The Role of Outer Membrane Protein(s) Harboring SLH/OprB-Domains in Extracellular Vesicles’ Production in Synechocystis sp. PCC 6803
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Genome of Synechocystis Harbors Six Genes Putatively Encoding SLH/OprB-Domains Containing Proteins
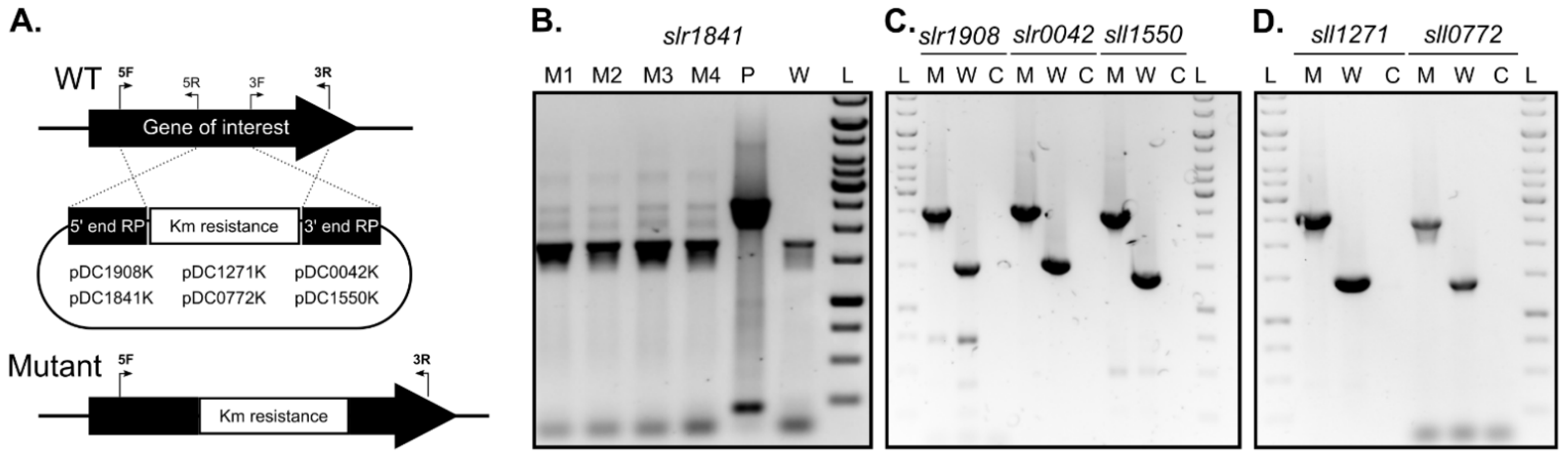
2.2. slr1841 Encodes an Essential Protein, While the Remaining SLH/OprB-Domain Containing Outer Membrane Proteins Are Not Essential for Growth
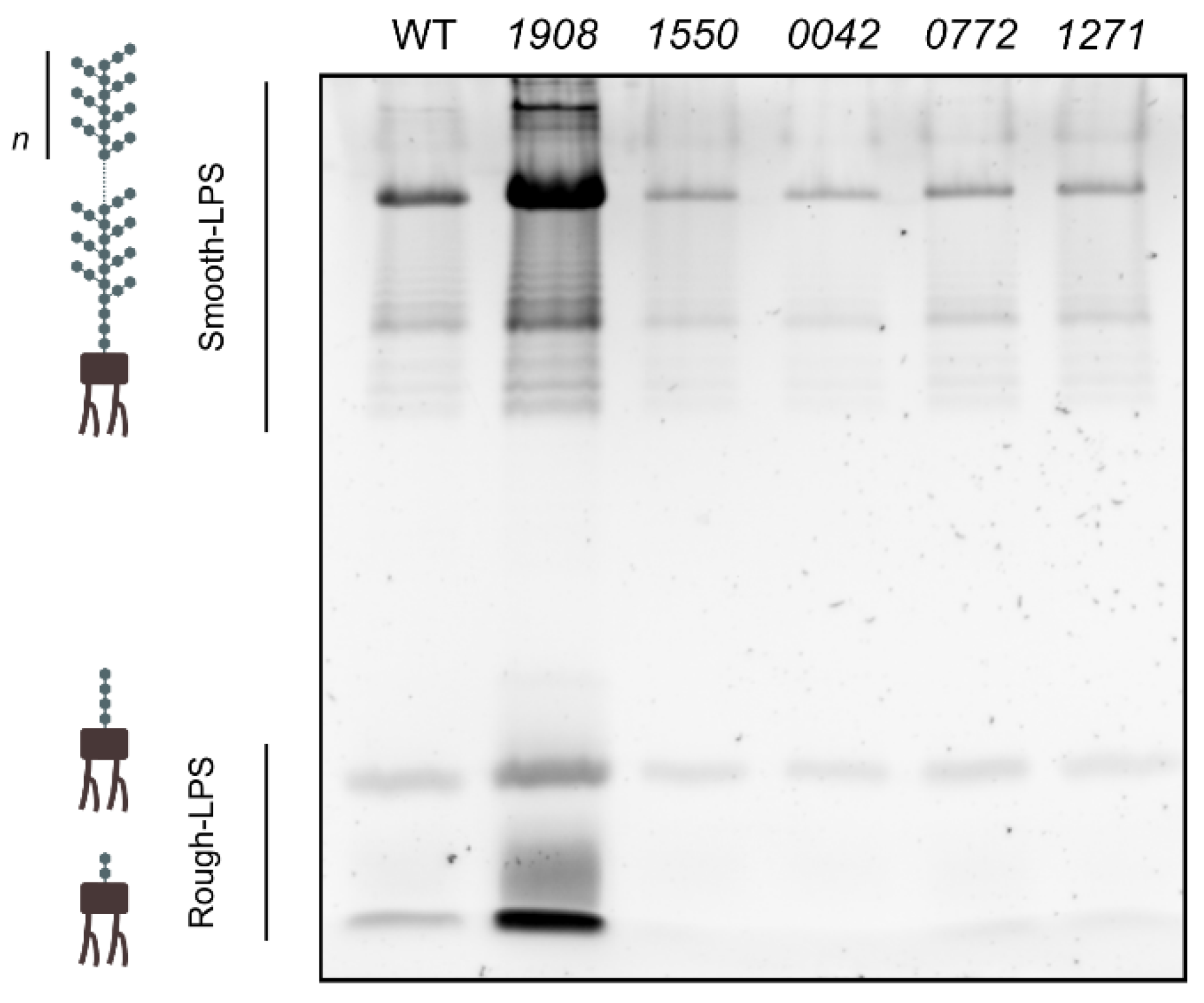
2.3. Disruption of slr1908 Results in Adjustments in the Outer Membrane and Extracellular Protein Composition Related to Iron Uptake
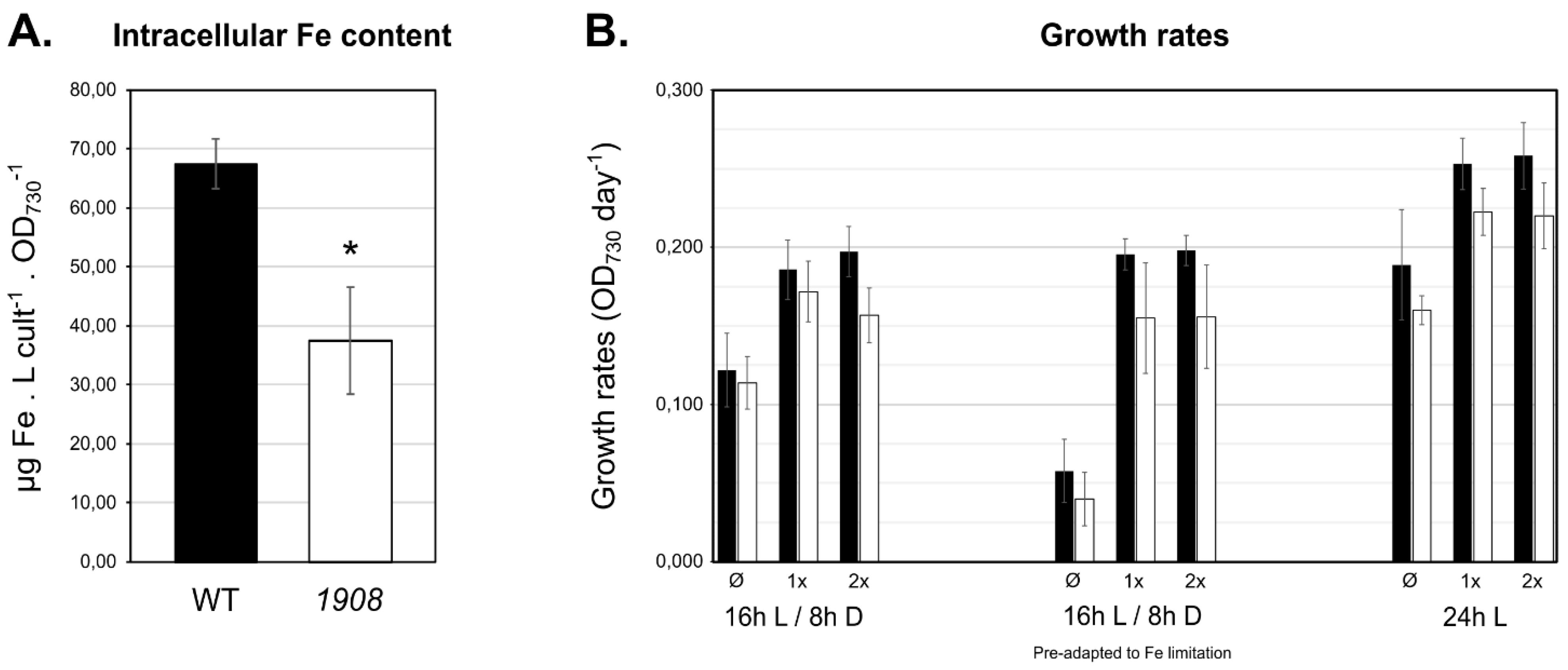
2.4. Mutant Strain 1908 Shows Significantly Lower Intracellular Iron Content
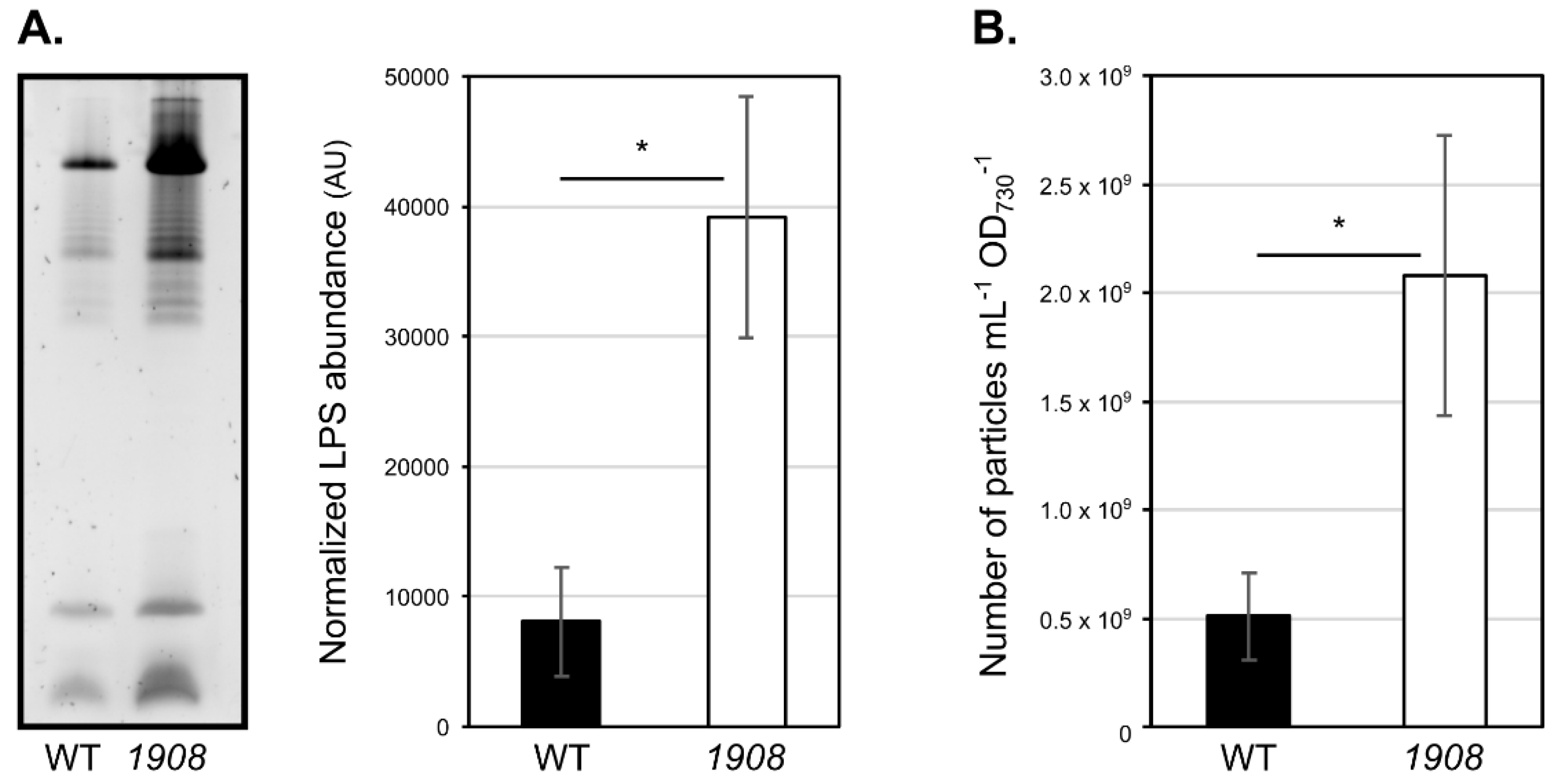
2.5. Disruption of slr1908 Results in Hypervesiculation
3. Conclusions and Future Perspectives
4. Materials and Methods
4.1. Strains and Maintenance Conditions
4.2. In Silico Identification of Genes Encoding SLH/OprB-Domain Containing Proteins
4.3. Isolation and Analysis of Synechocystis Outer Membrane Proteins
4.4. Protein Identification by NanoLC-MS/MS
4.5. Mutant Generation
4.6. Analysis of Synechocystis Wild-Type and Mutant Strains’ Growth Capacities
4.7. Quantification of Intracellular Iron Levels by Atomic Absorption Spectrometry
4.8. Isolation, Concentration and Analysis of Cyanobacterial Extracellular Media
4.9. Isolation and Characterization of Cyanobacterial Extracellular Vesicles
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coelho, C.; Casadevall, A. Answers to naysayers regarding microbial extracellular vesicles. Biochem. Soc. Trans. 2019, 47, 1005–1012. