Biosafe Management of Botrytis Grey Mold of Strawberry Fruit by Novel Bioagents
Abstract
1. Introduction
2. Results
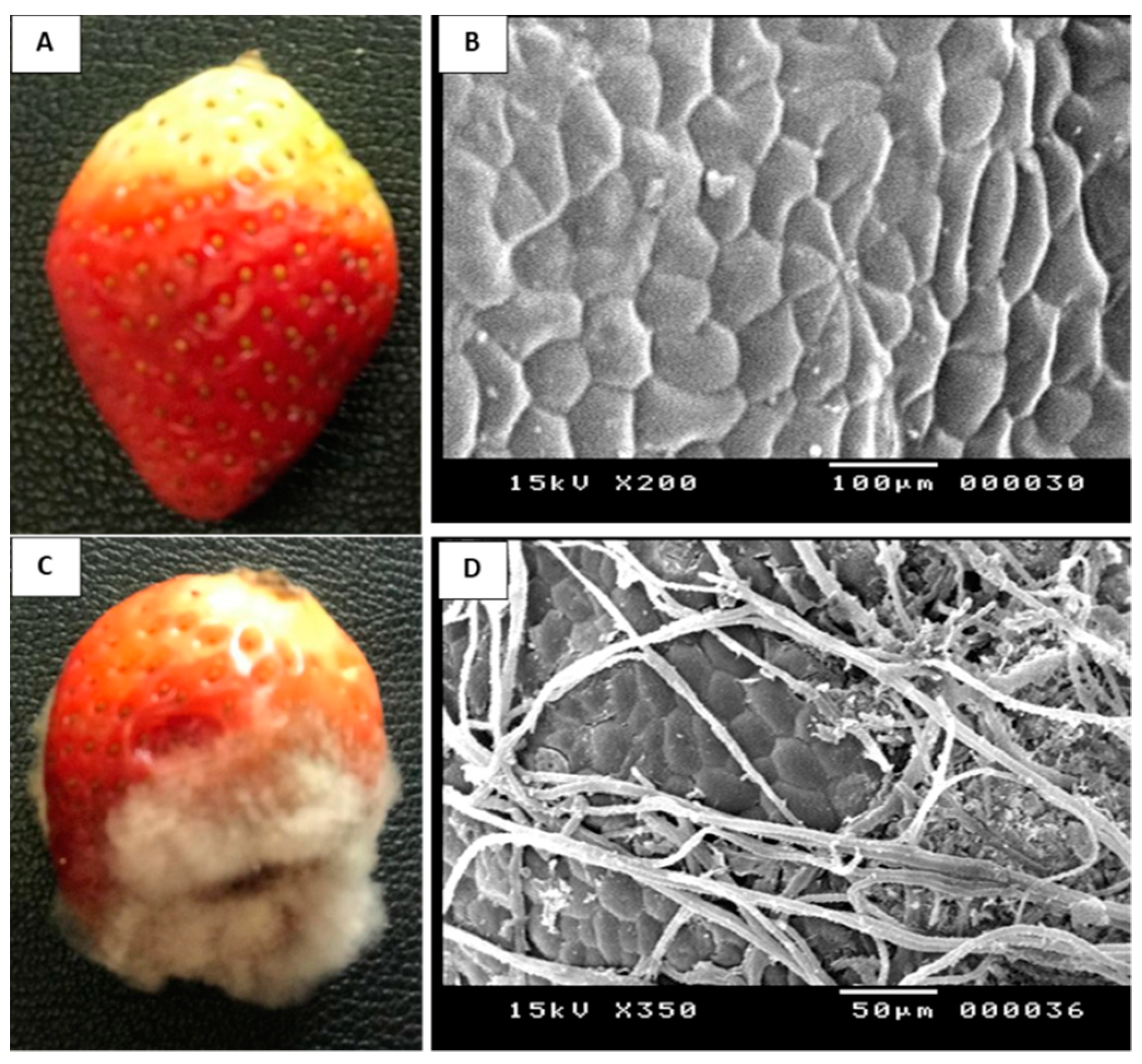
2.1. Identification and Pathogenicity of the Fungal Pathogen
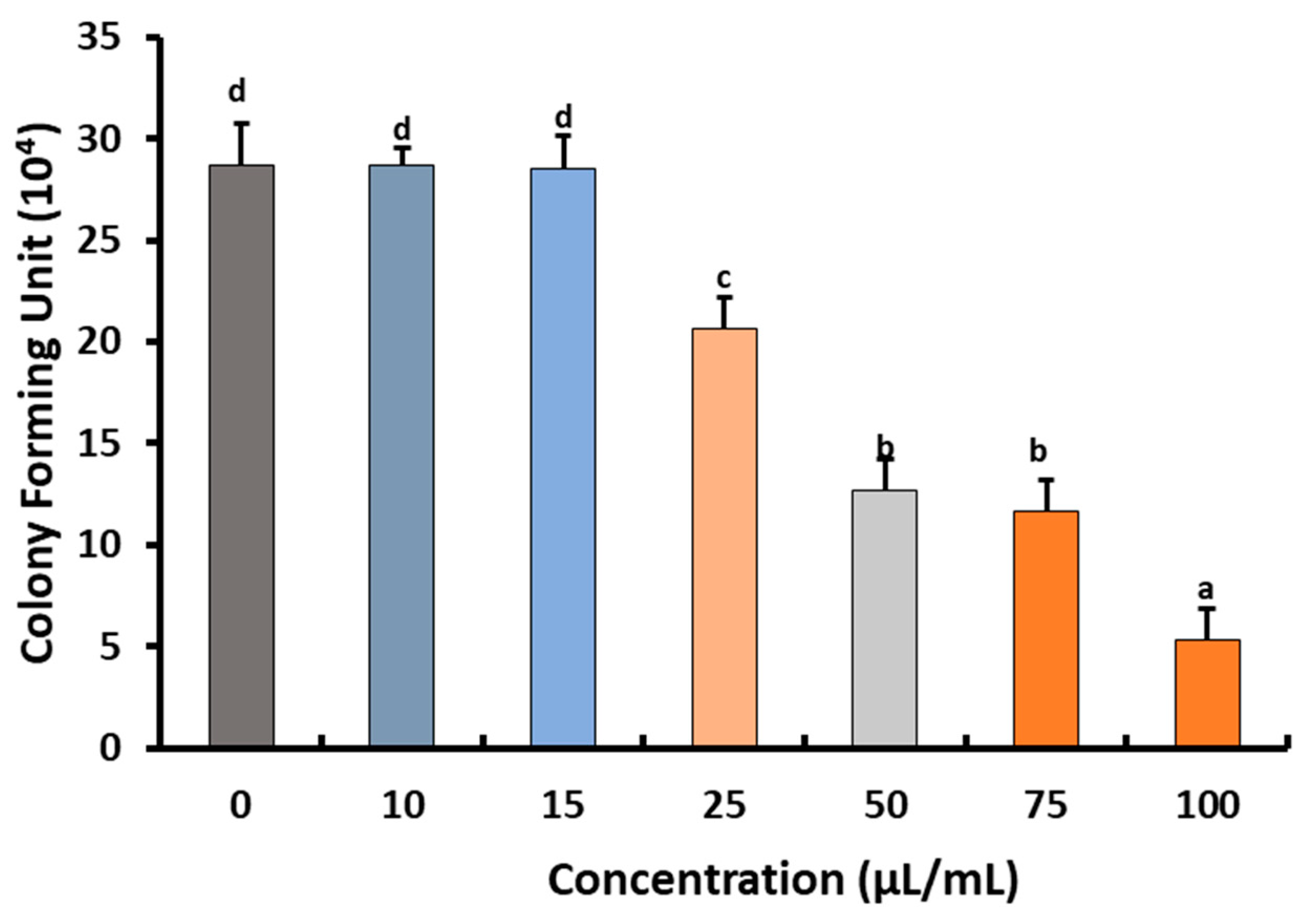
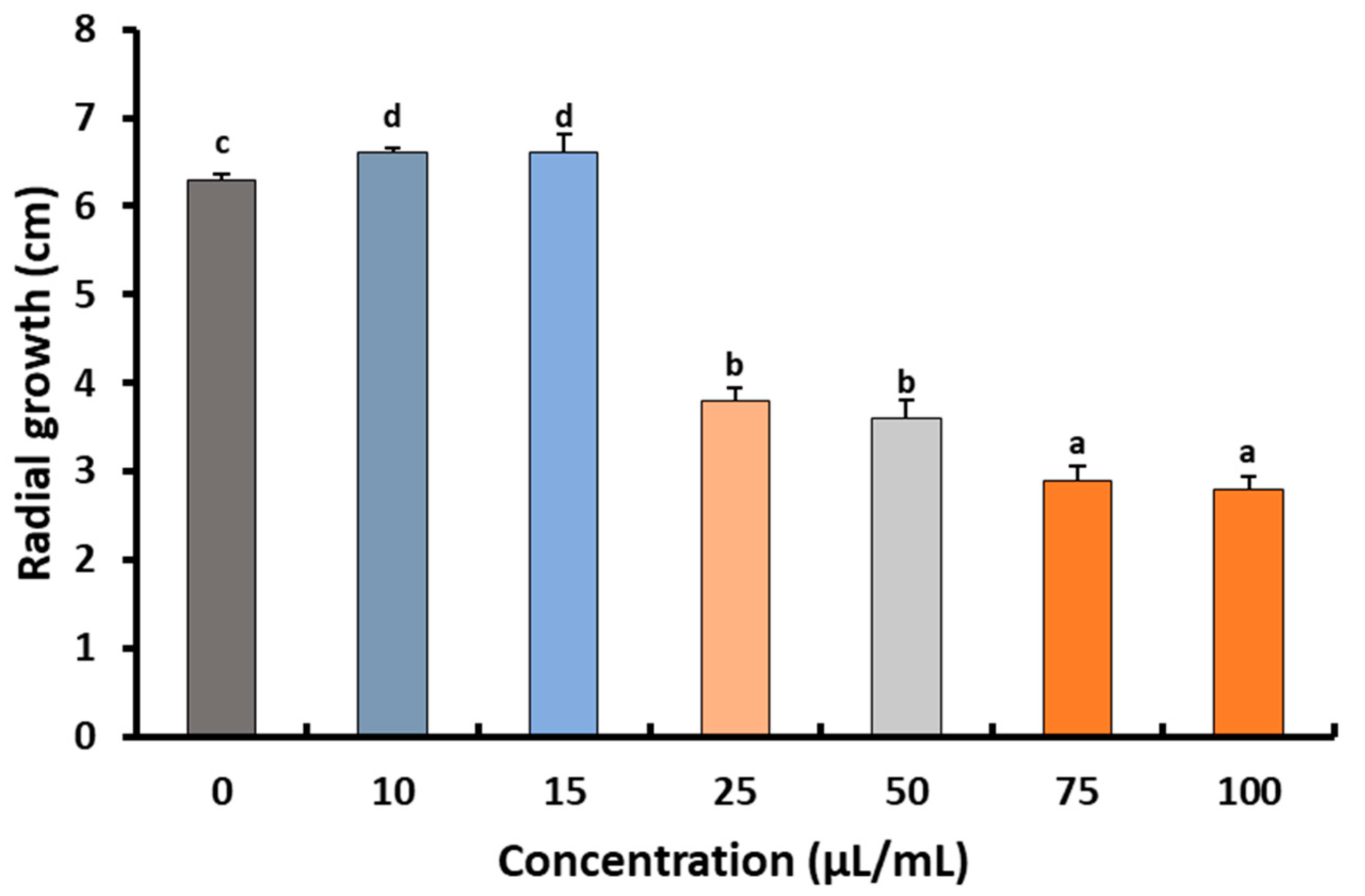
2.2. Fungicidal Effect of Preen Oil against B. cinerea Str5
2.3. Chemical Analysis of the Preen Oil Extract
