Herbicide Efficacy of Spot Spraying Systems in Fallow and Postharvest in the Pacific Northwest Dryland Wheat Production Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location
2.2. Experimental Trials and Design
2.3. Sprayer Information
2.4. Data Collection and Statistical Analyses
3. Results and Discussion
3.1. Fallow
3.2. Postharvest
3.3. Residue
3.4. Herbicide Savings with Spot Sprayers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmermann, C.; Gerhards, R.; Kühbauch, W. The economic impact of site-specific weed control. Precis. Agric. 2003, 4, 249–260. [Google Scholar] [CrossRef]
- Grisso, R.; Alley, M.; Thomason, W.; Holshouser, D.; Roberson, G.T. Precision Farming Tools: Variable-Rate Application; Virginia Cooperative Extension Publication: Blacksburg, VA, USA, 2011; pp. 442–505. [Google Scholar]
- Shanmugam, S.; Assunção, E.; Mesquita, R.; Verios, A.; Gaspar, P.D. Automated weed detection systems: A review. KnE Eng. 2020, 5, 271–284. [Google Scholar] [CrossRef]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Envrion. Sci. Eur. 2016, 28, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, J.M. The rise and future of glyphosate and glyphosate-resistant crops. Pest Manag. Sci. 2016, 74, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Barroso, J.; Gourlie, J.A.; Lutcher, L.K.; Liu, M.; Mallory-Smith, C.A. Identification of glyphosate resistance in Salsola tragus in north-eastern Oregon. Pest Manag. Sci. 2018, 74, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Spring, J.F.; Jha, P.; Lyon, D.J.; Burke, I.C. Glyphosate-resistant Russian-thistle (Salsola tragus) identified in Montana and Washington. Weed Technol. 2017, 31, 238–251. [Google Scholar] [CrossRef]
- Zuger, R.J.; Burke, I.C. Testing in Washington identifies widespread postemergence herbicide resistance in annual grasses. Crops Soils 2020, 53, 13–19. [Google Scholar] [CrossRef]
- The International Survey of Herbicide Resistant Weeds. Available online: www.weedscience.com (accessed on 28 June 2021).
- Clay, S.A. Near term challenges for global agriculture—Herbicide resistant weeds. Agron. J. 2021. [Google Scholar] [CrossRef]
- Beckie, H.J.; Ashworth, M.B.; Flower, K.C. Herbicide resistance management: Recent developments and trends. Plants 2019, 8, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, D.A.; Bennett, L. Light-Activated Sensor Sprayer for Reduced Herbicide Use in No-Till Fallow; 2008 Dryland Agricultural Research Annual Report; Columbia Basin Agricultural Research Center: Pendleton, OR, USA, 2008; p. 130. [Google Scholar]