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Kulp, A.; Kuehn, M.J. Biological Functions and Biogenesis of Secreted Bacterial Outer Membrane Vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Genet. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haurat, M.F.; Aduse-Opoku, A.; Rangarajan, M.; Dorobantu, L.; Gray, M.; A Curtis, M.; Feldman, M.F. Selective Sorting of Cargo Proteins into Bacterial Membrane Vesicles. J. Biol. Chem. 2011, 286, 1269–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakoff-Nahoum, S.; Coyne, M.J.; Comstock, L.E. An Ecological Network of Polysaccharide Utilization among Human Intestinal Symbionts. Curr. Biol. 2014, 24, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, S.; Matinha-Cardoso, J.; Tamagnini, P.; Oliveira, P. Extracellular Vesicles: An Overlooked Secretion System in Cyano-bacteria. Life 2020, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.B.; Garg, S.G.; Martin, W.F. Bacterial Vesicle Secretion and the Evolutionary Origin of the Eukaryotic Endomembrane System. Trends Microbiol. 2016, 24, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.; Nelson-Sathi, S.; Roettger, M.; Sousa, F.L.; Lockhart, P.J.; Bryant, D.; Hazkani-Covo, E.; McInerney, J.O.; Landan, G.; Martin, W.F. Endosymbiotic origin and differential loss of eukaryotic genes. Nat. Cell Biol. 2015, 524, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincón, J.; Zabala, L.L.; Jiao, N.; Karl, D.M.; Li, W.K.W.; Lomas, M.; Veneziano, D.; et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biller, S.J.; Schubotz, F.; Roggensack, S.E.; Thompson, A.W.; Summons, R.E.; Chisholm, S.W. Bacterial Vesicles in Marine Ecosystems. Science 2014, 343, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Hoiczyk, E.; Hansel, A. Cyanobacterial Cell Walls: News from an Unusual Prokaryotic Envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Hedman, E.; Funk, C.; Kieselbach, T.; Schröder, W.P.; Norling, B. Isolation of Outer Membrane of Synechocystis sp. PCC 6803 and Its Proteomic Characterization. Mol. Cell. Proteom. 