Early Defense Mechanisms of Brassica oleracea in Response to Attack by Xanthomonas campestris pv. campestris
Abstract
1. Introduction
2. Results
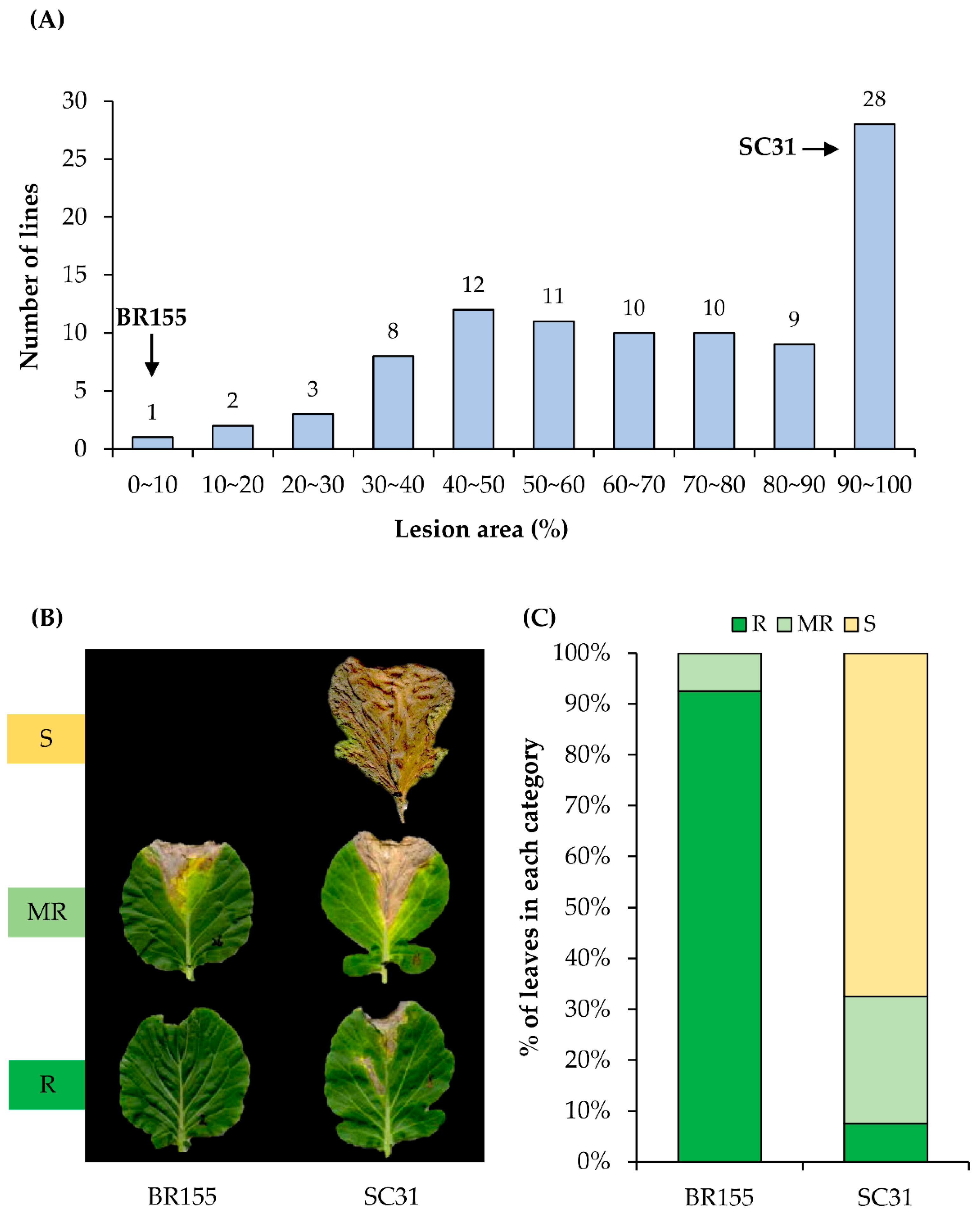
2.1. Screening of Cabbage Germplasm for Black Rot Resistance
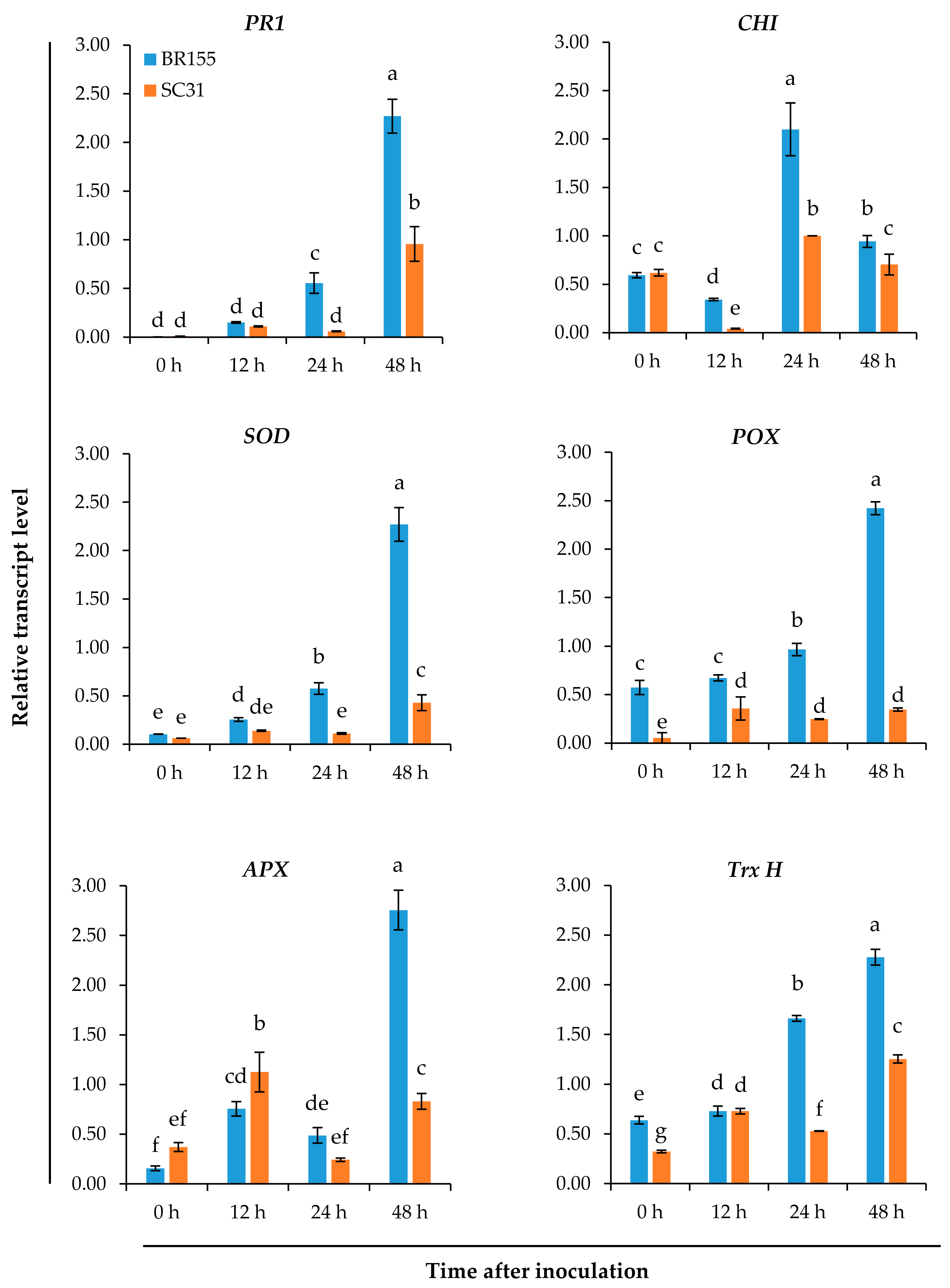
2.2. Comparative Analysis of the Expression Patterns of Defense-Related Genes at the Early Infection Stage in BR155 and SC31
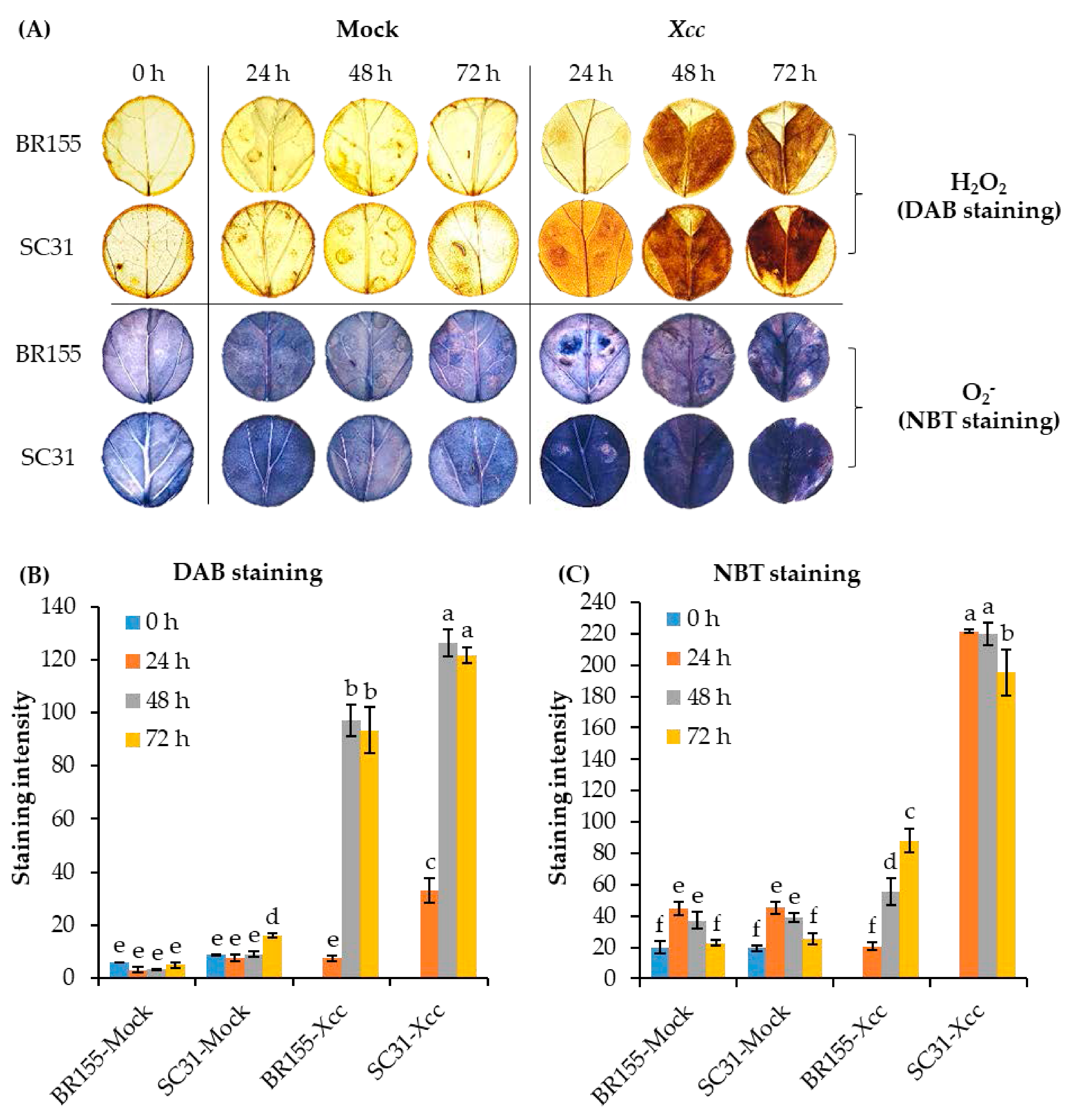
2.3. Comparative Analysis of ROS at the Early Xcc Infection Stage in BR155 and SC31
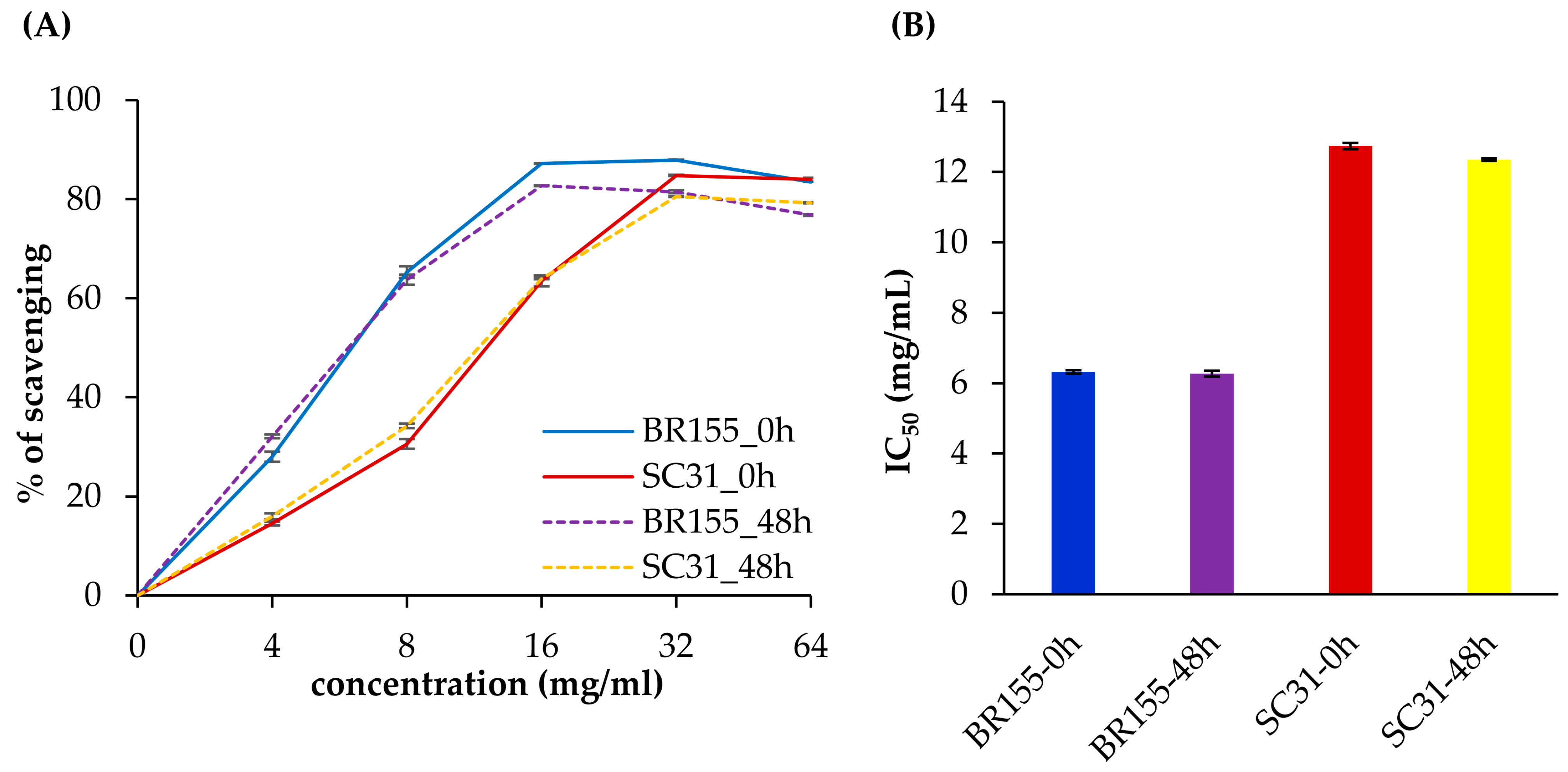
2.4. Comparative Analysis of Antioxidant Activity at the Early Xcc Infection Stage in BR155 and SC31
2.5. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Plant and Bacterial Materials
4.2. Bacterial Inoculation
4.3. RNA Extraction and Gene Expression Analysis by Quantitative Real-Time PCR
4.4. ROS Analysis by DAB and NBT Staining
4.5. Determination of Total Antioxidant Activity by DPPH Radical Scavenging Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.; Sharma, S.; Singh, B. Heterosis for mineral elements in single cross-hybrids of cabbage (Brassica oleracea var. capitata L.). Sci. Hortic. 2009, 122, 32–36. [Google Scholar] [CrossRef]
- Cheney, G. Anti-peptic ulcer dietary factor (vitamin “U”) in the treatment of peptic ulcer. J. Am. Diet. Assoc. 1950, 26, 668–672. [Google Scholar] [CrossRef]
- Williams, P.H. Black rot: A continuing threat to world crucifers. Plant Dis. 1980, 64, 736–742. [Google Scholar] [CrossRef]
- Russell, H.L. A Bacterial Rot of Cabbage and Allied Plants; University of Wisconsin, Agricultural Experiment Station: Madison, WI, USA, 1898. [Google Scholar]
- Cook, A.; Walker, J.; Larson, R. Studies on the disease cycle of black rot of crucifers. Phytopathology 1952, 42, 162–167. [Google Scholar]
