Comparative Metabolomics of Reproductive Organs in the Genus Aesculus (Sapindaceae) Reveals That Immature Fruits Are a Key Organ of Procyanidin Accumulation and Bioactivity
Abstract
:1. Introduction
2. Results
2.1. Metabolic Profiling of Reproductive Organs
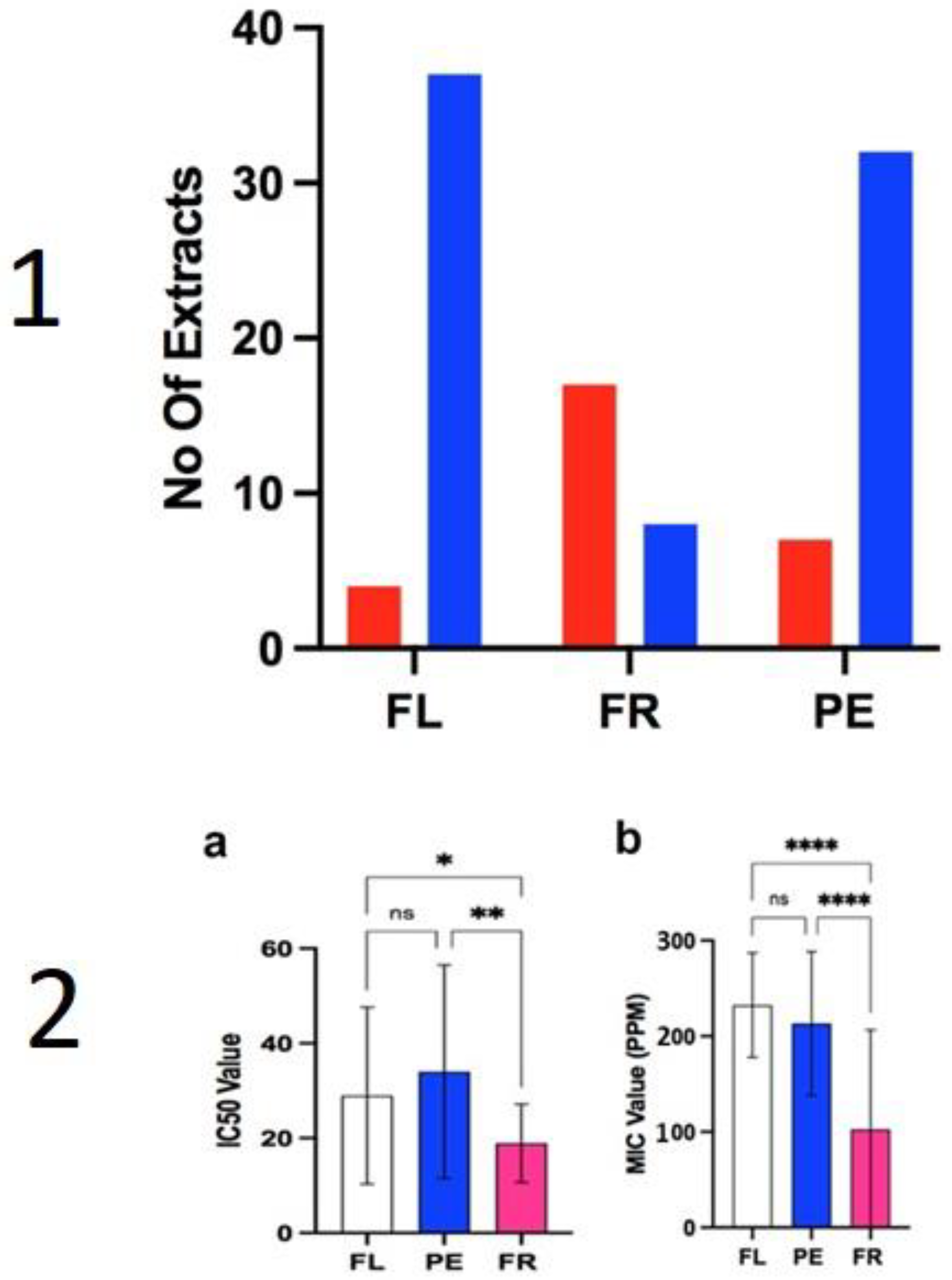
2.2. Anti-Fungal Assays
2.3. Antioxidant Assays
3. Discussion
3.1. Metabolomic Aspects of the Study
3.2. Antifungal Activity
3.3. Antioxidant Activity
4. Materials and Methods
4.1. Samples
4.2. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
4.3. Feature-Based Molecular Networking
4.4. Inhibition of Saccharomyces Cerevisiae
4.5. Antioxidant DPPH Radical Assay
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Z.Y.; Harris, A.J.; Xiang, Q.J. Phylogenomics, co-evolution of ecological niche and morphology, and historical biogeography of buckeyes, horsechestnuts, and their relatives (Hippocastaneae, Sapindaceae) and the value of RAD-Seq for deep evolutionary inferences back to the Late Cretaceous. Mol. Phylogenet. Evol. 2020, 145, 106726. [Google Scholar] [CrossRef] [PubMed]
- RBG-Kew. World Checklist of Vascular Plants. Available online: https://wcvp.science.kew.org/ (accessed on 6 April 2021).