2004, 3, 586–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, S.; Muramoto, K.; Kusano, T. Outer Membrane Proteins Derived from Non-cyanobacterial Lineage Cover the Pepti-doglycan of Cyanophora paradoxa Cyanelles and Serve as a Cyanelle Diffusion Channel. J. Biol. Chem. 2016, 291, 20198–20209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowata, H.; Tochigi, S.; Takahashi, H.; Kojima, S. Outer Membrane Permeability of Cyanobacterium Synechocystis sp. Strain PCC 6803: Studies of Passive Diffusion of Small Organic Nutrients Reveal the Absence of Classical Porins and Intrinsically Low Permeability. J. Bacteriol. 2017, 199, e00371-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schätzle, H.; Brouwer, E.-M.; Liebhart, E.; Stevanovic, M.; Schleiff, A.E. Comparative Phenotypic Analysis of Anabaena sp. PCC 7120 Mutants of Porin-like Genes. J. Microbiol. Biotechnol. 2021, 31, 645–658. [Google Scholar] [CrossRef]
- Kopf, M.; Klähn, S.; Scholz, I.; Matthiessen, J.K.; Hess, W.; Voß, B. Comparative Analysis of the Primary Transcriptome of Synechocystis sp. PCC 6803. DNA Res. 2014, 21, 527–539. [Google Scholar] [CrossRef]
- Qiu, G.; Jiang, H.; Lis, H.; Li, Z.; Deng, B.; Shang, J.; Sun, C.; Keren, N.; Qiu, B. A unique porin meditates iron-selective transport through cyanobacterial outer membranes. Environ. Microbiol. 2021, 23, 376–390. [Google Scholar] [CrossRef]