- Fischer, J.W.; Thorne, M.E.; Lyon, D.J. Weed sensing technology modifies fallow control of rush skeletonweed (Chondrilla juncea). Weed Technol. 2020, 34, 857–862. [Google Scholar] [CrossRef]
- Young, F.L.; Yenish, J.P.; Launchbaugh, G.K.; McGrew, L.L.; Alldredge, J.R. Postharvest control of Russian thistle (Salsola tragus) with a reduced herbicide applicator in the Pacific Northwest. Weed Technol. 2008, 22, 156–159. [Google Scholar] [CrossRef]
- Cook, T. Weed detecting technology: An excellent opportunity for advanced glyphosate resistance management. In Proceedings of the 18th Australasian Weeds Conference, Melbourne, VIC, Australia, 8–11 October 2012; Weed Society of Victoria: Bundoora, Australia, 2012; pp. 245–247. [Google Scholar]
- Visser, R.; Timmermans, A.J.M. WEED-IT: A new selective weed control system. In Proceedings of the SPIE 2907, Optics in Agriculture, Forestry, and Biological Processing II, Boston, MA, USA, 18 November 1996. [Google Scholar]
- Antuniassi, U.R.; da Silva Nery, M.; Carvalho, W.P.A.; Ruiz, E.R.S.; De León, M.J. Performance evaluation of an optical sensor for weed detection. In Proceedings of the ASABE Annual International Meeting, Las Vegas, NV, USA, 27–30 July 2003; American Society of Agricultural and Biological Engineers: St.Joseph, MO, USA, 2003; p. 11. [Google Scholar]
- Rizzardi, K.; Prostko, E.; Rains, G.; Vellidis, G.; Morari, F. Selective spraying of weeds in peanut. In Proceedings of the Sixth European Conference on Precision Agriculture, Skiathos, Greece, 3–6 June 2007; Aggelopoulou, A.S., Blackmore, T., Gemtos, T., Fountas, S., Eds.; p. 6. [Google Scholar]
- McCarthy, W. Validation of Commercial Precision Spraying Technology. Ph.D. Thesis, University of Southern Queensland, Toowoomba, Australia, 2016. [Google Scholar]
- Riar, D.S.; Ball, D.A.; Yenish, J.P.; Burke, I.C. Light-activated, sensor-controlled sprayer provides effective postemergence control of broadleaf weeds in fallow. Weed Technol. 2011, 25, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Simao, L.M.; Easterly, A.C.; Kruger, G.R.; Creech, C.F. Herbicide spray deposition in wheat stubble as affected by nozzle type and application direction. Agronomy 2020, 10, 1507. [Google Scholar] [CrossRef]