- Idris, S.; Mishra, A.; Khushtar, M. Phytochemical, ethanomedicinal and pharmacological applications of escin from Aesculus hippocastanum L. towards future medicine. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 1–13. [Google Scholar] [CrossRef]
- Yilmaz, R.; Sakcali, S.; Yarci, C.; Aksoy, A.; Ozturk, M. Use of Aesculus hippocastanum L. as a biomonitor of heavy metal pollution. Pak. J. Bot. 2006, 38, 1519–1527. [Google Scholar]
- Karatoprak, G.Ş. Chapter 3.26—Horse Chestnut. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 295–299. [Google Scholar] [CrossRef]
- Dudek-Makuch, M.; Etudzińska-Sroka, E. Horse chestnut—Efficacy and safety in chronic venous insufficiency: An overview. Rev. Bras. Farmacogn. 2015, 25, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Gallelli, L. Escin: A review of its anti-edematous, anti-inflammatory, and venotonic properties. Drug Des. Devel. Ther. 2019, 13, 3425–3437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, H.; Ogawa, S.; Ishihara, T.; Maruoka, M.; Tokuyama-Nakai, S.; Jisaka, M.; Yokota, K. Antioxidant activities and structural characterization of flavonol O-glycosides from seeds of Japanese horse chestnut (Aesculus turbinata BLUME). Food Chem. 2017, 228, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Oszmianski, J.; Kalisz, S.; Aneta, W. The content of phenolic compounds in leaf tissues of white (Aesculus hippocastanum L.) and red horse chestnut (Aesculus carea H.) colonized by the horse chestnut leaf miner (Cameraria ohridella Deschka & Dimic). Molecules 2014, 19, 14625–14636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oszmianski, J.; Kolniak-Ostek, J.; Biernat, A. The content of phenolic compounds in leaf tissues of Aesculus glabra and Aesculus parviflora walt. Molecules 2015, 20, 2176–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Wei, S.; Cao, S.; Zhang, Q.; Kang, N.; Ding, L.; Qiu, F. Bioactive Triterpenoid Saponins From the Seeds of Aesculus chinensis Bge. var. chekiangensis. Front. Chem. 2019, 7, 908. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R. Aescin: Pharmacology, pharmacokinetics and therapeutic profile. Pharmacol. Res. 2001, 44, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittler, M.H.; Ernst, E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst. Rev. 2012, 11, CD003230. [Google Scholar] [CrossRef] [PubMed]
- Cheong, D.H.J.; Arfuso, F.; Sethi, G.; Wang, L.; Hui, K.M.; Kumar, A.P.; Tran, T. Molecular targets and anti-cancer potential of escin. Cancer Lett. 2018, 422, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kovac, I.; Melegova, N.; Coma, M.; Takac, P.; Kovacova, K.; Holly, M.; Durkac, J.; Urban, L.; Gurbalova, M.; Svajdlenka, E.; et al. Aesculus hippocastanum L. Extract Does Not Induce Fibroblast to Myofibroblast Conversion but Increases Extracellular Matrix Production In Vitro Leading to Increased Wound Tensile Strength in Rats. Molecules 2020, 25, 1917. [Google Scholar] [CrossRef]
- Michelini, F.M.; Alché, L.E.; Bueno, C.A. Virucidal, antiviral and immunomodulatory activities of β-escin and Aesculus hippocastanum extract. J. Pharm. Pharmacol. 2018, 70, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Salinas, F.M.; Vazquez, L.; Gentilini, M.V.; Ailin, O.D.; Regueira, E.; Jodar, M.S.N.; Viegas, M.; Michelini, F.M.; Hermida, G.; Alche, L.E.; et al. Aesculus hippocastanum L. seed extract shows virucidal and antiviral activities against respiratory syncytial virus (RSV) and reduces lung inflammation In Vivo. Antivir. Res. 2019, 164, 1–11. [Google Scholar] [CrossRef]
- Yerer, M.B.; Dayan, S.; Han, M.I.; Sharma, A.; Tuli, H.S.; Sak, K. Nanoformulations of Coumarins and the Hybrid Molecules of Coumarins with Potential Anticancer Effects. Anticancer Agents Med. Chem. 2020, 20, 1797–1816. [Google Scholar] [CrossRef] [PubMed]
