Evaluation of Three Antimicrobial Peptides Mixtures to Control the Phytopathogen Responsible for Fire Blight Disease
Abstract
:1. Introduction
2. Results
2.1. Peptide Synthesis
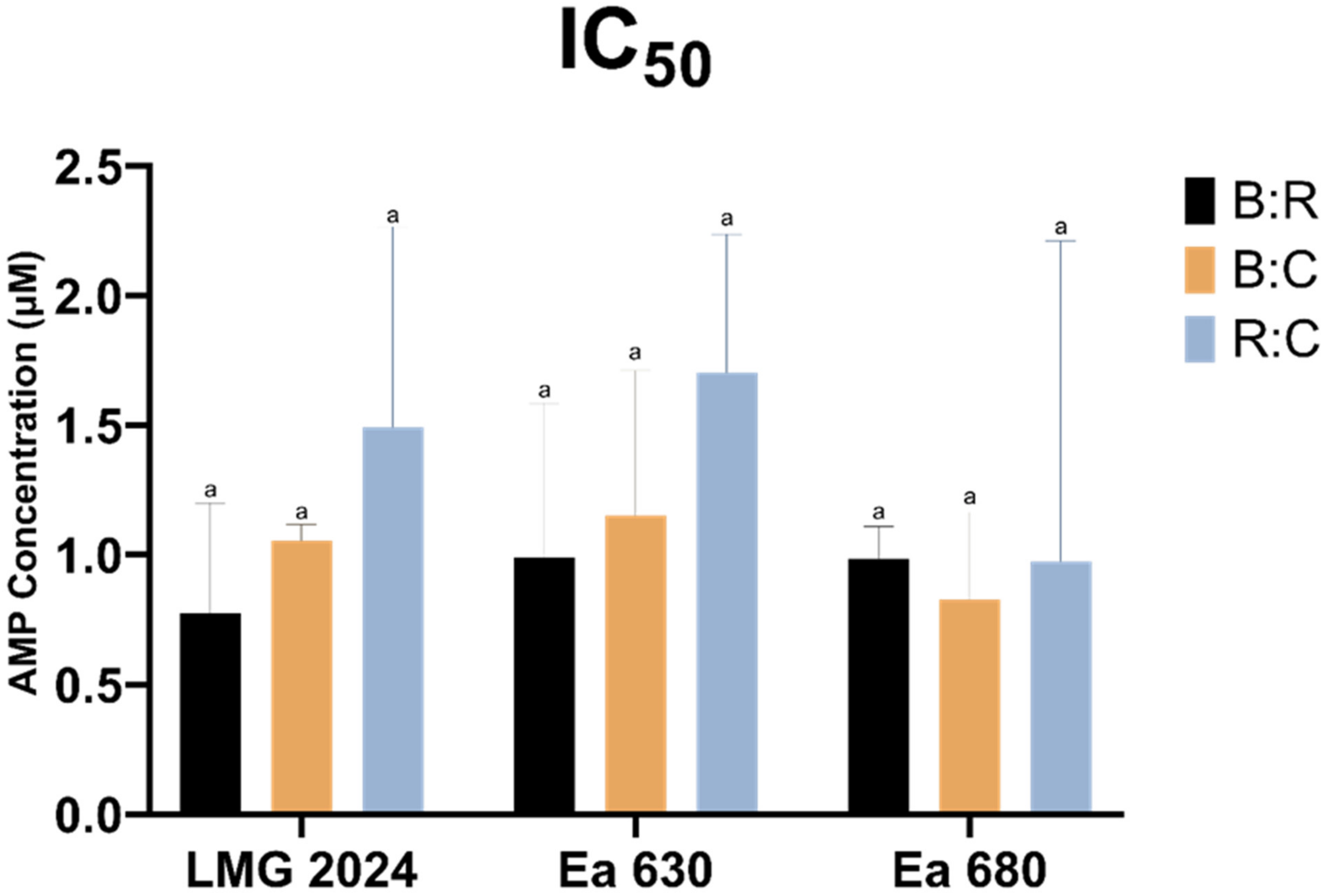
2.2. AMPs Antimicrobial Activity
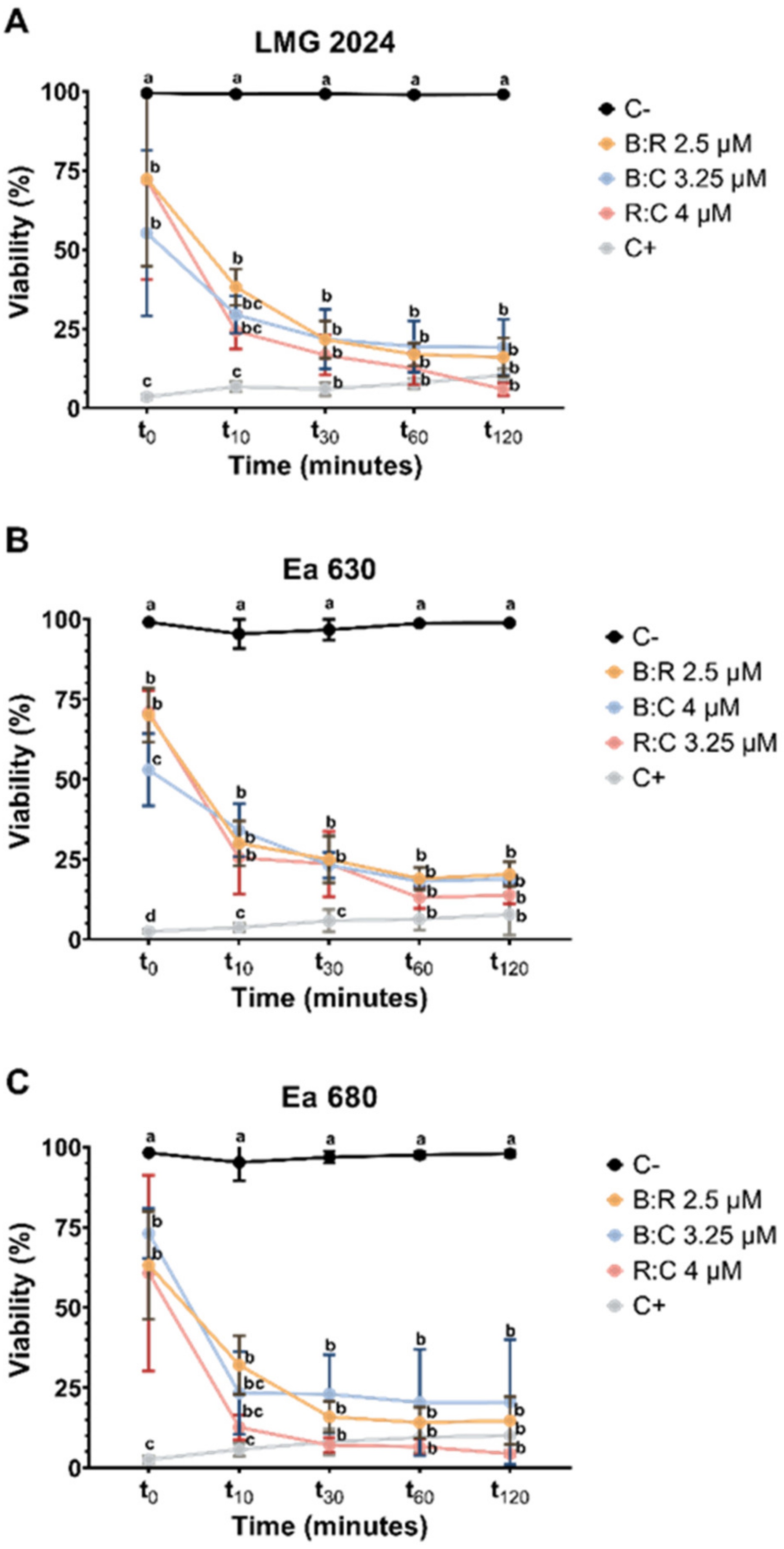
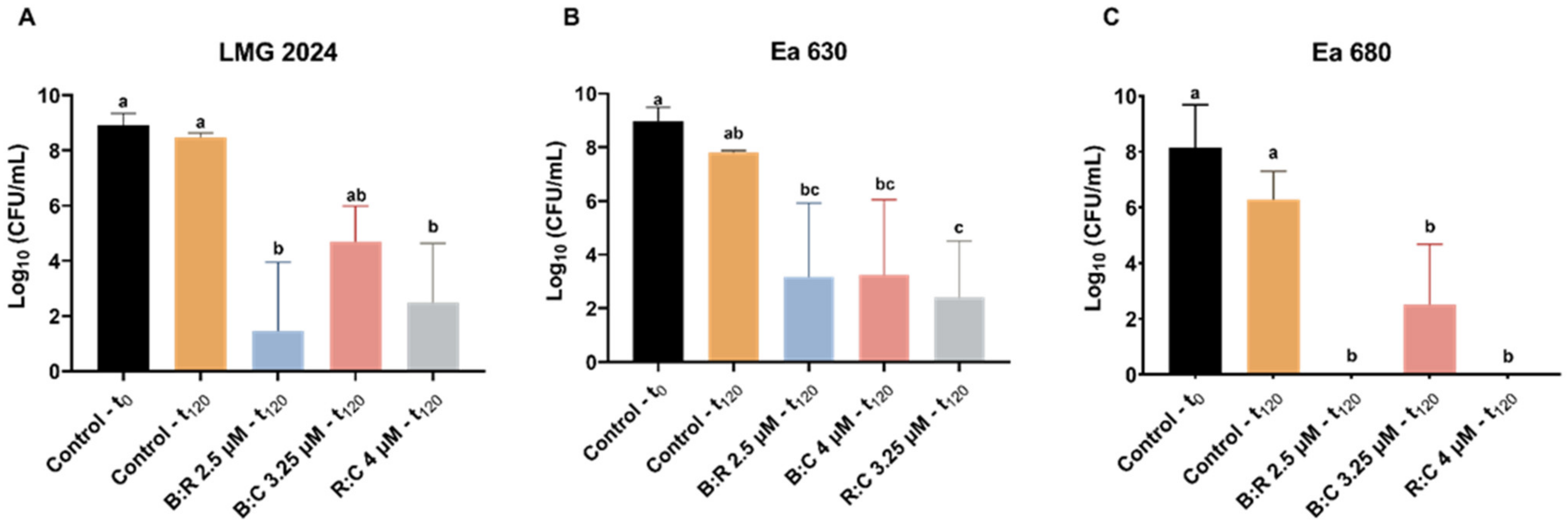
2.3. Viability Determination after Exposure to AMPs Mixture
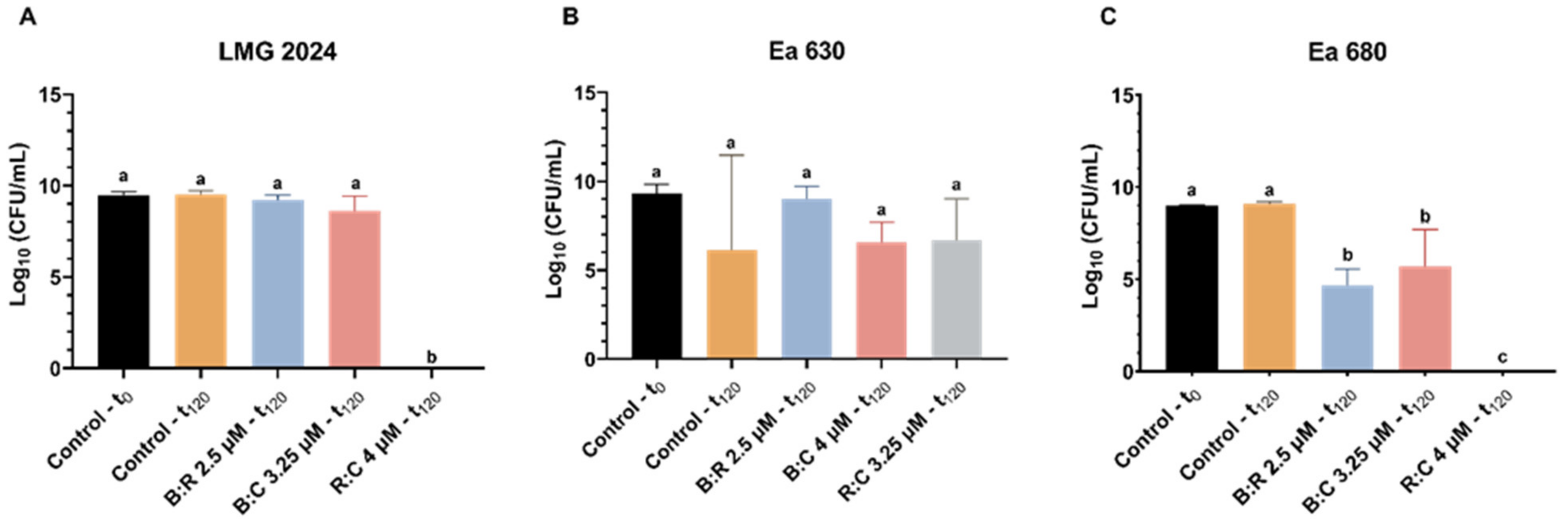
2.3.1. Flow Cytometry Assessment of Viability
2.3.2. AB Reduction Viability Assessment
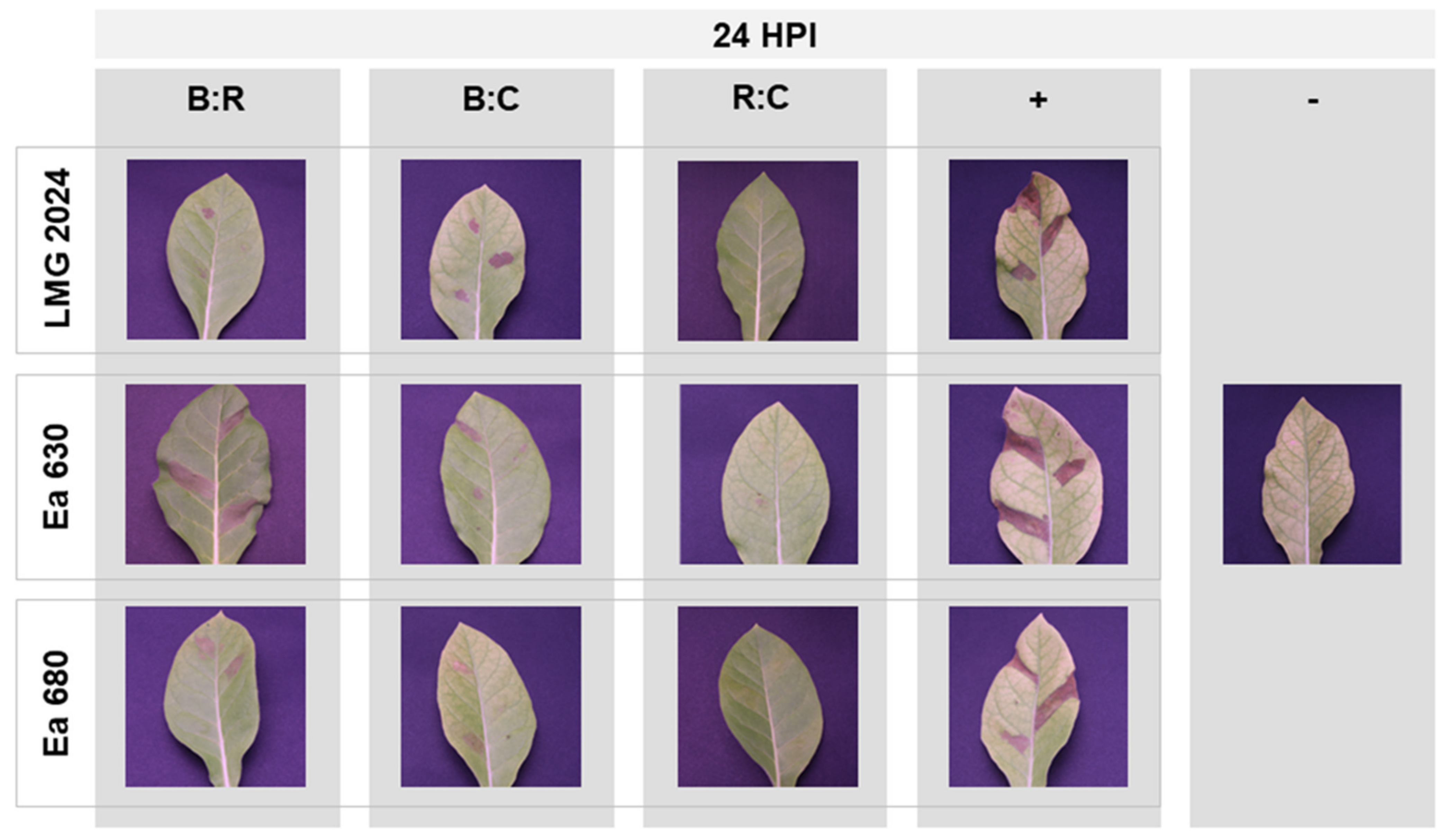
2.4. Hypersensitive Response to AMPs
3. Discussion
4. Materials and Methods
4.1. Peptide Selection and Synthesis
4.2. Bacterial Strains and Conditions
4.3. Bacterial Susceptibility to AMPs Mixtures
4.4. Bacterial Viability Assays
4.4.1. Membrane Permeability Analysis
4.4.2. alamarBlue™ Cell Viability Assessment
4.5. Hypersensitive Response in Tobacco Plants
4.6. Assessment of Colony Forming Units (CFU)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Zwet, T.; Orolaza-Halbrendt, N.; Zeller, W. Fire Blight, History, Biology and Management; APS Press: St. Paul, MN, USA, 2012. [Google Scholar]
- Sundin, G.W. Infectious Diseases. In Compendium of Apple and Pear Diseases and Pests, 2nd ed.; Sutton, T.B., Aldwinckle, H.S., Agnello, A.M., Walgenbach, J.F., Eds.; American Phytopathological Society: St. Paul, MN, USA, 2014; Chapter 1. [Google Scholar]