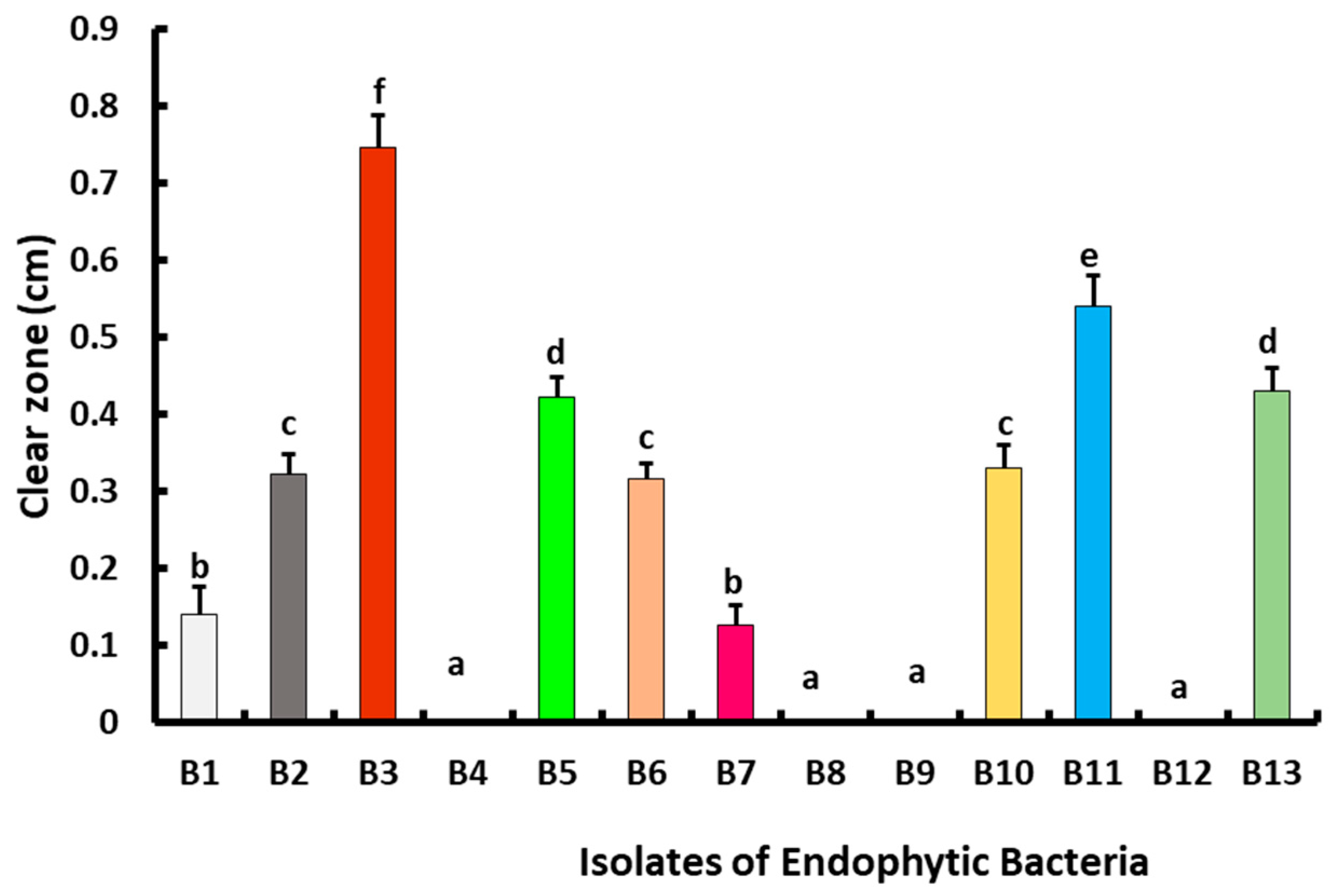
2.4. Screening for Antagonistic Activity of Endophytic Bacteria against B. cinerea Str5 Growth In Vitro
2.5. Hydrolytic Enzyme Activity of Antagonistic Bacteria
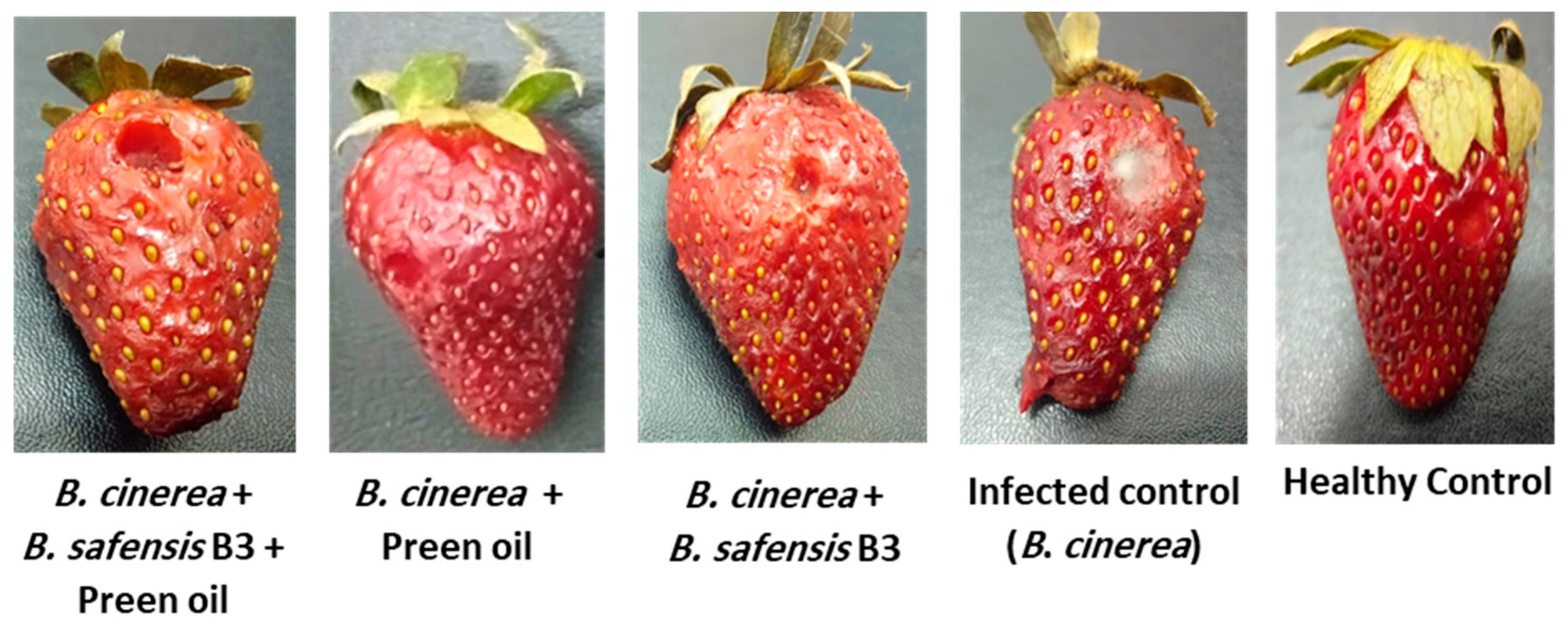
2.6. Efficiency of B. safensis B3 and/or Preen Oil in the Control of Postharvest B. cinerea Str5 Grey Mold on Strawberries, and Consequent Impact on the Fruit
2.6.1. Disease Severity