- Mokdad-Bzeouich, I.; Kovacic, H.; Ghedira, K.; Chebil, L.; Ghoul, M.; Chekir-Ghedira, L.; Luis, J. Esculin and its oligomer fractions inhibit adhesion and migration of U87 glioblastoma cells and in vitro angiogenesis. Tumour. Biol. 2016, 37, 3657–3664. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.; Cevik, O.; Sen, A.; Goger, F.; Sekerler, T.; Sener, A. Effect of Horse-chestnut seed extract on matrix metalloproteinase-1 and -9 during diabetic wound healing. J. Food Biochem. 2019, 43, e12758. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, A.; Kolodziejczyk-Czepas, J.; Woźniak-Serwata, J.; Magiera, A.; Kobiela, N.; Wąsowicz, K.; Olszewska, M.A. Potential Activity Mechanisms of Aesculus hippocastanum Bark: Antioxidant Effects in Chemical and Biological In Vitro Models. Antioxidants 2021, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, L.; Yang, C.; Li, Z.; Rong, S. Procyanidins and Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 5556–5567. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Nikitkova, A.; Abdelsalam, H.; Li, J.; Xiao, J. Activity of quercetin and kaemferol against Streptococcus mutans biofilm. Arch. Oral Biol. 2019, 98, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Žiarovská, J.; Padilla-González, G.F.; Viehmannová, I.; Fernández, E. Genetic and chemical diversity among yacon [Smallanthus sonchifolius (Poepp. et Endl.) H. Robinson] accessions based on iPBS markers and metabolomic fingerprinting. Plant Physiol. Biochem. 2019, 141, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Padilla-González, G.F.; Amrehn, E.; Frey, M.; Gómez-Zeledón, J.; Kaa, A.; Da Costa, F.B.; Spring, O. Metabolomic and gene expression studies reveal the diversity, distribution and spatial regulation of the specialized metabolism of yacon (Smallanthus sonchifolius, Asteraceae). Int. J. Mol. Sci. 2020, 21, 4555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Cao, S.; Huang, W.; Li, P.; Kang, N.; Ding, L.; Qiu, F. New Indole Glycosides from Aesculus chinensis var. chekiangensis and Their Neuroprotective Activities. Molecules 2019, 24, 4063. [Google Scholar] [CrossRef] [Green Version]
- Carullo, G.; Cappello, A.R.; Frattaruolo, L.; Badolato, M.; Armentano, B.; Aiello, F. Quercetin and derivatives: Useful tools in inflammation and pain management. Future Med. Chem. 2017, 9, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.Y.; Jung, Y.S.; Yoon, C.-S.; Oh, J.S.; Hong, J.H.; Kim, Y.-C.; Kim, S.Y. Fraxin Prevents Chemically Induced Hepatotoxicity by Reducing Oxidative Stress. Molecules 2017, 22, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadgrove, N.J.; Jones, G.L. From Petri Dish to Patient: Bioavailability Estimation and Mechanism of Action for Antimicrobial and Immunomodulatory Natural Products. Front. Microbiol. 2019, 10, 2470. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. Biomed Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Ma, B.; Shi, S.; Chen, Y.; Zhang, H.; Zhan, Y.; An, H. Procyanidin B1, a novel and specific inhibitor of Kv10.1 channel, suppresses the evolution of hepatoma. Biochem. Pharmacol. 2020, 178, 114089. [Google Scholar] [CrossRef]
- Bonomo, M.G.; Cafaro, C.; Russo, D.; Calabrone, L.; Milella, L.; Saturnino, C.; Capasso, A.; Salzano, G. Antimicrobial Activity, Antioxidant Properties and Phytochemical Screening of Aesculus hippocastanum Mother Tincture against Food-borne Bacteria. Lett. Drug Des. Discov. 2020, 17, 48–56. [Google Scholar] [CrossRef]
- Dantas-Medeiros, R.; Zanatta, A.C.; de Souza, L.B.F.C.; Fernandes, J.M.; Amorim-Carmo, B.; Torres-Rêgo, M.; de Freitas Fernandes-Pedrosa, M.; Vilegas, W.; de Sousa Araújo, T.A.; Michel, S.; et al. Antifungal and Antibiofilm Activities of B-Type Oligomeric Procyanidins From Commiphora leptophloeos Used Alone or in Combination With Fluconazole Against Candida spp. Front. Microbiol. 2021, 12, 613155. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla-González, G.F.; Sadgrove, N.J.; Ccana-Ccapatinta, G.V.; Leuner, O.; Fernandez-Cusimamani, E. Feature-Based Molecular Networking to Target the Isolation of New Caffeic Acid Esters from Yacon (Smallanthus sonchifolius, Asteraceae). Metabolites 2020, 10, 407. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Testing for Bacteria That Grow Aerobically, 8th ed.; M07-A8; Clinical Laboratory Standards Institute, 2009; Volume 29, pp. 1–66. Available online: https://community.clsi.org/media/1928/m07ed11_sample.pdf (accessed on 28 October 2021).