- Marco-Noales, E.; Peñalver, J.; Navarro, I.; Gorris, M.T.; Morente, M.C.; Balguerías, C.; López, M.M. Iberian wild pear (Pyrus bourgaeana) is a new host of Erwinia amylovora, the causal agent of fire blight. Plant Dis. 2017, 101, 502. [Google Scholar] [CrossRef]
- Rezzonico, F.; Smits, T.H.M.; Duffy, B. Diversity, evolution, and functionality of clustered regularly interspaced short palindromic repeat (CRISPR) regions in the fire blight pathogen Erwinia amylovora. App. Environ. Microbiol. 2011, 77, 3819–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.Y.; Yun, Y.H.; Kim, G.; Kim, S.H.; Lee, S.J.; Kim, J.F. Genome analysis of Erwinia amylovora strains responsible for a fire blight outbreak in Korea. Plant Dis. 2020, 105, 1143–1152. [Google Scholar] [CrossRef]
- Mendes, R.J.; Luz, J.P.; Santos, C.; Tavares, F. CRISPR genotyping as complementary tool for epidemiological surveillance of Erwinia amylovora outbreaks. PLoS ONE 2021, 16, e0250280. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 473/2002. Off. J. Eur. Communities 2002, 006, 463–486. [Google Scholar]
- Shtienberg, D.; Manulis-Sasson, S.; Zilberstaine, M.; Oppenheim, D.; Shwartz, H. The incessant battle against fire blight in pears: 30 years of challenges and successes in managing the disease in israel. Plant Dis. 2015, 99, 1048–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Sundin, G.W.; Wang, N. Antibiotic resistance in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef]