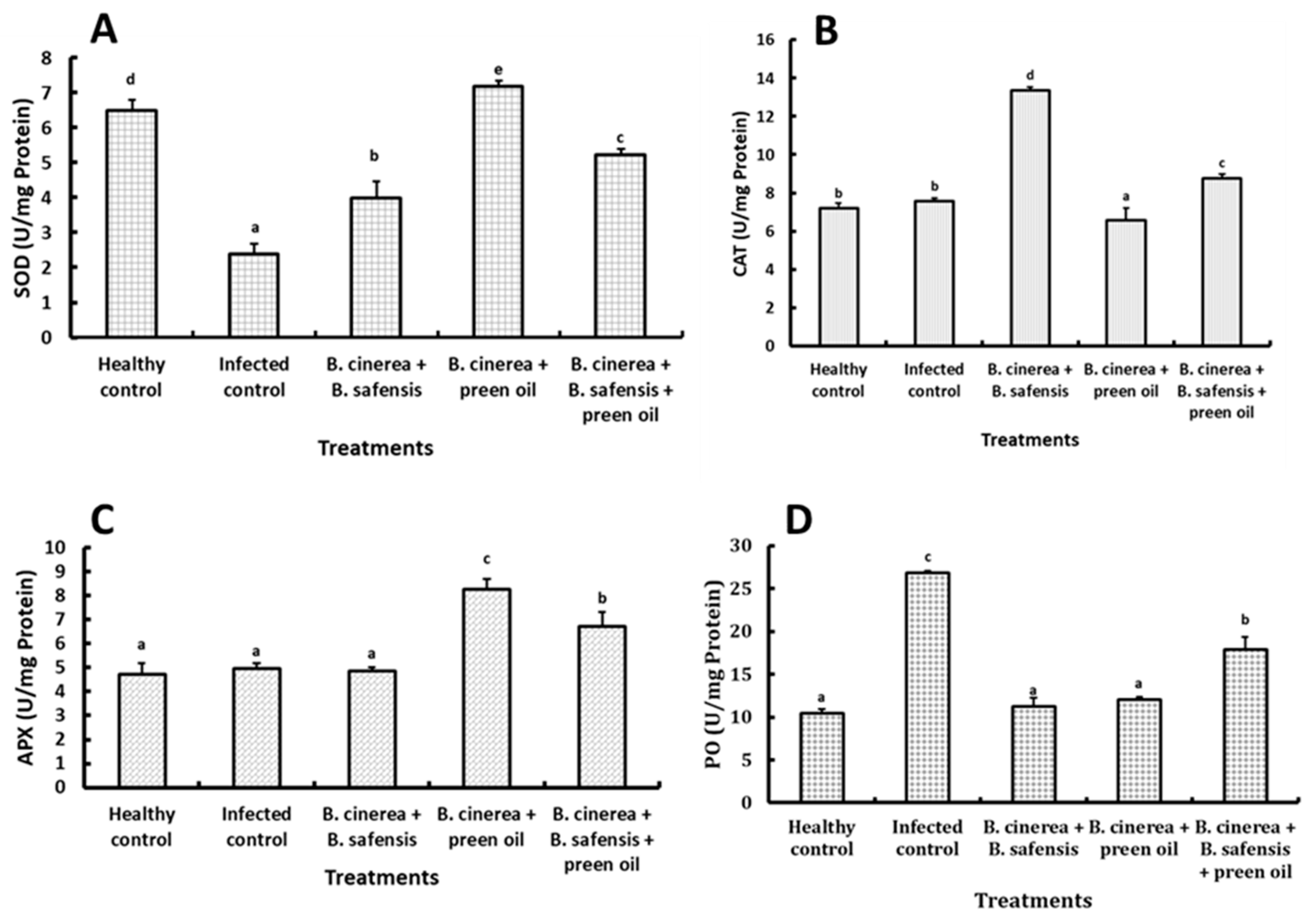
2.6.2. Biochemical Analysis
Total Phenolics
Antioxidant Enzymes
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Isolation of Fungal Pathogen
4.3. Assay for Pathogenicity Test of B. cinerea Str5 on Strawberry Fruit
4.4. Scanning Electron Microscopy Examination
4.5. Preparation and Assay of Biocontrol Agents
4.5.1. Preen Oil
Extraction and Fungicidal Activity of Preen Oil