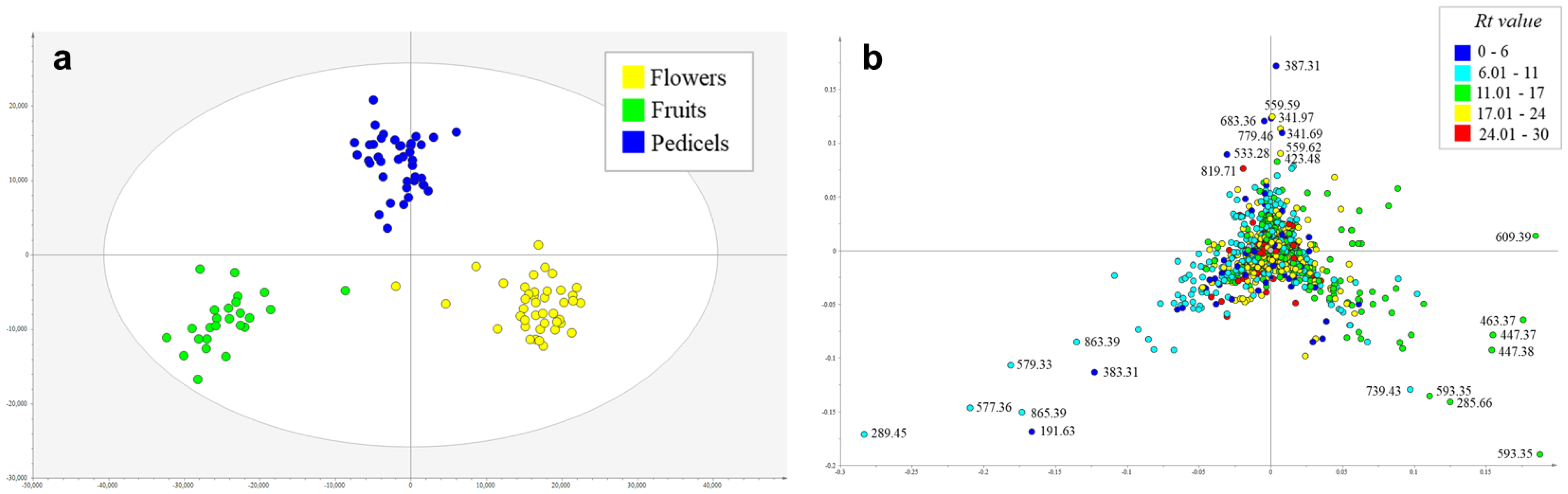
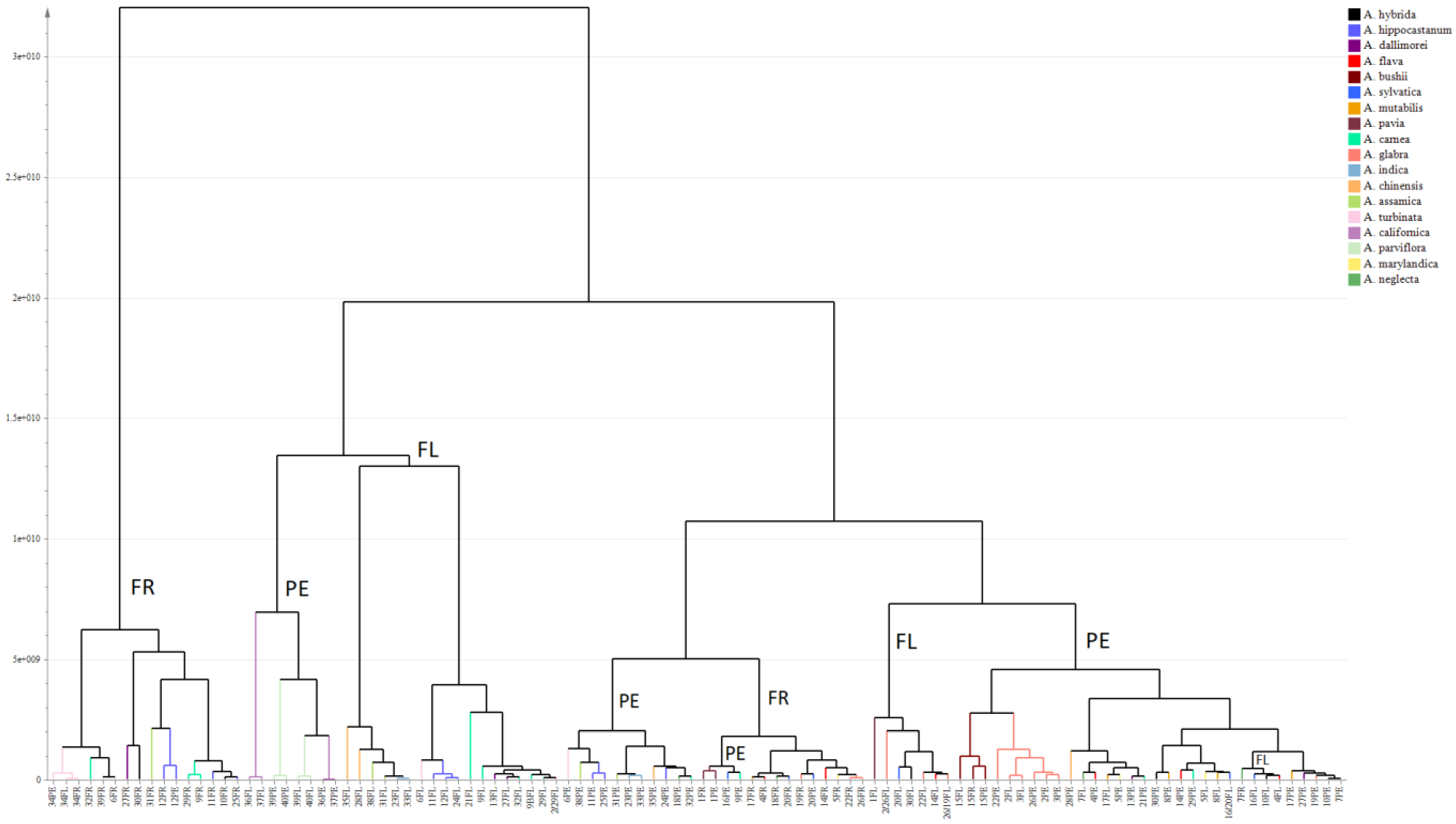
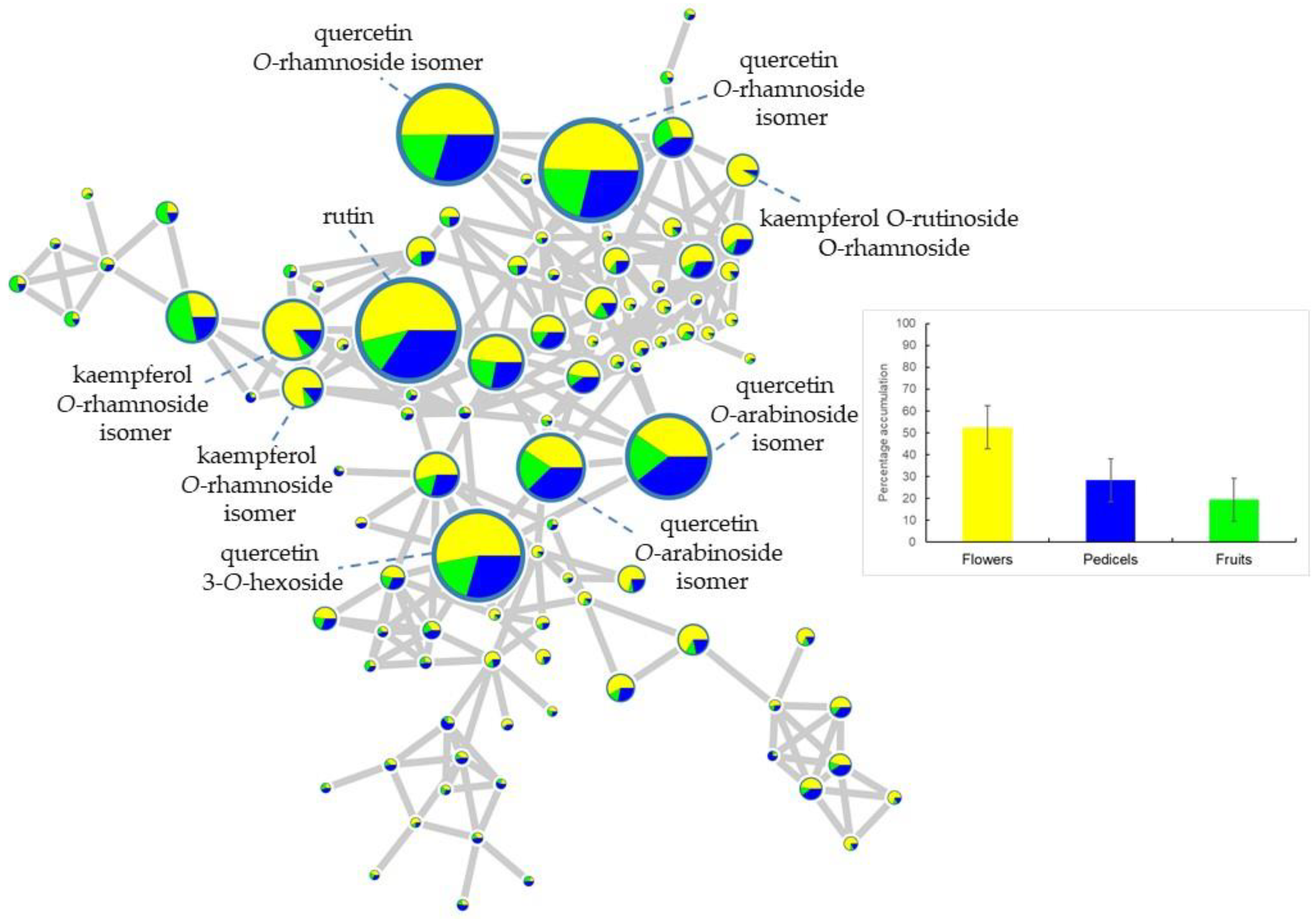
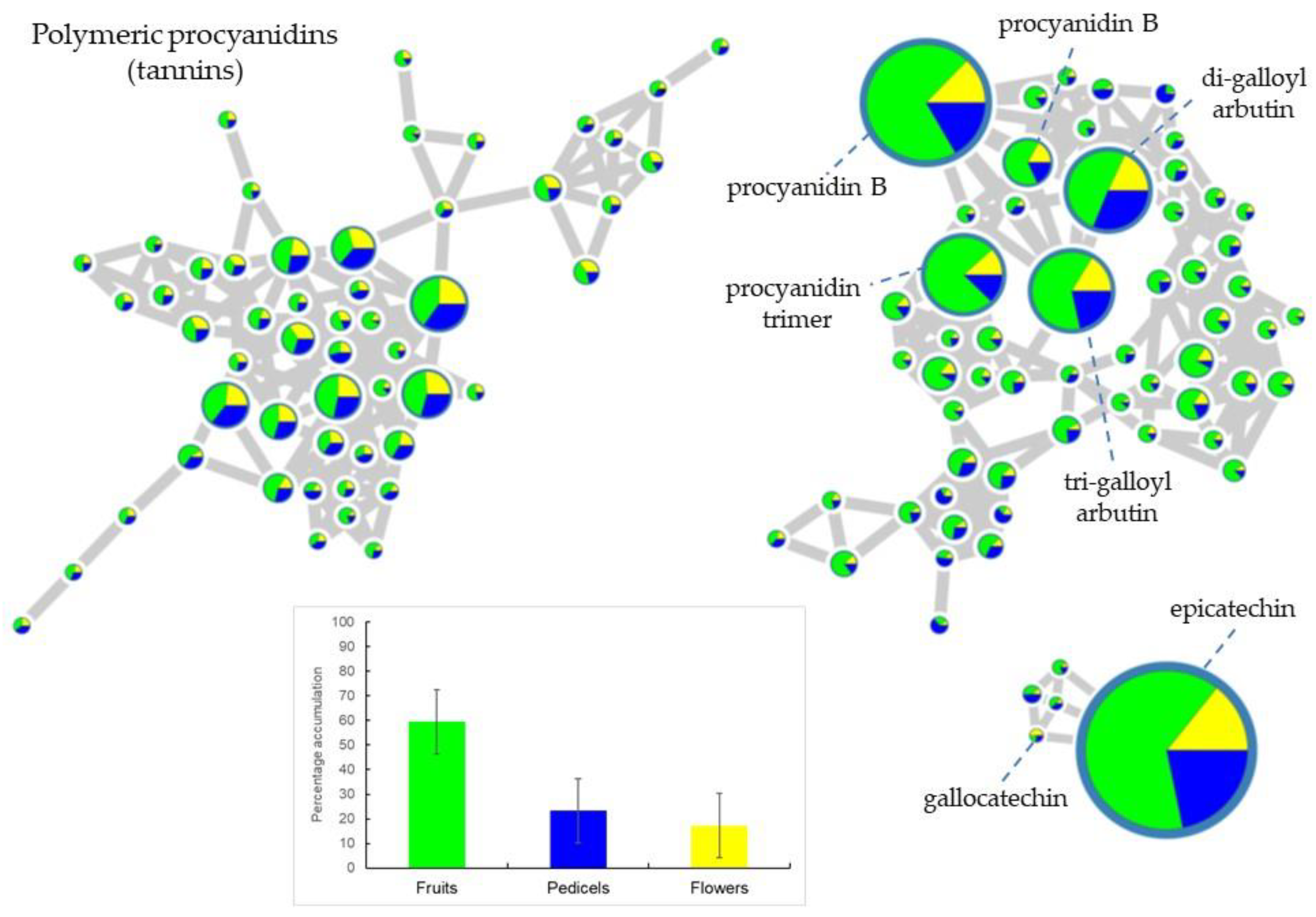
| Rt (min) | m/z | Organ | Tentative Identification | MSn Ions | Conf Level * |
|---|---|---|---|---|---|
| 11.13 | 609.39 | flowers | rutin | 609 → 343, 301 bp, 300, 271, 255, 179 | 2 |
| 12.39 | 593.35 | flowers | kaempferol O-rutinoside isomer 2 | 593 → 285 bp → 267, 257 bp, 241, 229, 213 | 3 |
| 11.38 | 463.37 | flowers | quercetin 3-O-hexoside | 463 → 301 bp → 271, 255, 179 bp, 151 | 2 |
| 12.55 | 447.37 | flowers | quercetin 3-O-rhamnoside | 447 → 301 bp → 271, 255, 179 bp, 151 | 2 |
| 16.04 | 285.67 | flowers | kaempferol | 285 → 284, 257, 229, 213, 151 | 2 |
| 12.22 | 593.35 | flowers | kaempferol O-rutinoside isomer 1 | 593 → 285 bp → 267, 257, 255 bp, 241, 229, 213 | 3 |
| 10.29 | 739.42 | flowers | kaempferol O-rutinoside-O-rhamnoside | 739 → 693, 593, 575 bp, 473, 393, 327, 285, 227, 255, 285 → 255 bp, 241, 227, 213, 151 | 3 |
| 7.93 | 289.45 | fruits | epicatechin | 289 → 271, 245 bp, 205, 179 | 2 |
| 6.82 | 577.36 | fruits | procyanidin B | 577 → 559, 451, 425 bp, 407, 299, 289, 245 | 3 |
| 7.94 | 579.32 | fruits | dimer of epicatechin | 579 → 289 bp → 271, 245 bp, 205, 179 | 2 |