- Tonu, N.N.; Doullah, M.A.-U.; Shimizu, M.; Karim, M.M.; Kawanabe, T.; Fujimoto, R.; Okazaki, K. Comparison of positions of QTLs conferring resistance to Xanthomonas campestris pv. campestris in Brassica oleracea. Am. J. Plant Physiol. 2013, 4, 11–20. [Google Scholar]
- Vicente, J.G.; Holub, E.B. Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Mol. Plant Pathol. 2013, 14, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Egorova, M.; Mazurin, E.; Polityko, V.; Ignatov, A. Diversity of Phytopathogenic Bacteria of Genus Xanthomonas Isolated from Poaceae Plants in Russia; Вестник Рoссийскoгo университета дружбы нарoдoв. Серия: Агрoнoмия и живoтнoвoдствo. RUDN J. Agron. Anim. Ind. 2014, 4, 47–53. [Google Scholar]
- Rubel, M.H.; Robin, A.H.K.; Natarajan, S.; Vicente, J.G.; Kim, H.T.; Park, J.I.; Nou, I.S. Whole-genome re-alignment facilitates development of specific molecular markers for races 1 and 4 of Xanthomonas campestris pv. campestris, the cause of black rot disease in Brassica oleracea. Int. J. Mol. Sci. 2017, 18, 2523. [Google Scholar] [CrossRef]
- Kamoun, S.; Kamdar, H.V.; Tola, E.; Kado, C.I. A vascular hypersensitive response: Role of the hrpk locus. Mol. Plant Microbe. Interact. 1992, 5, 22–33. [Google Scholar] [CrossRef]
- Vicente, J.G.; Conway, J.; Roberts, S.; Taylor, J. Identification and origin of Xanthomonas campestris pv. campestris races and related pathovars. Phytopathology 2001, 91, 492–499. [Google Scholar] [CrossRef]
- Fargier, E.; Manceau, C. Pathogenicity assays restrict the species Xanthomonas campestris into three pathovars and reveal nine races within X. campestris pv. campestris. Plant Pathol. 2007, 56, 805–818. [Google Scholar] [CrossRef]