- Ghadiri, H.; Shea, P.J.; Wicks, G.A. Interception and retention of atrazine by wheat (Triticum aestivum L.) stubble. Weed Sci. 1984, 32, 24–27. [Google Scholar] [CrossRef]
- Lyon, D.J.; Burke, I.C. Integrated Management of Prickly Lettuce in Wheat Production Systems; Pacific Northwest Extension Publication. Washington State University: Pullman, WA, USA, 2021; pp. 1–8. [Google Scholar]
| Year | Trial | Active Ingredient | Herbicide Rate a | Tank Mixture | Delivered Rate a | ||
|---|---|---|---|---|---|---|---|
| Uniform | WEED-IT | WeedSeeker | |||||
| 2019 | Fallow | Glyphosate | 833 g a.e. ha−1 | Glystar Plus + 20 g Ammonium Sulfate (AMS) L−1 | 1× | 1× | 1× |
| Bromoxynil + Pyrasulfotole | 230 g a.i. ha−1 + 40 g a.i. ha−1 | Huskie + 157 g a.i. ha−1 Section 3 (clethodim) + 2.3 L Crop oil concentrate (COC) ha−1 + 9.4 L Solution 32 ha−1 | 1× | 1× | 1× | ||
| Postharvest | Glyphosate | 833 g a.e. ha−1 | Glystar Plus + 20 g AMS L−1 | 1× | 1× | 1× | |
| Bromoxynil + Pyrasulfotole | 230 g a.i. ha−1 + 40 g a.i. ha−1 | Huskie + 157 g a.i. ha−1 Section 3 (clethodim) + 2.3 L COC ha−1 + 9.4 L Solution 32 ha−1 | 1× | 1× | 1× | ||
| Residue | Bromoxynil + 2,4-D | 877 g a.i. ha−1 + 1097 g a.i. ha−1 | Deadbolt + 701 g In-Place ha−1 | 1× | 1× | 1× | |
| 2020 | Fallow | Glyphosate | 1117 g a.e. ha−1 | GlyStar 5 Extra + 20 g AMS L−1 | 1× | 3× | 1× |
| Bromoxynil + Pyrasulfotole | 230 g a.i. ha−1 + 40 g a.i. ha−1 | Huskie + 157 g a.i. ha−1 Section 3 (clethodim) + 2.3 L COC ha−1 + 9.4 L Solution 32 ha−1 | 1× | 3× | 1× | ||
| Postharvest | Glyphosate | 1117 g a.e. ha−1 | GlyStar 5 Extra + 20 g AMS L−1 | 1× | 3× | 1× | |
| Bromoxynil + Pyrasulfotole | 230 g a.i. ha−1 + 40 g a.i. ha−1 | Huskie + 157 g a.i. ha−1 Section 3 (clethodim) + 2.3 L COC ha−1 + 9.4 L Solution 32 ha−1 | 1× | 3× | 1× | ||
| Residue | Glyphosate + Bromoxynil + Pyrasulfotole | 1117 g a.e. ha−1 + 215 g a.i. ha−1 + 38 g a.i. ha−1 | Huskie + GlyStar 5 Extra + 136 g Non-ionic surfactant ha−1 + 1.9 L Solution 32 ha−1 | 1× | 3× | 1× | |
| Glyphosate | Bromoxynil + Pyrasulfotole | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | Factor | Level | Pre-Spray Density (Plants/m2) | Pre-Spray Cover (%) | Density Reduction Efficacy (%) | Cover Reduction Efficacy (%) | Pre-Spray Density (Plants m2) | Pre-Spray Cover (%) | Density Reduction Efficacy (%) | Cover Reduction Efficacy (%) |