Effects of Preen Oil on the Fungal Pathogen (Linear Growth and Fungal Colony-Forming Units)
Analysis of Preen Oil Composition
4.5.2. Antagonistic Bacteria
Isolation of the Endophytic Bacteria
Assay for Antagonistic Activity
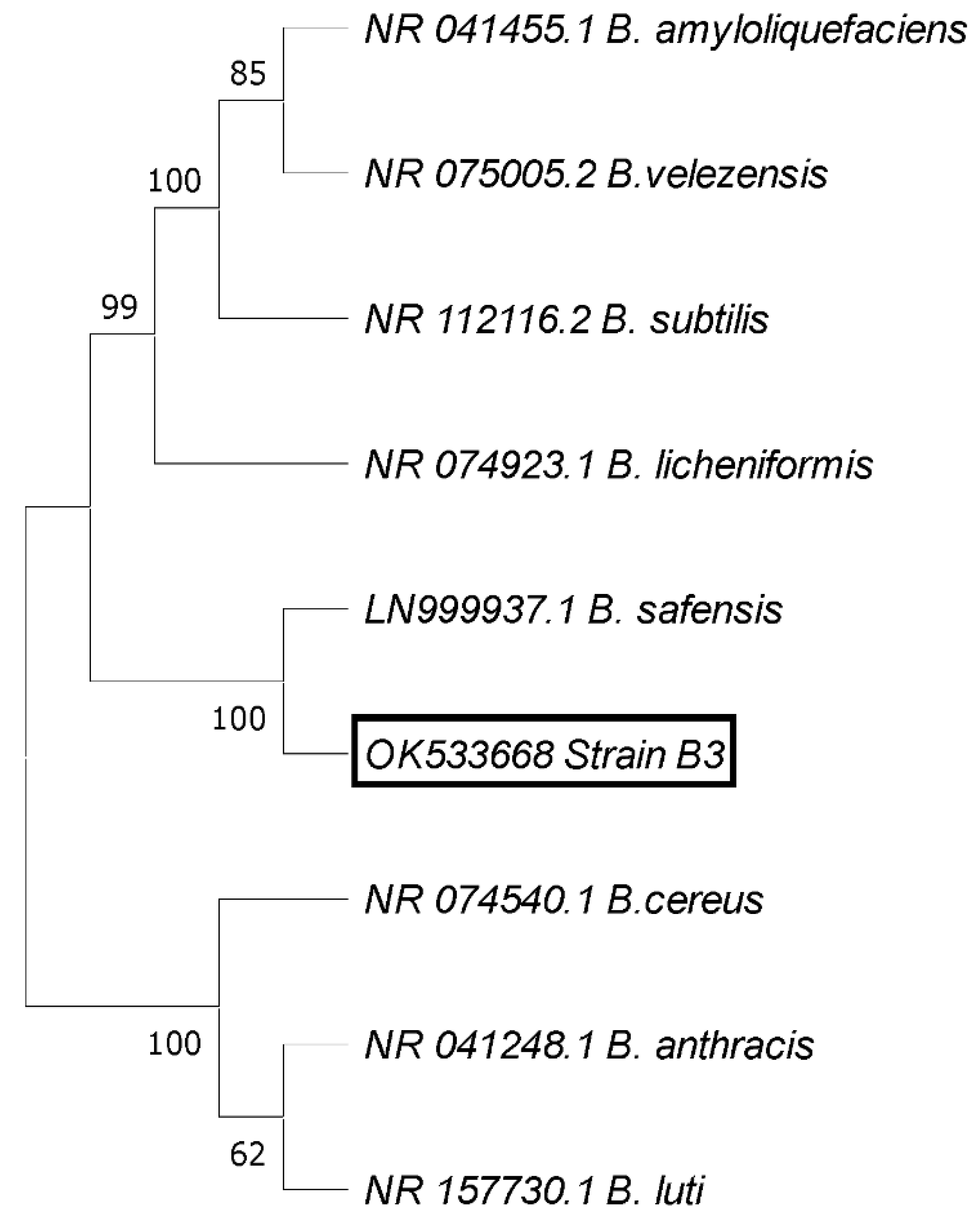
Identification of the Most Antagonistic Endophytic Bacterial Isolate
Assay of Hydrolytic Activity of the Most Antagonistic Bacteria
- Determination of chitinase activity
- b.