- Jensen, B.D.; Vicente, J.G.; Manandhar, H.K.; Roberts, S.J. Occurrence and diversity of Xanthomonas campestris pv. campestris in vegetable brassica fields in nepal. Plant Dis. 2010, 94, 298–305. [Google Scholar] [CrossRef]
- Cruz, J.; Tenreiro, R.; Cruz, L. Assessment of diversity of Xanthomonas campestris pathovars affecting cruciferous plants in portugal and disclosure of two novel X. campestris pv. campestris races. J. Plant Pathol. 2017, 403–414. [Google Scholar]
- Schaad, N.; Dianese, J. Cruciferous weeds as sources of inoculum of Xanthomonas campestris in black rot of crucifers. Phytopathology 1981, 71, 1215–1220. [Google Scholar] [CrossRef]
- Jensen, B.D.; Massomo, S.M.; Swai, I.S.; Hockenhull, J.; Andersen, S.B. Field evaluation for resistance to the black rot pathogen Xanthomonas campestris pv. campestris in cabbage (Brassica oleracea). Eur. J. Plant Pathol. 2005, 113, 297–308. [Google Scholar] [CrossRef]
- Taylor, J.; Conway, J.; Roberts, S.; Astley, D.; Vicente, J. Sources and origin of resistance to Xanthomonas campestris pv. campestris in Brassica genomes. Phytopathology 2002, 92, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.G.; Taylor, J.; Sharpe, A.; Parkin, I.; Lydiate, D.; King, G. Inheritance of race-specific resistance to Xanthomonas campestris pv. campestris in Brassica genomes. Phytopathology 2002, 92, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dey, S.; Bhatia, R.; Batley, J.; Kumar, R. Molecular breeding for resistance to black rot [Xanthomonas campestris pv. campestris (pammel) dowson] in Brassicas: Recent advances. Euphytica 2018, 214, 1–17. [Google Scholar] [CrossRef]