| 2019 | Spray system | *** | *** | ** | ** | |||||
| Uniform | 30.8 | 23.9 | 86.6 ± 13 a | 68.3 ± 24 a | 25.2 | 25.6 | 58.1 ± 25 a | 34.4 ± 37 a | ||
| WEED-IT | 34.4 | 35.9 | 73.5 ± 11 b | 19.4 ± 27 b | 38.0 | 38.1 | 38.0 ± 20 b | 5.9 ± 13 b | ||
| Weed Community | ns | * | ** | ns | ||||||
| Natural | 33.2 | 22.8 | 81.6 ± 16 | 61.2 ± 30 a | 23.4 | 19.0 | 40.9 ± 26 b | 25.4 ± 36 | ||
| Kochia | 34.6 | 24.9 | 83.3 ± 12 | 44.2 ± 37 ab | 32.0 | 25.2 | 40.4 ± 26 b | 16.5 ± 29 | ||
| Russian thistle (RT) | 29.8 | 41.9 | 75.3 ± 12 | 26.0 ± 32 b | 39.2 | 51.4 | 62.9 ± 14 a | 18.5 ± 29 | ||
| Interaction | *** | *** | ** | ns | ||||||
| Uniform_Natural | 28.4 | 12.3 | 83.4 ± 21 ab | 78.8 ± 16 a | 18.2 | 12.4 | 53.7 ± 30 ab | 40.0 ± 44 | ||
| Uniform_Kochia | 39.0 | 24.0 | 91.7 ± 7 a | 74.5 ± 20 a | 32.0 | 22.2 | 51.4 ± 25 ab | 30.3 ± 36 | ||
| Uniform_RT | 24.8 | 35.4 | 84.7 ± 8 abc | 51.2 ± 27 ab | 25.2 | 42.4 | 69.2 ± 15 a | 32.8 ± 36 | ||
| WEED-IT_Natural | 38.0 | 33.4 | 80.0 ± 11 abc | 43.7 ± 32 ab | 28.4 | 25.6 | 28.1 ± 12 b | 10.9 ± 20 | ||
| WEED-IT_Kochia | 30.0 | 25.9 | 74.9 ± 9 bc | 13.7 ± 19 b | 32.0 | 28.2 | 29.3 ± 23 b | 2.7 ± 6 | ||
| WEED-IT_RT | 35.0 | 48.5 | 65.8 ± 8 c | 0.8 ± 2 b | 53.2 | 60.5 | 56.6 ± 12 ab | 4.2 ± 7.8 | ||
| 2020 | Spray system | ns | *** | ** | ** | |||||
| Uniform | 54.6 | 19.2 | 43.5 ± 24 | 23.4 ± 30 a | 32.0 | 8.1 | 62.9 ± 21 a | 9.6 ± 28 a | ||
| WeedSeeker | 66.8 | 14.7 | 27.6 ± 28 | 1.7 ± 6 b | 31.6 | 10.9 | 31.1 ± 28 b | 0.0 ± 0 b | ||
| WEED-IT | 55.0 | 8.3 | 31.4 ± 27 | 0.0 ± 0 b | 39.6 | 6.9 | 47.3 ± 30 ab | 1.6 ± 8 ab | ||
| Weed Community | ns | ns | ns | ns | ||||||
| Natural | 40.0 | 12.5 | 30.3 ± 25 | 9.2 ± 24 | 26.6 | 8.4 | 46.8 ± 31 | 6.3 ± 18 | ||
| Kochia | 51.2 | 12.3 | 34.7 ± 25 | 5.6 ± 14 | 29.4 | 9.1 | 53.9 ± 24 | 1.0 ± 5 | ||
| RT | 85.2 | 17.5 | 37.5 ± 31 | 10.2 ± 22 | 47.2 | 8.4 | 40.5 ± 31 | 3.9 ± 13 | ||
| Interaction | ns | ** | ns | * | ||||||
| Uniform_Natural | 37.0 | 17.5 | 35.7 ± 22 | 27.7 ± 36 a | 22.2 | 8.3 | 63.3 ± 27 | 14.1 ± 28 a | ||
| Uniform_Kochia | 45.2 | 14.8 | 42.6 ± 22 | 12.7 ± 22 ab | 29.8 | 6.8 | 66.6 ± 17 | 2.9 ± 8 ab | ||
| Uniform_RT | 81.6 | 25.2 | 52.1 ± 28 | 29.7 ± 31 a | 44.2 | 9.2 | 58.7 ± 18 | 11.8 ± 28 a | ||
| WeedSeeker_Natural | 45.0 | 12.9 | 22.2 ± 26 | 0.0 ± 0 b | 26.6 | 10.3 | 24.5 ± 29 | 0.0 ± 0 b | ||
| WeedSeeker_Kochia | 72.0 | 15.6 | 37.7 ± 28 | 4.0 ± 10 b | 24.8 | 11.1 | 39.6 ± 21 | 0.0 ± 0 b | ||