- Determination of protease activity
- c.
- Determination of lipase activity
4.6. Biocontrol Efficiency of B. safensis B3 and Preen Oil against Postharvest Botrytis Grey Mold on Strawberries In Vivo
4.6.1. Assay of Disease Severity
4.6.2. Biochemical Analysis of the Inoculated Strawberry Fruit
- Total phenol content
- b.
- Antioxidant enzymes
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, H.; Wei, Y.; Wang, X.; Xu, C.; Shao, X. The marine yeast Sporidiobolus pararoseus ZMY-1 has antagonistic properties against Botrytis cinerea in vitro and in strawberry fruit. Postharvest Biol. Technol. 2019, 150, 1–8. [Google Scholar] [CrossRef]
- Bhaskara Reddy, M.; Belkacemi, K.; Corcuff, R.; Castaigne, F.; Arul, J. Effect of pre-harvest chitosan sprays on post-harvest infection by Botrytis cinerea and quality of strawberry fruit. Postharvest Biol. Technol. 2000, 20, 39–51. [Google Scholar] [CrossRef]
- Schestibratov, K.A.; Dolgov, S.V. Transgenic strawberry plants expressing a thaumatin II gene demonstrate enhanced resistance to Botrytis cinerea. Sci. Hortic 2005, 106, 177–189. [Google Scholar] [CrossRef]
- Barakat, R.M.; Al-Masri, M.I. Effect of Trichoderma harzianum in combination with fungicides in controlling grey mould disease (Botrytis cinerea) of strawberry. Am. J. Plant Sci. 2017, 8, 651. [Google Scholar] [CrossRef][Green Version]
- Rasiukevičiūtė, N.; Rugienius, R.; Šikšnianienė, J.B. Genetic diversity of Botrytis cinerea from strawberry in Lithuania. Zemdirbyste-Agric. 2018, 105, 265–270. [Google Scholar] [CrossRef]
- Rosero-Hernandez, E.D.; Moraga, J.; Collado, I.G.; Echeverri, F. Natural compounds that modulate the development of the fungus Botrytis cinerea and protect Solanum lycopersicum. Plants 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Vorotnikova, E.; Van Sickle, J.J.; Borisova, T. The Economic value of the precision disease management system for anthracnose and Botrytis fruit rot for the Florida strawberry industry. In Proceedings of the Southern Agricultural Economics Association Annual Meeting, Birmingham, Alabama, 4–7 February 2012; p. 20. [Google Scholar]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)—Prospects and challenges. Biocontrol. Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L.; Agraria, D.; Mediterranea, U.; Feo, L.; Calabria, R. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Wang, X.; Shi, J.; Wang, R. Effect of Burkholderia contaminans on postharvest diseases and induced resistance of strawberry fruits. Plant Pathol. J. 2018, 34, 403–411. [Google Scholar] [CrossRef]
- Minova, S.; Seskena, R.; Voitkane, S.; Metla, Z.; Daugavietis, M.; Jankevica, L. Impact of pine (Pinus sylvestris L.) and spruce (Picea abies (L.) Karst.) bark extracts on important strawberry pathogens. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2015, 69, 62–67. [Google Scholar] [CrossRef]
- Mirmajlessi, S.M.; Mand, M.; Najdabbasi, N.; Larena, I.; Loit, E. Screening of native Trichoderma harzianum isolates for their ability to control Verticillium wilt of strawberry. Zemdirbyste-Agric. 2016, 103, 397–404. [Google Scholar] [CrossRef]
- Valiuškaitė, A.; Uselis, N.; Kviklys, D.; Lanauskas, J.; Rasiukevičiūtė, N. The effect of sustainable plant protection and apple tree management on fruit quality and yield. Zemdirbyste-Agric. 2017, 104, 353–358. [Google Scholar] [CrossRef]
- Gilles, M.; Zhao, J.; An, M.; Agboola, S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 2010, 119, 731–737. [Google Scholar] [CrossRef]
- Czirják, G.Á.; Pap, P.L.; Vágási, C.I.; Giraudeau, M.; Mureşan, C.; Mirleau, P.; Heeb, P. Preen gland removal increases plumage bacterial load but not that of feather-degrading bacteria. Naturwissenschaften 2013, 100, 145–151. [Google Scholar] [CrossRef]
- Carneiro, D.; Czirják, G.Á.; Rowe, M. Innate and adaptive immune proteins in the preen gland secretions of male house sparrows. J. Avian Biol. 2020, 51, 51. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Chen, P.H.; Chen, R.Y.; Chou, J.Y. Screening and evaluation of yeast antagonists for biological control of Botrytis cinerea on strawberry fruits. Mycobiology 2018, 46, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahim, I.R.; Abo-Elyousr, K.A.M. Using of endophytic Saccharomycopsis fibuligera and thyme oil for management of grey mold rot of guava fruits. Biol. Control. 2017, 110, 124–131. [Google Scholar] [CrossRef]
- Wallace, R.L.; Hirkala, D.L.; Nelson, L.M. Mechanisms of action of three isolates of Pseudomonas fluorescens active against postharvest grey mold decay of apple during commercial storage. Biol. Control. 2018, 117, 13–20. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. plant path. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Hewitt, H.G. Fungicides in Crop Protection; CAB International: New York, NY, USA, 1988. [Google Scholar]
- Mehrotra, R.S.; Aneja, K.R.; Aggarwal, A. Fungal control agents. In Environmentally Safe Approaches to Disease Control; Lewis Publishers: New York, NY, USA, 1997; pp. 111–137. [Google Scholar]