- Saha, P.; Kalia, P.; Sharma, P.; Sharma, T. Race-specific genetics of resistance to black rot disease [Xanthomonas campestris pv. campestris (Xcc)(pammel) dowson] and the development of three random amplified polymorphic DNA markers in cauliflower. J. Hortic. Sci. Biotechnol. 2014, 89, 480–486. [Google Scholar] [CrossRef]
- MAJI, A.; NATH, R. Pathogenecity test by using different inoculation methods on Xanthomonas campestris pv. campestris caused of black rot of cabbage. IMPACT IJRANSS 2015, 3, 53–58. [Google Scholar]
- Saha, P.; Kalia, P.; Sharma, M.; Singh, D. New source of black rot disease resistance in Brassica oleracea and genetic analysis of resistance. Euphytica 2016, 207, 35–48. [Google Scholar] [CrossRef]
- Afrin, K.S.; Rahim, M.A.; Park, J.-I.; Natarajan, S.; Rubel, M.H.; Kim, H.-T.; Nou, I.-S. Screening of cabbage (Brassica oleracea L.) germplasm for resistance to black rot. Plant Breed Biotechnol. 2018, 6, 30–43. [Google Scholar] [CrossRef]
- Peňázová, E.; Kopta, T.; Jurica, M.; Pečenka, J.; Eichmeier, A.; Pokluda, R. Testing of inoculation methods and susceptibility testing of perspective cabbage breeding lines (Brassica oleracea convar. capitata) to the black rot disease caused by Xanthomonas campestris pv. campestris. Acta Univ. Agric. Silvic. Mendel. Brun. 2018, 66, 139–148. [Google Scholar] [CrossRef]
- Griffiths, P.D.; Marek, L.F.; Robertson, L.D. Identification of Crucifer accessions from the NC-7 and NE-9 plant introduction collections that are resistant to black rot (Xanthomonas campestris pv. campestris) races 1 and 4. HortScience 2009, 44, 284–288. [Google Scholar] [CrossRef]
- Islam, M.T.; Al Mamun, M.; Lee, B.-R.; Jung, W.-J.; Bae, D.-W.; Kim, T.-H. Role of salicylic acid signaling in the biotrophy-necrotrophy transition of Xanthomonas campestris pv. campestris infection in Brassica napus. Physiol. Mol. Plant Pathol. 2021, 113, 101578. [Google Scholar] [CrossRef]