| WeedSeeker_RT | 83.6 | 15.8 | 22.8 ± 29 | 1.0 ± 3 b | 43.6 | 11.2 | 29.3 ± 35 | 0.0 ± 0 b | ||
| WEED-IT_Natural | 36.8 | 7.0 | 32.9 ± 28 | 0.0 ± 0 b | 31.2 | 6.6 | 52.7 ± 26 | 4.9 ± 14 ab | ||
| WEED-IT_Kochia | 37.8 | 6.4 | 23.8 ± 23 | 0.0 ± 0 b | 34.0 | 9.4 | 55.7 ± 27 | 0.0 ± 0 b | ||
| WEED-IT_RT | 90.2 | 11.5 | 37.5 ± 31 | 0.0 ± 0 b | 53.8 | 4.8 | 33.4 ± 34 | 0.0 ± 0 b |
| Glyphosate | Bromoxynil + Pyrasulfotole | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | Factors | Level | Pre-Spray Density (Plants/m2) | Pre-Spray Cover (%) | Density Reduction Efficacy (%) | Cover Reduction Efficacy (%) | Pre-Spray Density (Plants m2) | Pre-Spray Cover (%) | Density Reduction Efficacy (%) | Cover Reduction Efficacy (%) |
| 2019 | Spray system | ns | ** | ** | ** | |||||
| Uniform | 13.4 | 20.6 | 46.1 ± 35 | 50.4 ± 36 a | 13.4 | 22.5 | 62.0 ± 36 a | 74.6 ± 34 a | ||
| WeedSeeker | 8.9 | 15.6 | 30.2 ± 29 | 24.1 ± 35 b | 13.3 | 25.2 | 30.5 ± 31 b | 35.9 ± 38 b | ||
| WEED-IT | 13.9 | 19.3 | 28.9 ± 29 | 23.9 ± 30 b | 13.1 | 18.0 | 40.0 ± 38 ab | 42.6 ± 40 b | ||
| Weed Community | * | ns | ns | * | ||||||
| Natural | 3.8 | 7.8 | 20.9 ± 27 b | 29.3 ± 35 | 5.8 | 5.6 | 33.8 ± 37 | 35.3 ± 42 b | ||
| Kochia | 9.3 | 6.0 | 43.9 ± 33 a | 26.1 ± 34 | 11.0 | 12.2 | 42.8 ± 36 | 49.8 ± 38 ab | ||
| Russian thistle (RT) | 23.0 | 41.8 | 40.5 ± 32 ab | 43.1 ± 37 | 22.9 | 47.9 | 55.8 ± 35 | 68.0 ± 37 a | ||
| Interaction | ** | * | ns | ** | ||||||
| Uniform_Natural | 3.1 | 3.7 | 15.5 ± 22 b | 34.6 ± 35 ab | 5.8 | 4.4 | 50.9 ± 48 | 63.3 ± 45 ab | ||
| Uniform_Kochia | 6.8 | 5.0 | 54.6 ± 35 ab | 45.0 ± 42 ab | 10.8 | 10.6 | 61.7 ± 33 | 71.5 ± 36 ab | ||
| Uniform _RT | 30.2 | 53.2 | 68.2 ± 26 a | 71.6 ± 23 a | 23.6 | 52.4 | 73.4 ± 23 | 89.0 ± 12 a | ||
| WeedSeeker_Natural | 3.4 | 7.4 | 33.9 ± 29 ab | 30.6 ± 39 ab | 5.8 | 9.4 | 23.0 ± 28 | 26.9 ± 39 ab | ||
| WeedSeeker_Kochia | 7.8 | 6.4 | 34.0 ± 33 ab | 15.4 ± 29 b | 10.3 | 13.5 | 27.6 ± 31 | 29.7 ± 30 ab | ||
| WeedSeeker_RT | 15.3 | 33.1 | 22.7 ± 26 ab | 26.2 ± 38 ab | 23.7 | 52.7 | 40.9 ± 33 | 51.2 ± 43 ab | ||
| WEED-IT_Natural | 4.8 | 12.4 | 13.2 ± 28 b | 22.7 ± 33 ab | 5.9 | 3.0 | 27.5 ± 30 | 15.9 ± 29 b | ||
| WEED-IT_Kochia | 13.3 | 6.6 | 43.1 ± 31 ab | 17.8 ± 25 ab | 12.1 | 12.5 | 39.2 ± 39 | 48.1 ± 40 ab | ||
| WEED-IT_RT | 23.5 | 39.0 | 30.5 ± 24 ab | 31.4 ± 34 ab | 21.3 | 38.4 | 53.2 ± 43 | 63.8 ± 39 ab | ||