- Mendes, R.J.; Mariz-Ponte, N.; Correia, C.V.; Dias, M.C.; Sousa, M.L.; Tavares, F.; Santos, C. Fire blight management: Physiological assessment of cultural control by pruning in pear orchards. Agriculture 2020, 66, 128–136. [Google Scholar] [CrossRef]
- Dagher, F.; Nickzad, A.; Zheng, J.; Hoffmann, M.; Déziel, E. Characterization of the biocontrol activity of three bacterial isolates against the phytopathogen Erwinia amylovora. MicrobiologyOpen 2021, 10, e1202. [Google Scholar] [CrossRef] [PubMed]
- Grace, E.R.; Rabiey, M.; Friman, V.P.; Jackson, R.W. Seeing the forest for the trees: Use of phages to treat bacterial tree diseases. Plant Pathol. 2021. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Lupascu, L.; Aricu, A.; Dragalin, I.; Popescu, V.; Geana, E.I.; Ionete, R.E.; Vornicu, N.; Duliu, O.G.; Hristozova, G.; et al. Chemical composition and assessment of antimicrobial activity of lavender essential oil and some by-products. Plants 2021, 10, 1829. [Google Scholar] [CrossRef] [PubMed]
- Caravaca-Fuentes, P.; Camó, C.; Oliveras, À.; Baró, A.; Francés, J.; Badosa, E.; Planas, M.; Feliu, L.; Montesinos, E.; Bonaterra, A. A bifunctional peptide conjugate that controls infections of Erwinia amylovora in pear plants. Molecules 2021, 26, 3426. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.F.; Qutb, A.M.; Dong, W. Prediction and activity of a cationic α-helix antimicrobial peptide zm-804 from maize. Int. J. Mol. Sci. 2021, 22, 2643. [Google Scholar] [CrossRef]
- Mendes, R.J.; Regalado, L.; Luz, J.P.; Tassi, N.; Teixeira, C.; Gomes, P.; Tavares, F.; Santos, C. In Vitro evaluation of five antimicrobial peptides against the plant pathogen Erwinia amylovora. Biomolecules 2021, 11, 554. [Google Scholar] [CrossRef]
- Li, W.; Separovic, F.; O’Brien-Simpson, N.M.; Wade, J. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem. Soc. Rev. 2021, 50, 4932. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.U. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Shi, G.; Kang, X.; Dong, F.; Liu, Y.; Zhu, N.; Hu, Y.; Xu, H.; Lao, X.; Zheng, H. DRAMP 3.0: An enhanced comprehensive data repository of antimicrobial peptides. Nucleic Acids Res. 2021, 14, gkab651. [Google Scholar] [CrossRef]
- Goyal, R.K.; Mattoo, A.K. Multitasking antimicrobial peptides in plant development and host defense against biotic/abiotic stress. Plant Sci. 2014, 228, 135–149. [Google Scholar] [CrossRef]
- Ilyas, H.; Datta, A.; Bhunia, A. An approach towards structure based antimicrobial peptide design for use in development of transgenic plants: A strategy for plant disease management. Curr. Med. Chem. 2017, 24, 1350–1364. [Google Scholar] [CrossRef] [PubMed]