| 7.62 | 865.39 | fruits | procyanidin trimer | 856 → 847, 739, 713, 695 bp, 587, 577, 575, 543, 451, 449, 425, 407, 287, 245 | 3 |
| 1.80 | 191.63 | fruits | quinic acid | 191 → 173, 155, 127, 111, 99, 93, 85 bp, 71, 59 | 2 |
| 7.30 | 863.39 | fruits | aesculitannin or isomer | 863 → 711 bp, 693, 573, 559, 541, 531, 451, 411, 299, 289, 285 | 3 |
| 1.80 | 383.31 | fruits | dimer of quinic acid | 383 → 191 bp → 173, 155, 127, 111, 99, 93, 85 bp, 71, 59 | 2 |
| 1.69 | 387.31 | pedicels | sucrose [FA adduct] | 341 → 179 bp, 161, 143, 131, 119, 113, 101 | 2 |
| 22.25 | 559.59 | pedicels | n.i. | 559 → 513 bp, 277, 253 | 4 |
| 1.69 | 341.97 | pedicels | sucrose | 341 → 179 bp, 161, 143, 131, 119, 113, 101 | 2 |
| 1.69 | 683.36 | pedicels | dimer of sucrose | 683 → 341 bp → 179 bp, 161, 143, 131, 119, 113, 101 | 2 |
| 17.07 | 779.46 | pedicels | n.i. [FA adduct] | 733 → 651, 633 bp, 375, 357 | 4 |
| 1.77 | 533.28 | pedicels | quinic acid glucoside | 533 → 191 bp → 173, 155, 127, 111, 99, 93, 85 bp, 71, 59 | 2 |
| 13.18 | 423.48 | pedicels | n.i. | 423 → 279 bp, 249, 205, 169, 139 | 4 |
| 26.57 | 819.70 | pedicels | n.i. | 819 → 773, 513, 277 bp | 4 |
| Flowers | Fruits | Pedicel | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Authority | Replicate No. | Code | DPPH | MIC | DPPH | MIC | DPPH | MIC |
| A. hippocastanum | L. | 1 | 11 | 20.5 | n.i * | 11.6 | n.i | 33.7 | n.i |