- Katoh, H.; Hagino, N.; Grossman, A.R.; Ogawa, T. Genes Essential to Iron Transport in the Cyanobacterium Synechocystis sp. Strain PCC 6803. J. Bacteriol. 2001, 183, 2779–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noinaj, N.; Guillier, M.; Barnard, T.J.; Buchanan, S.K. TonB-Dependent Transporters: Regulation, Structure, and Function. Annu. Rev. Microbiol. 2010, 64, 43–60. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.-W.; Lou, W.-J.; Sun, C.-Y.; Yang, N.; Li, Z.-K.; Li, D.-L.; Zang, S.-S.; Fu, F.-X.; Hutchins, D.A.; Jiang, H.-B.; et al. Outer Membrane Iron Uptake Pathways in the Model Cyanobacterium Synechocystis sp. Strain PCC 6803. Appl. Environ. Microbiol. 2018, 84, 01512–01518. [Google Scholar] [CrossRef] [Green Version]
- Badarau, A.; Firbank, S.J.; Waldron, K.; Yanagisawa, S.; Robinson, N.; Banfield, M.; Dennison, C. FutA2 Is a Ferric Binding Protein from Synechocystis PCC 6803. J. Biol. Chem. 2008, 283, 12520–12527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Parmryd, I.; Nilsson, F.; Persson, A.L.; Pakrasi, H.B.; Andersson, B.; Norling, B. Proteomics of Synechocystis sp. Strain PCC 6803. Mol. Cell. Proteom. 2002, 267, 956–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tölle, J.; Michel, K.-P.; Kruip, J.; Kahmann, U.; Preisfeld, A.; Pistorius, E.K. Localization and function of the IdiA homologue Slr1295 in the cyanobacterium Synechocystis sp. strain PCC 6803. Microbiology 2002, 148, 3293–3305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Prieto, M.A.; Semeniuk, T.A.; Giner, J.; Futschik, M.E. The Transcriptional Landscape of the Photosynthetic Model Cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2016, 6, 22168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rosa, E.; Checchetto, V.; Franchin, C.; Bergantino, E.; Berto, P.; Szabò, I.; Giacometti, G.M.; Arrigoni, G.; Costantini, P. [NiFe]-hydrogenase is essential for cyanobacterium Synechocystis sp. PCC 6803 aerobic growth in the