- Alt, G.; Mägi, M.; Lodjak, J.; Mänd, R. Experimental study of the effect of preen oil against feather bacteria in passerine birds. Oecologia 2020, 192, 723–733. [Google Scholar] [CrossRef]
- Magallanes, S.; Møller, A.P.; García-Longoria, L.; de Lope, F.; Marzal, A. Volume and antimicrobial activity of secretions of the uropygial gland are correlated with malaria infection in house sparrows. Parasites Vectors 2016, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.S.; Sporer, F.; Zimmermann, S.; Wink, M. Birds, feather-degrading bacteria and preen glands: The antimicrobial activity of preen gland secretions from turkeys (Meleagris gallopavo) is amplified by keratinase. FEMS Microbiol. Ecol. 2018, 94, 117. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.E.; Sand, J.; Olson, J.M.; Barry, T.P. Wisconsin Alumni Research Foundation: Compositions containing preen oil and methods of use thereof. U.S. Patent 10,675,241, 9 June 2020. [Google Scholar]
- Kumar, A.; Shukla, R.; Singh, P.; Prasad, C.S.; Dubey, N.K. Assessment of Thymus vulgaris L. essential oil as a safe botanical preservative against post harvest fungal infestation of food commodities. Innov. Food Sci. Emerg. Technol. 2008, 9, 575–580. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Antifungal activities of thyme, clove and oregano essential oils. J. Food Saf. 2007, 27, 91–101. [Google Scholar] [CrossRef]
- Lattanzio, V.; Di Venere, D.; Linsalata, V.; Lima, G.; Ippolito, A.; Salerno, M. Antifungal activity of 2, 5-dimethoxybenzoic acid on postharvest pathogens of strawberry fruits. Postharvest Biol. Technol. 1996, 9, 325–334. [Google Scholar] [CrossRef]
- Hussein, M.M.; Abo-Elyousr, K.A.; Hassan, M.A.; Hashem, M.; Hassan, E.A.; Alamri, S.A. Induction of defense mechanisms involved in disease resistance of onion blight disease caused by Botrytis allii. Egypt J. Biol Pest Control 2018, 28, 1–11. [Google Scholar] [CrossRef]
- Avis, T.J. Antifungal compounds that target fungal membranes: Applications in plant disease control. Can. J. Plant Pathol. 2007, 29, 323–329. [Google Scholar] [CrossRef]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Dukare, A.; Paul, S. Effect of chitinolytic biocontrol bacterial inoculation on soil microbiological activities and Fusarium population in rhizophere of Pigeon pea (Cajanus cajan). Ann. Plant Prot. Sci. 2018, 26, 98–103. [Google Scholar] [CrossRef]
- Suman, A.; Yadav, A.N.; Verma, P. Endophytic microbes in crops: Diversity and beneficial impact for sustainable agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; pp. 117–143. [Google Scholar]
- Gladieux, P. What makes a specialized endophyte special. Mol. Ecol. 2018, 27, 3037–3039. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M. The fruit microbiome: A new frontier for postharvest biocontrol and postharvest biology. Postharvest Biol. Technol. 2018, 140, 107–112. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, J.; Fu, Y.; Cheng, J.; Qu, Z.; Zhao, Z.; Cheng, S.; Chen, T.; Li, B.; Wang, Q.; et al. A 2-kb mycovirus converts a pathogenic fungus into a beneficial endophyte for brassica protection and yield enhancement. Mol. Plant 2020, 13, 1420–1433. [Google Scholar] [CrossRef]
- Wu, T.; Xu, J.; Liu, J.; Guo, W.H.; Li, X.B.; Xia, J.B.; Xie, W.J.; Yao, Z.G.; Zhang, Y.M.; Wang, R.Q. Characterization and initial application of endophytic Bacillus safensis strain ZY16 for improving phytoremediation of oil-contaminated saline soils. Front. Microbiol. 2019, 10, 991. [Google Scholar] [CrossRef] [PubMed]
- Branquinho, R.; Meirinhos-Soares, L.; Carriço, J.A.; Pintado, M.; Peixe, L.V. Phylogenetic and clonality analysis of Bacillus pumilus isolates uncovered a highly heterogeneous population of different closely related species and clones. FEMS Microbiol. Ecol. 2014, 90, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, A.; Romero, D.; De Vicente, A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef]
- Lateef, A.; Adelere, I.A.; Gueguim-Kana, E.B. The biology and potential biotechnological applications of Bacillus safensis. Biologia 2015, 70, 411–419. [Google Scholar] [CrossRef]
- Ting, A.S.Y.; Chai, J.Y. Chitinase and β-1, 3-glucanase activities of Trichoderma harzianum in response towards pathogenic and non-pathogenic isolates: Early indications of compatibility in consortium. Biocatal. Agric. Biotechnol. 2015, 4, 109–113. [Google Scholar] [CrossRef]
- Schönbichler, A.; Díaz-Moreno, S.M.; Srivastava, V.; McKee, L.S. Exploring the potential for fungal antagonism and cell wall attack by Bacillus subtilis natto. Front. Microbiol. 2020, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Sipos, B.; Benkő, Z.; Dienes, D.; Réczey, K.; Viikari, L.; Siika-aho, M. Characterisation of specific activities and hydrolytic properties of cell-wall-degrading enzymes produced by Trichoderma reesei Rut C30 on different carbon sources. Appl. Biochem. Biotechnol. 2010, 161, 347–364. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef]