| 2 | 12 | 41 | n.i | 14.8 | n.i | 20 | n.i | ||
| 3 | 24 | 33.9 | n.i | - * | - | 138 | n.i | ||
| 4 | 25 | - | n.i | 11.7 | 125 | 12.1 | n.i | ||
| A. hybrida | DC. | 1 | 10 | 38.5 | n.i | 19.9 | n.i | 42.2 | n.i |
| 2 | 30 | 29 | n.i | 12 | 20.85 | 30 | n.i | ||
| A. flava | Sol. | 1 | 14 | 6 | n.i | 46.2 | 125 | 43.2 | n.i |
| 2 | 4 | 9.8 | n.i | 19.6 | 62.5 | 40.5 | n.i | ||
| A. indica | (Wall. ex Cambess.) Hook | 1 | 33 | 29.8 | 208.35 | - | - | 14.3 | n.i |
| 2 | 23 | 22.1 | n.i | - | - | 23.1 | n.i | ||
| A. chinensis | (Rehder) Turland and N.H.Xia | 1 | 28 | 22.6 | n.i | - | - | 5.5 | n.i |
| 2 | 35 | 14.1 | n.i | - | - | 35.4 | n.i | ||
| A. assamica | Griff | 1 | 38 | 41.5 | n.i | - | - | 52.1 | n.i |
| 2 | 31 | 35.7 | n.i | 15.2 | 62.5 | 16.7 | n.i | ||
| A. turbinata | Blume | 1 | 34 | 9.1 | n.i | 15.4 | n.i | 12.2 | n.i |
| 2 | 6 | 52.3 | n.i | 13.6 | n.i | 23.5 | n.i | ||
| A. californica | (Spach) Nutt. | 1 | 36 | 45.2 | 166.65 | - | - | 32.2 | n.i |
| 2 | 37 | 48.8 | 125 | - | - | 1289.7 | n.i | ||
| A. parviflora | Walter | 1 | 39 | 53.3 | n.i | 16.2 | n.i | 34.1 | n.i |
| 2 | 40 | 47 | n.i | - | - | 43.3 | n.i | ||
| A. neglecta | Lindl. | 1 | 7 | 11.1 | 62.5 | 33.9 | n.i | 5.5 | 166.65 |
| 2 | 18 | - | - | 19.4 | 83.35 | 33.2 | n.i | ||
