon (Hufnagel, 1766) | 18–44 c | Chewing | Generalist | Unknown | [5,7,10,16] |
| Agrotis malefida (Guenée, 1852) | 32–56 | Chewing | Generalist | Neotropic | [7,10] |
| Copitarsia spp. (species complex) | 18–56 a,b,c,d | Chewing | Generalist | Neotropic | [5,10,11,12,16,29] |
| Chrysodeixis includens (Walker, 1858) (=Pseudoplusia includens (Walker)) (=Phytometra oo (Cramer)) | 18–26 | Chewing | Generalist | Neotropic—Nearctic | [5,10,12] |
| Feltia subterranea (Fabricius, 1794) (=Agrotis subterranea) | 18–40 b,c | Chewing | Generalist | Neotropic—Nearctic | [5,10] |
| Helicoverpa atacamae Hardwick, 1965 | 18–41 a | Chewing | Generalist | Neotropic | [5,16,30] |
| Helicoverpa gelotopoeon (Dyar, 1921) | 18–40 a | Chewing | Generalist | Neotropic | [5,12,15,30] |
| Helicoverpa zea (Boddie, 1850) | 18–44 | Chewing | Generalist | Neotropic—Nearctic | [5,7,10,12,16,18] |
| Peridroma saucia (Hübner, 1808) | 18–56 | Chewing | Generalist | Unknown | [5,7,10,16] |
| Rachiplusia nu (Guenée, 1852) | 18–44 | Chewing | Generalist | Neotropic—Nearctic | [5,10,11,15] |
| Spodoptera eridania (Stoll, 1782) | 18–33 | Chewing | Generalist | Neotropic—Nearctic | [5,7,10,16] |
| Spodoptera frugiperda (J.E. Smith, 1797) | 18–22 | Chewing | Generalist | Neotropic—Nearctic | [5,7,10,15,16] |
| Spodoptera ochrea (Hampson, 1909) | 18–22 | Chewing | Generalist | Neotropic | [5,10] |
| Trichoplusia ni (Hübner, 1803) | 18–44 b | Chewing | Generalist | Unknown | [12,18] |
| Acari: Tetranychidae | |||||
| Tetranychus urticae Koch, 1836 | 18–44 b | Cell puncturing | Generalist | Unknown | [5,10] |
| Cluster Group | Geographical Limits of Each Cluster (° S) | Species Present within the Delimited Area * |
|---|---|---|
| 1 | 17.5–25.8 | 32 species:Achyra similalis, Agrotis experta, Agrotis ipsilon, Anacuerna centrolinea, Aphis craccivora, Aphis gossypii, Chrysodeixis includens, Copitarsia spp., Eurysacca quinoae, Feltia subterranea, Frankliniella occidentalis, Helicoverpa atacamae, Helicoverpa gelotopoeon, Helicoverpa zea, Liorhyssus hialinus, Liriomyza huidobrensis, Macrosiphum euphorbiae, Myzus persicae, Nezara viridula, Oncopeltus miles, Peridroma saucia, Pseudomeloe sp., Rachiplusia nu, Smynthurodes betae, Spodoptera eridania, Spodoptera frugiperda, Spodoptera ochrea, Spoladea recurvalis, Tetranychus urticae, Thrips tabaci, Trichocyphus rubricollis, Trichoplusia ni |
| 2 | 25.9—32.0 | 27 species:Achyra similalis, Agrotis ipsilon, Aphis craccivora, Aphis gossypii, Copitarsia spp., Dichroplus maculipennis, Feltia subterranea, Frankliniella occidentalis, Helicoverpa atacamae, Helicoverpa gelotopoeon, Helicoverpa zea, Heterotrioza chenopodii, Leptoglossus chilensis, Liorhyssus hialinus, Liorhyssus lineatoventris, Liriomyza huidobrensis, Macrosiphum euphorbiae, Myzus persicae, Nezara viridula, Nysius simulans, Oncopeltus miles, Peridroma saucia, Rachiplusia nu, Spodoptera eridania, Tetranychus urticae, Thrips tabaci, Trichoplusia ni |
| 3 | 32.1—39.5 | 34 species:Achyra similalis, Agrotis ipsilon, Agrotis malefida, Aphis craccivora, Aphis gossypii, Coleophora versurella, Copitarsia spp., Dichroplus maculipennis, Epitrix sp., Eurysacca media, Feltia subterranea, Frankliniella occidentalis, Helicoverpa atacamae, Helicoverpa gelotopoeon, Helicoverpa zea, Heterotrioza chenopodii, Leptoglossus chilensis, Liorhyssus hialinus, Liorhyssus lineatoventris, Liriomyza huidobrensis, Macrosiphum euphorbiae, Myzus persicae, Nezara viridula, Nysius simulans, Oncopeltus miles, Orthotylus flavosparsus, Paratanus exitiosus, Peridroma saucia, Rachiplusia nu, Smynthurodes betae, Spodoptera eridania, Tetranychus urticae, Thrips tabaci, Trichoplusia ni |
| 4 | 39.5—43.7 | 22 species:Achyra similalis, Agrotis ipsilon, Agrotis malefida, Aphis craccivora, Aphis gossypii, Copitarsia spp., Dichroplus maculipennis, Frankliniella occidentalis, Helicoverpa atacamae, Helicoverpa zea, Leptoglossus chilensis, Liorhyssus lineatoventris, Liriomyza huidobrensis, Macrosiphum euphorbiae, Myzus persicae, Nezara viridula, Paratanus exitiosus, Peridroma saucia, Rachiplusia nu, Smynthurodes betae, Tetranychus urticae, Trichoplusia ni |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chorbadjian, R.A.; Ahumada, M.I.; Urra, F.; Elgueta, M.; Gilligan, T.M. Biogeographical Patterns of Herbivore Arthropods Associated with Chenopodium quinoa Grown along the Latitudinal Gradient of Chile. Plants 2021, 10, 2811. https://doi.org/10.3390/plants10122811
Chorbadjian RA, Ahumada MI, Urra F, Elgueta M, Gilligan TM. Biogeographical Patterns of Herbivore Arthropods Associated with Chenopodium quinoa Grown along the Latitudinal Gradient of Chile. Plants. 2021; 10(12):2811. https://doi.org/10.3390/plants10122811
Chicago/Turabian StyleChorbadjian, Rodrigo A., María I. Ahumada, Francisco Urra, Mario Elgueta, and Todd M. Gilligan. 2021. "Biogeographical Patterns of Herbivore Arthropods Associated with Chenopodium quinoa Grown along the Latitudinal Gradient of Chile" Plants 10, no. 12: 2811. https://doi.org/10.3390/plants10122811
APA StyleChorbadjian, R. A., Ahumada, M. I., Urra, F., Elgueta, M., & Gilligan, T. M. (2021). Biogeographical Patterns of Herbivore Arthropods Associated with Chenopodium quinoa Grown along the Latitudinal Gradient of Chile. Plants, 10(12), 2811. https://doi.org/10.3390/plants10122811