- Abdel-Rahim, I.R.; Abo-Elyousr, K.A. Talaromyces pinophilus strain AUN-1 as a novel mycoparasite of Botrytis cinerea, the pathogen of onion scape and umbel blights. Microbiol. Res. 2018, 212, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, S.; Gao, Y.; Hu, W.; Hu, M.; Zhong, G. Isolation of a novel beta-cypermethrin degrading strain Bacillus subtilis BSF01 and its biodegradation pathway. Appl. Microbial. Biotechnol. 2015, 99, 2849–2859. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.A. Biological control of plant diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Veliz, E.A.; Martínez-Hidalgo, P.; Hirsch, A.M. Chitinase-producing bacteria and their role in biocontrol. AIMS Microbiol. 2017, 3, 689. [Google Scholar] [CrossRef]
- Hura, G.L.; Budworth, H.; Dyer, K.N.; Rambo, R.P.; Hammel, M.; McMurray, C.T.; Tainer, J.A. Comprehensive macromolecular conformations mapped by quantitative SAXS analyses. Nat. Methods 2013, 10, 453–454. [Google Scholar] [CrossRef]
- Benhamou, N.; Gagné, S.; Le Quéré, D.; Dehbi, L. Bacterial-mediated induced resistance in cucumber: Beneficial effect of the endophytic bacterium Serratia plymuthica on the protection against infection by Pythium ultimum. Phytopathology 2000, 90, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Ahmad, I.; Mohmood, I.; Pacheco, M.; Duarte, A.C.; Pereira, E.; Prasad, M.N.V. Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—A review. Environ. Exp. Bot. 2012, 75, 307–324. [Google Scholar] [CrossRef]
- Osbourn, A.E. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 1996, 8, 1821. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Khaledi, N.; Taheri, P.; Tarighi, S. Antifungal activity of various essential oils against Rhizoctonia solani and Macrophomina phaseolina as major bean pathogens. J. Appl. Microbiol. 2015, 118, 704–717. [Google Scholar] [CrossRef]
- Baysal, D.; Balyk, R.; Otto, D.; Luciak-Corea, C.; Beaupre, L. Functional outcome and health-related quality of life after surgical repair of full-thickness rotator cuff tear using a mini-open technique. Am. J. Sports Med. 2005, 33, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Seleim, M.A.; Abo-Elyousr, K.A.M.; Mohamed, A.A.A.; Al-Marzoky, H.A. Peroxidase and polyphenoloxidase activities as biochemical markers for biocontrol efficacy in the control of tomato bacterial wilt. J. Plant Physiol. Pathol. 2014, 2, 2. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol. 1994, 35, 785–791. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.I.; Abo-Elyousr, K.A.; Abdel-Rahim, I.R. Leaf surface and endophytic fungi associated with onion leaves and their antagonistic activity against Alternaria porri. Czech Mycol. 2015, 67, 1–22. [Google Scholar] [CrossRef]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi; Academic Press: London, UK, 1980. [Google Scholar]
- Hashem, M.; Alamri, S. The biocontrol of postharvest disease (Botryodiplodia theobromae) of guava (Psidium guajava L.) by the application of yeast strains. Postharvest Biol. Technol. 2009, 53, 123–130. [Google Scholar]
- Hassan, E.A.; Bagy, H.M.M.; Bashandy, S.R. Efficacy of potent antagonistic yeast Wickerhamiella versatilis against soft rot disease of potato caused by Pectobacterium carotovorum subsp. Carotovorum. Arch. Phytopathol. Plant Prot. 2019, 52, 1125–1148. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.M.; Asran, M.R. Antibacterial activity of certain plant extracts against bacterial wilt of tomato. Arch. Phytopathol. Plant Prot. 2009, 42, 573–578. [Google Scholar] [CrossRef]
- Bauer, A.W. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrobial. Chemoth. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Singh, J.; Tripathi, N.N. Inhibition of storage fungi of blackgram (Vigna mungo L.) by some essential oils. Flavour Fragr. J. 1999, 14, 1–4. [Google Scholar] [CrossRef]
- Bouziane, Z.; Dehimat, L.; Aziz, W.A.; Benabdelkader, M.; Chaouche, N.K. The antagonism between Trichoderma viride and other pathogenic fungal strains in Zea mays. Agric. Biol. J. North AM 2011, 2, 584–590. [Google Scholar] [CrossRef]
- Najjar, A.; Hassan, E.A.; Zabermawi, N.; Saber, S.H.; Bajrai, L.H.; Almuhayawi, M.S.; Abujamel, T.S.; Almasaudi, S.B.; Azhar, L.E.; Moulay, M.; et al. Optimizing the catalytic activities of methanol and thermotolerant Kocuria flava lipases for biodiesel production from cooking oil wastes. Sci. Rep. 2021, 11, 1–19. [Google Scholar]
- Sanders, E.R. Aseptic laboratory techniques: Plating methods. J. Vis. Exp. 2012, 63, 3064. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T.; Garrity, G.M. Bergey’s Manual of Systematic Bacteriology; Volume 2 The Proteobacteria; East Lansing; Springer: New York, NY, USA, 2005; p. 183. [Google Scholar]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Nat. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426. [Google Scholar] [CrossRef]
- Chen, J.P.; Lee, M.S. Simultaneous production and partition of chitinase during growth of Serratia marcescens in an aqueous two-phase system. Biotechnol. Tech. 1994, 8, 783–788. [Google Scholar] [CrossRef]