dark. Sci. Rep. 2015, 5, 12424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, C.F.; Pacheco, C.C.; Tamagnini, P.; Oliveira, P. Identification of inner membrane translocase components of TolC-mediated secretion in the cyanobacterium Synechocystis sp. PCC 6803. Environ. Microbiol. 2018, 20, 2354–2369. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Martins, N.M.; Santos, M.; Pinto, F.; Büttel, Z.; Couto, N.A.S.; Wright, P.C.; Tamagnini, P. The versatile TolC-like Slr1270 in the cyanobacterium Synechocystis sp. PCC 6803. Environ. Microbiol. 2016, 18, 486–502. [Google Scholar] [CrossRef]
- Pardo, Y.A.; Florez, C.; Baker, K.M.; Schertzer, J.W.; Mahler, G.J. Detection of outer membrane vesicles in Synechocystis PCC 6803. FEMS Microbiol. Lett. 2015, 362, 163. [Google Scholar] [CrossRef] [Green Version]
- Prados-Rosales, R.; Weinrick Brian, C.; Piqué Daniel, G.; Jacobs William, R.; Casadevall, A.; Rodriguez, G.M. Role for Myco-bacterium tuberculosis Membrane Vesicles in Iron Acquisition. J. Bacteriol. 2014, 196, 1250–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesnage, S.; Fontaine, T.; Mignot, T.; Delepierre, M.; Mock, M.; Fouet, A. Bacterial SLH domain proteins are non-covalently anchored to the cell surface via a conserved mechanism involving wall polysaccharide pyruvylation. EMBO J. 2000, 19, 4473–4484. [Google Scholar] [CrossRef] [Green Version]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Carlone, G.M.; Thomas, M.L.; Rumschlag, H.S.; O Sottnek, F. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J. Clin. Microbiol. 1986, 24, 330–332. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.L.; Allen, R.; Luo, Y.; Curtiss, R., III. Export of Extracellular Polysaccharides Modulates Adherence of the Cyano-bacterium Synechocystis. PLoS ONE 2013, 8, e74514. [Google Scholar] [CrossRef] [Green Version]