- Ryals, J.A.; Neuenschwander, U.H.; Willits, M.G.; Molina, A.; Steiner, H.-Y.; Hunt, M.D. Systemic acquired resistance. Plant Cell. 1996, 8, 1809. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, B.N.; Brooks, D.M. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef]
- Islam, M.; Lee, B.-R.; Park, S.-H.; La, V.H.; Bae, D.-W.; Kim, T.-H. Cultivar variation in hormonal balance is a significant determinant of disease susceptibility to Xanthomonas campestris pv. campestris in Brassica napus. Front. Plant Sci. 2017, 8, 2121. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.; Islam, M.; Lee, B.-R.; La, V.H.; Bae, D.-W.; Kim, T.-H. Genotypic variation in resistance gene-mediated calcium signaling and hormonal signaling involved in effector-triggered immunity or disease susceptibility in the Xanthomonas campestris pv. Campestris—Brassica napus pathosystem. Plants 2020, 9, 303. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. Antioxidants and Reactive Oxygen Species in Plants; Blackwell Pub.: Oxford, UK; Ames, IA, USA, 2005; Volume xii, p. 302. [Google Scholar]
- Pandhair, V.; Sekhon, B.S. Reactive oxygen species and antioxidants in plants: An overview. J. Plant Biochem. Biotechnol. 2006, 15, 71–78. [Google Scholar] [CrossRef]
- Amaral, L.S.; Debona, D.; Costa, L.C.; Silva, A.L.R.; Oliveira, J.R.; Rodrigues, F.A. Biochemical insights into basal and induced resistance in cabbage to black rot. J. Phytopathol. 2019, 167, 390–403. [Google Scholar] [CrossRef]
- Roohie, R.K.; Umesha, S. Identification of genes associated with black rot resistance in cabbage through suppression subtractive hybridization. 3 Biotech. 2015, 5, 1089–1100. [Google Scholar] [CrossRef][Green Version]