| 2020 | Spray system | ns | * | ns | ns | |||||
| Uniform | 9.8 | 6.5 | 12.3 ± 18 | 34.6 ± 32 a | 10.0 | 4.2 | 6.6 ± 10 | 24.6 ± 36 | ||
| WeedSeeker | 7.9 | 5.6 | 5.2 ± 13 | 15.6 ± 23 b | 11.4 | 4.9 | 1.5 ± 4 | 8.5 ± 18 | ||
| WEED-IT | 6.8 | 7.1 | 22.2 ± 31 | 47.4 ± 39 a | 8.1 | 4.8 | 3.5 ± 9 | 15.7 ± 28 | ||
| Weed Community | *** | *** | *** | *** | ||||||
| Natural | 4.0 | 3.4 | 1.3 ± 5 b | 20.7 ± 25 b | 4.2 | 1.0 | 0.3 ± 1.6 b | 0.0 ± 0 b | ||
| Kochia | 1.7 | 2.7 | 1.9 ± 6 b | 18.1 ± 28 b | 4.8 | 1.0 | 0.0 ± 0 b | 0.9 ± 4 b | ||
| RT | 18.8 | 13.0 | 36.5 ± 26 a | 58.8 ± 33 a | 20.5 | 11.9 | 11.2 ± 12 a | 47.9 ± 31 a | ||
| Interaction | *** | *** | *** | *** | ||||||
| Uniform_Natural | 4.4 | 4.1 | 0.0 ± 0 c | 21.8 ± 26 bc | 4.1 | 1.0 | 0.0 ± 0 b | 0.0 ± 0 b | ||
| Uniform_Kochia | 2.1 | 2.2 | 2.5 ± 7 c | 12.4 ± 18 bc | 6.7 | 1.1 | 0.0 ± 0 b | 2.7 ± 8 b | ||
| Uniform_RT | 23.0 | 13.4 | 34.3 ± 12 ab | 69.5 ± 14 ab | 19.3 | 10.5 | 19.7 ± 8 a | 71.1 ± 20 a | ||
| WeedSeeker_Natural | 5.8 | 3.8 | 3.8 ± 8 bc | 16.9 ± 25 bc | 5.0 | 1.1 | 1.0 ± 3 b | 0.0 ± 0 b | ||
| WeedSeeker_Kochia | 1.6 | 2.5 | 0.0 ± 0 c | 8.2 ± 16 c | 4.3 | 1.1 | 0.0 ± 0 b | 0.0 ± 0 b | ||
| WeedSeeker_RT | 16.2 | 10.6 | 11.7 ± 20 bc | 21.8 ± 27 bc | 24.8 | 12.6 | 3.4 ± 7 b | 25.4 ± 25 ab | ||
| WEED-IT_Natural | 1.8 | 2.5 | 0.0 ± 0 c | 23.3 ± 27 bc | 3.4 | 0.8 | 0.0 ± 0 b | 0.0 ± 0 b | ||
| WEED-IT_Kochia | 1.3 | 3.6 | 3.1 ± 9 c | 33.7 ± 41 abc | 3.5 | 0.9 | 0.0 ± 0 b | 0.0 ± 0 b | ||
| WEED-IT_RT | 17.2 | 15.1 | 63.6 ± 11 a | 85.2 ± 9 a | 17.3 | 12.6 | 10.6 ± 14 ab | 47.1 ± 30 a |
| Year | Factor | Level | Pre-Spray Cover (%) | Cover Reduction Efficacy (%) |
|---|---|---|---|---|
| 2019 | Spray system | *** | ||
| Uniform | 12.0 | 76.8 ± 29 a | ||
| WeedSeeker | 13.6 | 43.2 ± 33 b | ||
| WEED-IT | 9.8 | 21.0 ± 28 b | ||
| Stubble height | ns | |||
| Short | 5.7 | 32.7 ± 40 | ||
| Tall | 16.1 | 51.9 ± 35 | ||
| Tarp | 13.5 | 56.3 ± 35 | ||
| Interaction | *** | |||
| Uniform_Short | 5.5 | 64.4 ± 42 ab | ||
| Uniform_Tall | 22.4 | 80.5 ± 20 a | ||
| Uniform_Tarp | 8.1 | 85.4 ± 16 a | ||
| WeedSeeker_Short | 7.6 | 27.5 ± 36 ab | ||
| WeedSeeker_Tall | 15.7 | 46.4 ± 30 ab | ||
| WeedSeeker_Tarp | 17.5 | 55.7 ± 30 ab | ||
| WEED-IT_Short | 4.1 | 6.3 ± 12 b | ||
| WEED-IT_Tall | 10.2 | 28.9 ± 34 ab | ||
| WEED-IT_Tarp | 15.1 | 27.7 ± 31 ab | ||
| 2020 | Spray system | ** | ||