- Palanivel, P.; Ashokkumar, L.; Balagurunathan, R. Production, purification and fibrinolytic characterization of alkaline protease from extremophilic soil fungi. Int. J. Pharm. BioSci. 2013, 4, 101–110. [Google Scholar]
- Pereira, M.G.; Vici, A.C.; Facchini, F.D.A.; Tristão, A.P.; Cursino-Santos, J.R.; Sanches, P.R.; Jorge, J.A.; Polizeli, M.d.L.T.d.M. Screening of filamentous fungi for lipase production: Hypocrea pseudokoningii a new producer with a high biotechnological potential. Biocatal. Biotransform. 2014, 32, 74–83. [Google Scholar] [CrossRef]
- Prazeres, J.N.d.; Cruz, J.A.B.; Pastore, G.M. Characterization of alkaline lipase from Fusarium oxysporum and the effect of different surfactants and detergents on the enzyme activity. Braz. J. Microbiol. 2006, 37, 505–509. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bobev, S.G.; Zveibil, A.; Freeman, S. First Report of Colletotrichum acutatum on Strawberry in Bulgaria. Plant Dis. 2002, 86, 1178. [Google Scholar] [CrossRef] [PubMed]
- Yaganza, E.S.; Rioux, D.; Simard, M.; Arul, J.; Tweddell, R.J. Ultrastructural alterations of Erwinia carotovora subsp. atroseptica caused by treatment with aluminum chloride and sodium metabisulfite. Appl. Environ. Microbiol. 2004, 70, 6800–6808. [Google Scholar]
- Kofalvi, S.A.; Nassuth, A. Influence of wheat streak mosaic virus infection on phenylpropanoid metabolism and the accumulation of phenolics and lignin in wheat. Physiol. Mol. Plant Path. 1995, 47, 365–377. [Google Scholar] [CrossRef]
- Sun, T.; Powers, J.R.; Tang, J. Evaluation of the antioxidant activity of asparagus, broccoli and their juices. Food Chem. 2007, 105, 101–106. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Phys. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Abo-Elyousr, K.A.; Ibrahim, Y.E.; Balabel, N.M. Induction of Disease Defensive Enzymes in Response to Treatment with acibenzolar-S-methyl (ASM) and Pseudomonas fluorescens Pf2 and Inoculation with Ralstonia solanacearum race 3, biovar 2 (phylotype II). J. Phytopath. 2012, 160, 382–389. [Google Scholar] [CrossRef]
- Snedecor, G.; Cochran, W. Statistical Methods; Iowa State University: Ames, IA, USA, 1980. [Google Scholar]

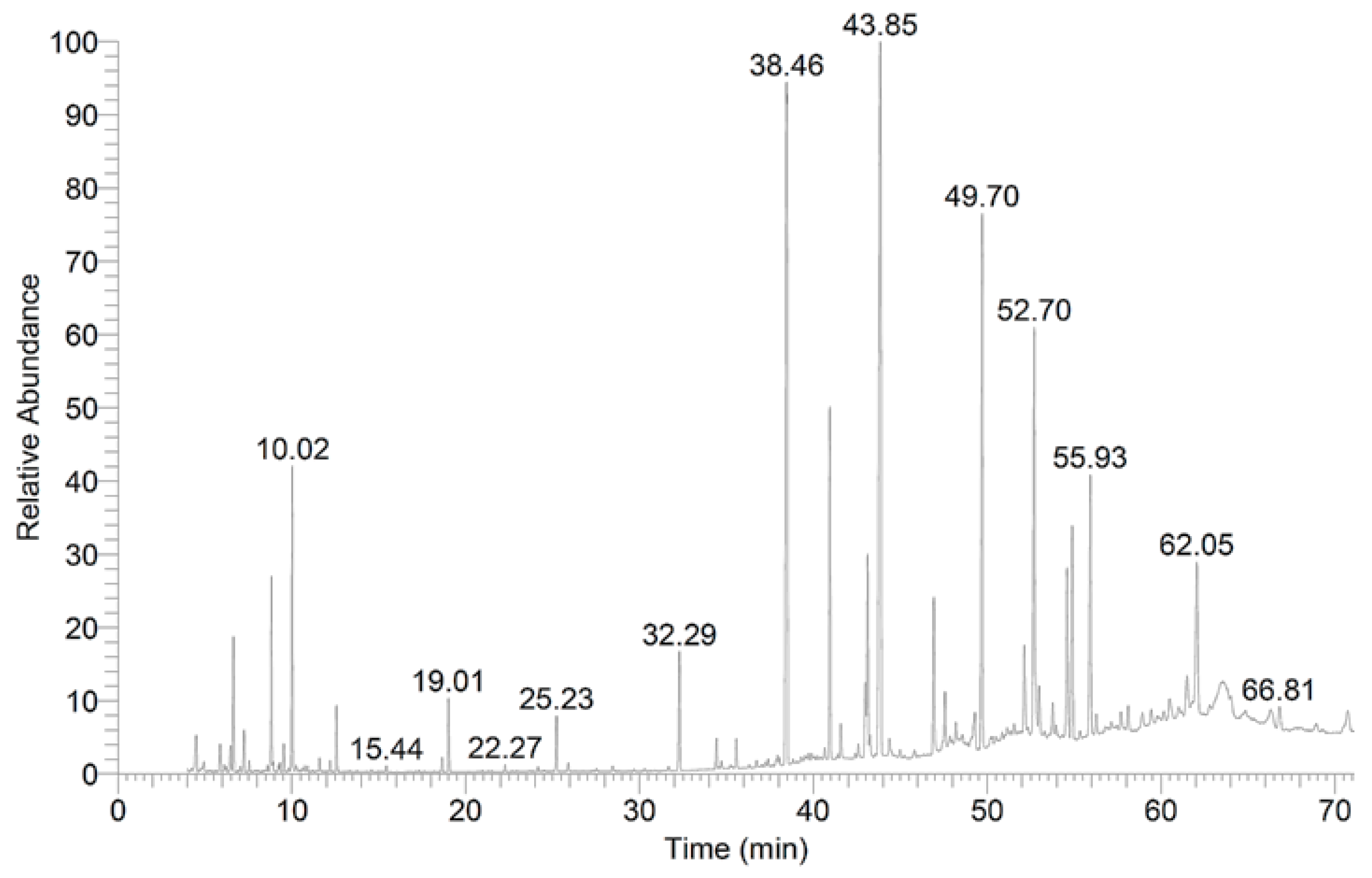

| RT | The Components | % of Total Constituents |
|---|---|---|
| 6.65 | Benzene, 1,3-dimethyl- | 1.78 |
| 8.83 | Cyclotetrasiloxane, octamethyl- | 1.90 |
| 10.02 | Benzene, 1-chloro-4-methyl- | 3.25 |
| 32.29 | Methyl 12-methyl-tridecanoate | 1.75 |
| 38.46 | Hexadecanoic acid | 14.79 |
| 40.95 | Heptadecanoic acid | 4.74 |
| 42.99 | 9,12-Octadecadienoic acid (Z,Z)- | 1.02 |
| 43.12 | trans-13-Octadecenoic acid | 2.88 |
| 43.86 | Octadecanoic acid | 15.93 |
| 46.93 | Octadecanoic acid, 17-methyl- | 2.27 |
| 49.70 | Methyl 18-methylnonadecanoate | 8.40 |
| 52.13 | Methyl 14-methyl-eicosanoate | 1.43 |
| 52.70 | Dodecanoic acid | 6.01 |
| 54.60 | Octadecanoic acid, 9,10-dichloro- | 2.78 |
| 54.89 | Methyl 20-methyl-heneicosanoate | 3.35 |
| 55.93 | 1,2-Benzenedicarboxylic acid | 4.34 |
| 62.05 | Methyl 14-methyl-eicosanoate | 2.69 |
| Biochemical Test | Value |
|---|---|
| Colonial characteristics | |
| Gram staining | + * |
| Colony color | White |
| Colony shape | Round with irregular margins |
| Cell shape | Rod-shaped |
| Biochemical characteristics | |
| Carbon source utilization | |
| D-Fructose | + |
| Citrate | + |
| D-Sorbitol | + |
| D-Galactose | − |
| Glycerol | + |
| Glucose | + |
| Maltose | + |
| Catalase test | + |
| Gelatin hydrolysis | + |
| Casein hydrolysis | + |
| Growth at 4 °C | – |
| Growth at 37 °C | + |
| Growth at 45 °C | + |
| Enzyme | Enzyme Activity (U/mL) | Protein Concentration (mg/mL) | Enzyme-Specific Activity (U/mg Protein) |
|---|---|---|---|
| Chitinase | 3.69 ± 0.31 * | 0.340 ± 0.29 | 9.66 ± 1.04 |
| Protease | 13.28 ± 0.65 | 0.705 ± 0.20 | 18.85 ± 3.26 |
| Lipase | 10.65 ± 0.51 | 0.650 ± 0.14 | 16.45 ± 3.55 |
| Treatment | Disease Severity (%) |
|---|---|
| Healthy control | 0.00 ± 0.0 *,a |
| Infected control | 86.11 ± 0.80 c |
| Botrytis + endophytic bacteria | 13.48 ± 0.92 b |
| Botrytis + preen oil | 12.57 ± 1.74 b |
| Botrytis + preen oil + endophytic bacteria | 22.72 ± 5.13 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, E.A.; Mostafa, Y.S.; Alamri, S.; Hashem, M.; Nafady, N.A. Biosafe Management of Botrytis Grey Mold of Strawberry Fruit by Novel Bioagents. Plants 2021, 10, 2737. https://doi.org/10.3390/plants10122737
Hassan EA, Mostafa YS, Alamri S, Hashem M, Nafady NA. Biosafe Management of Botrytis Grey Mold of Strawberry Fruit by Novel Bioagents. Plants. 2021; 10(12):2737. https://doi.org/10.3390/plants10122737
Chicago/Turabian StyleHassan, Elhagag A., Yasser S. Mostafa, Saad Alamri, Mohamed Hashem, and Nivien A. Nafady. 2021. "Biosafe Management of Botrytis Grey Mold of Strawberry Fruit by Novel Bioagents" Plants 10, no. 12: 2737. https://doi.org/10.3390/plants10122737
APA StyleHassan, E. A., Mostafa, Y. S., Alamri, S., Hashem, M., & Nafady, N. A. (2021). Biosafe Management of Botrytis Grey Mold of Strawberry Fruit by Novel Bioagents. Plants, 10(12), 2737. https://doi.org/10.3390/plants10122737