- Heidorn, T.; Camsund, D.; Huang, H.-H.; Lindberg, P.; Oliveira, P.; Stensjö, K.; Lindblad, P. Chapter Twenty-Four -Synthetic Biology in Cyanobacteria: Engineering and Analyzing Novel Functions. In Methods in Enzymology; Voigt, C., Ed.; Academic Press: London, UK, 2011; Volume 497, pp. 539–579. [Google Scholar] [CrossRef]
- Oliveira, P.; Pinto, F.; Pacheco, C.C.; Mota, R.; Tamagnini, P. HesF, an exoprotein required for filament adhesion and aggregation in Anabaena sp. PCC 7120. Environ. Microbiol. 2015, 17, 1631–1648. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, D.; Lima, S.; Matinha-Cardoso, J.; Tamagnini, P.; Oliveira, P. The Role of Outer Membrane Protein(s) Harboring SLH/OprB-Domains in Extracellular Vesicles’ Production in Synechocystis sp. PCC 6803. Plants 2021, 10, 2757. https://doi.org/10.3390/plants10122757
Cardoso D, Lima S, Matinha-Cardoso J, Tamagnini P, Oliveira P. The Role of Outer Membrane Protein(s) Harboring SLH/OprB-Domains in Extracellular Vesicles’ Production in Synechocystis sp. PCC 6803. Plants. 2021; 10(12):2757. https://doi.org/10.3390/plants10122757
Chicago/Turabian StyleCardoso, Delfim, Steeve Lima, Jorge Matinha-Cardoso, Paula Tamagnini, and Paulo Oliveira. 2021. "The Role of Outer Membrane Protein(s) Harboring SLH/OprB-Domains in Extracellular Vesicles’ Production in Synechocystis sp. PCC 6803" Plants 10, no. 12: 2757. https://doi.org/10.3390/plants10122757
APA StyleCardoso, D., Lima, S., Matinha-Cardoso, J., Tamagnini, P., & Oliveira, P. (2021). The Role of Outer Membrane Protein(s) Harboring SLH/OprB-Domains in Extracellular Vesicles’ Production in Synechocystis sp. PCC 6803. Plants, 10(12), 2757. https://doi.org/10.3390/plants10122757