| Uniform | 46.4 | 93.8 ± 5 ab | ||
| WeedSeeker | 53.7 | 88.2 ± 10 b | ||
| WEED-IT | 47.6 | 94.9 ± 5 a | ||
| Stubble height | ns | |||
| Short | 54.0 | 94.2 ± 5 | ||
| Tall | 50.0 | 91.0 ± 7 | ||
| Tarp | 43.8 | 91.8 ± 10 | ||
| Interaction | * | |||
| Uniform_Short | 45.8 | 94.2 ± 6 ab | ||
| Uniform_Tall | 46.8 | 91.3 ± 6 abc | ||
| Uniform_Tarp | 46.7 | 95.9 ± 4 a | ||
| WeedSeeker_Short | 59.1 | 92.4 ± 6 abc | ||
| WeedSeeker_Tall | 53.9 | 89.2 ± 8 bc | ||
| WeedSeeker_Tarp | 48.5 | 83.1 ± 13 c | ||
| WEED-IT_Short | 57.1 | 95.9 ± 4 a | ||
| WEED-IT_Tall | 49.5 | 92.4 ± 7 abc | ||
| WEED-IT_Tarp | 36.2 | 96.4 ± 4 a |
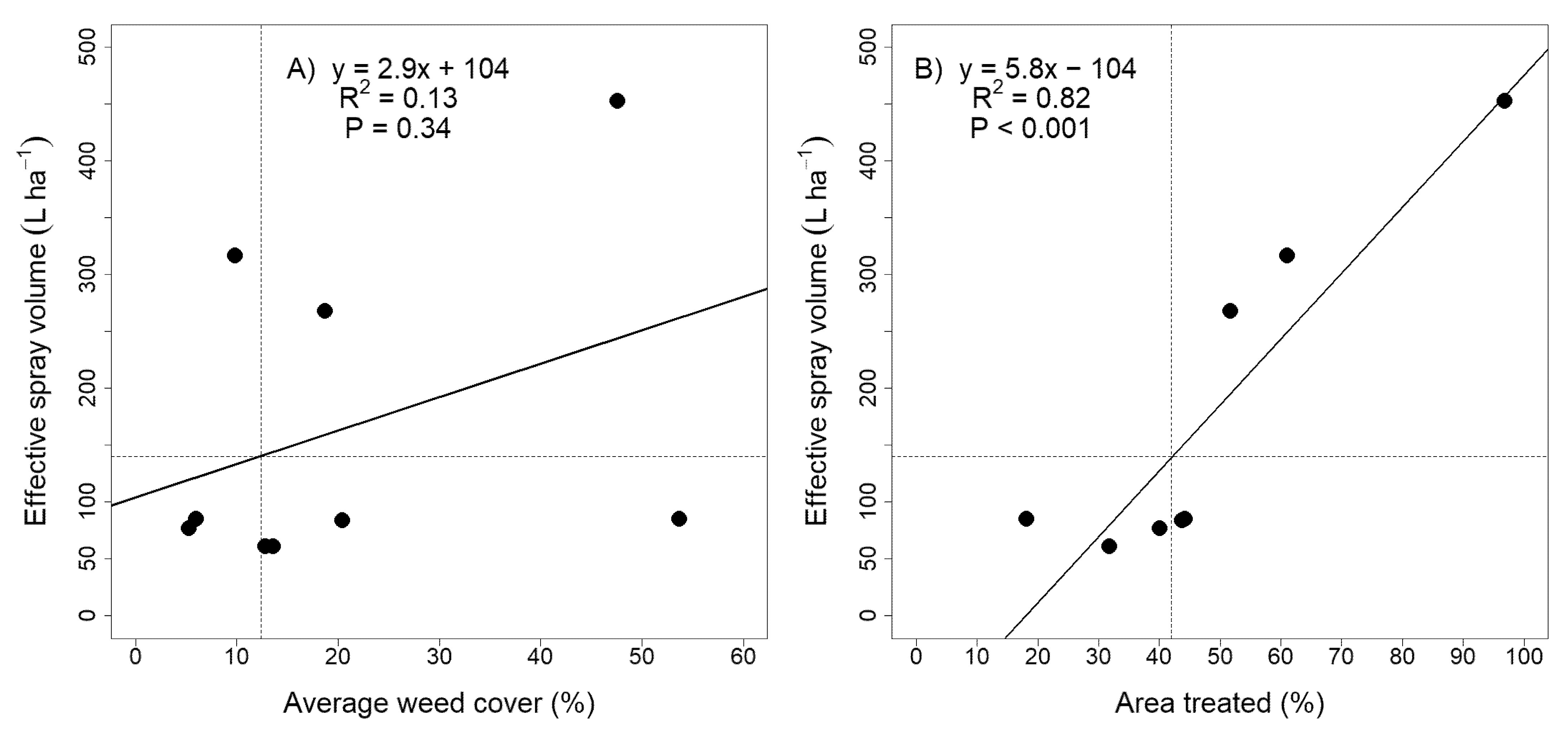
| Year | Trial | Sprayer | Continuous Spray Volume (L ha−1) | Effective Spray Volume b (L ha−1) | Average Weed Coverage (%) | Area Treated c (%) | Herbicide Volume Saved (%) |
|---|---|---|---|---|---|---|---|
| 2019 | Postharvest | WeedSeeker | 192 | 84 | 20.4 | 44 | 56 |
| WEED-IT | 519 | 268 | 18.7 | 52 | 48 | ||
| Residue | WeedSeeker | 192 | 61 | 13.6 | 32 | 68 | |
| WEED-IT | 519 | 317 | 9.8 | 61 | 39 | ||
| 2020 | Fallow | WeedSeeker | 192 | 61 | 12.8 | 32 | 68 |
| Postharvest | WeedSeeker | 192 | 77 | 5.3 | 40 | 60 | |
| WEED-IT | 468 | 85 | 6.0 | 18 | 82 | ||
| Residue | WeedSeeker | 192 | 85 | 53.7 | 44 | 56 | |
| WEED-IT | 468 | 453 | 47.6 | 97 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genna, N.G.; Gourlie, J.A.; Barroso, J. Herbicide Efficacy of Spot Spraying Systems in Fallow and Postharvest in the Pacific Northwest Dryland Wheat Production Region. Plants 2021, 10, 2725. https://doi.org/10.3390/plants10122725
Genna NG, Gourlie JA, Barroso J. Herbicide Efficacy of Spot Spraying Systems in Fallow and Postharvest in the Pacific Northwest Dryland Wheat Production Region. Plants. 2021; 10(12):2725. https://doi.org/10.3390/plants10122725
Chicago/Turabian StyleGenna, Nicholas G., Jennifer A. Gourlie, and Judit Barroso. 2021. "Herbicide Efficacy of Spot Spraying Systems in Fallow and Postharvest in the Pacific Northwest Dryland Wheat Production Region" Plants 10, no. 12: 2725. https://doi.org/10.3390/plants10122725
APA StyleGenna, N. G., Gourlie, J. A., & Barroso, J. (2021). Herbicide Efficacy of Spot Spraying Systems in Fallow and Postharvest in the Pacific Northwest Dryland Wheat Production Region. Plants, 10(12), 2725. https://doi.org/10.3390/plants10122725