- Tonguc, M.; Griffiths, P.D. Development of black rot resistant interspecific hybrids between Brassica oleracea L. cultivars and Brassica accession A 19182, using embryo rescue. Euphytica 2004, 136, 313–318. [Google Scholar] [CrossRef]
- Jiang, H.M.; Song, W.Q.; Li, A.; Yang, X.A.; Sun, D.L. Identification of genes differentially expressed in cauliflower associated with resistance to Xanthomonas campestris pv. campestris. Mol. Biol. Rep. 2011, 38, 621–629. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takeda, K. Proteomic analysis of specific proteins in the root of salt-tolerant barley. Biosci. Biotechnol. Biochem. 2009, 73, 2762–2765. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- ThordalChristensen, H.; Zhang, Z.G.; Wei, Y.D.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 2005, 37, 1130–1134. [Google Scholar] [CrossRef]
- Jabs, T.; Dietrich, R.A.; Dangl, J.L. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 1996, 273, 1853–1856. [Google Scholar] [CrossRef]
- Shigenaga, A.M.; Berens, M.L.; Tsuda, K.; Argueso, C.T. Towards engineering of hormonal crosstalk in plant immunity. Curr. Opin. Plant Biol. 2017, 38, 164–172. [Google Scholar] [CrossRef]
- Leah, R.; Tommerup, H.; Svendsen, I.; Mundy, J. Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J. Biol. Chem. 1991, 266, 1564–1573. [Google Scholar] [CrossRef]
- Selitrennikoff, C.P. Antifungal proteins. Appl. Environ. Microbiol. 2001, 67, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, X.; Wang, Q.; Chen, X.; Lv, D.; Xu, K.; Qu, S.; Zhang, Z. Expression of pathogenesis related genes in response to salicylic acid, methyl jasmonate and 1-aminocyclopropane-1-carboxylic acid in Malus hupehensis (Pamp.) Rehd. BMC Res. Notes 2010, 3, 208. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessay 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Yang, S.-R.; Songzhuzhao; Boo, H.O. Antioxidant activity of several cabbage (Brassica oleracea L.) cultivars. Korean J. Plant Res. 2015, 28, 312–320. [Google Scholar] [CrossRef][Green Version]
- Doullah, M.; Mohsin, G.; Ishikawa, K.; Hori, H.; Okazaki, K. Construction of a linkage map and QTL analysis for black rot resistance in Brassica oleracea L. IJNS 2011, 1, 1–6. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. BIO-PROTOCOL 2014, 4, e1108. [Google Scholar] [CrossRef]
- Juszczak, I.; Baier, M. Quantification of superoxide and hydrogen peroxide in leaves. In Plant Cold Acclimation; Springer: New York, NY, USA, 2014; pp. 217–224. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Monakhos, S.G.; Lim, Y.P.; Yi, S.Y. Early Defense Mechanisms of Brassica oleracea in Response to Attack by Xanthomonas campestris pv. campestris. Plants 2021, 10, 2705. https://doi.org/10.3390/plants10122705
Lu L, Monakhos SG, Lim YP, Yi SY. Early Defense Mechanisms of Brassica oleracea in Response to Attack by Xanthomonas campestris pv. campestris. Plants. 2021; 10(12):2705. https://doi.org/10.3390/plants10122705
Chicago/Turabian StyleLu, Lu, Sokrat G. Monakhos, Yong Pyo Lim, and So Young Yi. 2021. "Early Defense Mechanisms of Brassica oleracea in Response to Attack by Xanthomonas campestris pv. campestris" Plants 10, no. 12: 2705. https://doi.org/10.3390/plants10122705
APA StyleLu, L., Monakhos, S. G., Lim, Y. P., & Yi, S. Y. (2021). Early Defense Mechanisms of Brassica oleracea in Response to Attack by Xanthomonas campestris pv. campestris. Plants, 10(12), 2705. https://doi.org/10.3390/plants10122705