| A. mutabilis | (Spach) Scheele | 1 | 8 | 15.4 | n.i | - | - | 22.2 | 166.65 |
| 2 | 17 | 24.8 | n.i | 23.5 | 41.65 | 31.7 | n.i | ||
| A. carnea | Zeyh ** | 1 | 21 | 42.3 | n.i | 29.3 | 31.25 | 22 | n.i |
| 2 | 32 | 19.3 | n.i | 15.9 | 62.5 | 7.8 | n.i | ||
| 3 | 9 | 28.2 | n.i | 15.2 | n.i | 43.3 | 208.35 | ||
| 4 | 9 | 37.2 | n.i | - | - | - | - | ||
| 5 | 29 | 46.1 | n.i | 9.4 | 83.35 | 37.4 | n.i | ||
| A. sylvatica | W.Bartram | 1 | 16/20 | 19.3 | n.i | - | - | - | - |
| 2 | 20 | 19.7 | n.i | - | - | 28.4 | 83.35 | ||
| 3 | 16 | 8.2 | n.i | - | - | 48.1 | 125 | ||
| A. glabra | (Buckley) Rob. | 1 | 29 | 26 | n.i | - | - | - | - |
| 2 | 26 | 45.3 | n.i | 18.3 | 62.5 | 39.9 | n.i | ||
| 3 | 19 | 18.1 | n.i | 23.2 | 31.25 | 48 | n.i | ||
| 4 | 22 | 40.3 | n.i | 10.5 | 62.5 | 24.4 | n.i | ||
| 5 | 2 | 4.1 | n.i | - | - | 24.6 | n.i | ||
| 6 | 3 | 5.6 | n.i | - | - | 69.3 | 166.65 | ||
| A. dallimorei | Sealy ** | 1 | 13 | 98.6 | n.i | - | - | 48 | n.i |
| 2 | 27 | 16.2 | n.i | 16.7 | 125 | 51.8 | n.i | ||
| A. marylandica | Booth ex Dippel | 1 | 5 | 3.7 | n.i | 27.5 | 62.5 | 30.6 | n.i |
| A. bushii | C.K.Schneid | 1 | 15 | 45.8 | n.i | 19.2 | 41.65 | - | 83.35 |
| A. pavia | L. | 1 | 1 | 12.5 | n.i | 15 | 41.65 | 24 | 62.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Green, A.; Padilla-Gonzalez, G.F.; Phumthum, M.; Simmonds, M.S.J.; Sadgrove, N.J. Comparative Metabolomics of Reproductive Organs in the Genus Aesculus (Sapindaceae) Reveals That Immature Fruits Are a Key Organ of Procyanidin Accumulation and Bioactivity. Plants 2021, 10, 2695. https://doi.org/10.3390/plants10122695
Green A, Padilla-Gonzalez GF, Phumthum M, Simmonds MSJ, Sadgrove NJ. Comparative Metabolomics of Reproductive Organs in the Genus Aesculus (Sapindaceae) Reveals That Immature Fruits Are a Key Organ of Procyanidin Accumulation and Bioactivity. Plants. 2021; 10(12):2695. https://doi.org/10.3390/plants10122695
Chicago/Turabian StyleGreen, Alison, Guillermo Federico Padilla-Gonzalez, Methee Phumthum, Monique S. J. Simmonds, and Nicholas J. Sadgrove. 2021. "Comparative Metabolomics of Reproductive Organs in the Genus Aesculus (Sapindaceae) Reveals That Immature Fruits Are a Key Organ of Procyanidin Accumulation and Bioactivity" Plants 10, no. 12: 2695. https://doi.org/10.3390/plants10122695
APA StyleGreen, A., Padilla-Gonzalez, G. F., Phumthum, M., Simmonds, M. S. J., & Sadgrove, N. J. (2021). Comparative Metabolomics of Reproductive Organs in the Genus Aesculus (Sapindaceae) Reveals That Immature Fruits Are a Key Organ of Procyanidin Accumulation and Bioactivity. Plants, 10(12), 2695. https://doi.org/10.3390/plants10122695