- Spohn, R.; Daruka, L.; Lázár, V.; Martins, A.; Vidovics, F.; Grézal, G.; Méhi, O.; Kintses, B.; Számel, M.; Jangir, P.K.; et al. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat. Commun. 2019, 10, 4538. [Google Scholar] [CrossRef]
- Baró, A.; Badosa, E.; Montesinos, L.; Feliu, L.; Planas, M.; Montesinos, E.; Bonaterra, A. Screening and identification of BP100 peptide conjugates active against Xylella fastidiosa using a viability-qPCR method. BMC Microbiol. 2020, 20, 229. [Google Scholar] [CrossRef]
- Camó, C.; Bonaterra, A.; Badosa, E.; Baró, A.; Montesinos, L.; Montesinos, E.; Planas, M.; Feliu, L. Antimicrobial peptide KSL-W and analogues: Promising agents to control plant diseases. Peptides 2019, 112, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Mariz-Ponte, N.; Regalado, L.; Gimranov, E.; Tassi, N.; Moura, L.; Gomes, P.; Tavares, F.; Santos, C.; Teixeira, C. A Synergic Potential of Antimicrobial Peptides against Pseudomonas syringae pv. actinidiae. Molecules 2021, 26, 1461. [Google Scholar] [CrossRef]
- Badosa, E.; Ferre, R.; Planas, M.; Feliu, L.; Besaluâ, E.; Cabrefiga, J.; Bardají, E.; Montesinos, E. A library of linear undecapeptides with bactericidal activity against phytopathogenic bacteria. Peptides 2007, 28, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Güell, I.; Cabrefiga, J.; Badosa, E.; Ferre, R.; Talleda, M.; Bardají, E.; Planas, M.; Feliu, L.; Montesinos, E. Improvement of the efficacy of linear undecapeptides against plant-pathogenic bacteria by incorporation of D-amino acids. Appl. Environ. Microb. 2011, 77, 2667–2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badosa, E.; Montesinos, L.; Montesinos, E.; Feliu, L.; Planas, M.; Bardají, E. Prospects and limitations of synthetic antimicrobial peptides for fire blight control. Acta Hortic. 2014, 1056, 111–116. [Google Scholar] [CrossRef]
- Badosa, E.; Montesinos, L.; Camó, C.; Ruz, L.; Cabrefiga, J.; Francés, J.; Gascón, B.; Planas, M.; Feliu, L.; Montesinos, E. Control of fire blight infections with synthetic peptides that elicit plant defense responses. J. Plant Pathol. 2017, 99, 65–73. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Tarighi, S.; Taheri, P. Effect of antimicrobial peptides and monoterpenes on control of fire blight. Span. J. Agric. Res. 2020, 18, 1–15. [Google Scholar] [CrossRef]
- López-Rojas, R.; Docobo-Pérez, F.; Pachón-Ibáñez, M.E.; De La Torre, B.G.; Fernández-Reyes, M.; March, C.; Bengoechea, J.A.; Andreu, D.; Rivas, L.; Pachón, J. Efficacy of cecropin A-melittin peptides on a sepsis model of infection by pan-resistant Acinetobacter baumannii. Eur. J. Clin. Microbiol. 2011, 20, 1391–1398. [Google Scholar] [CrossRef]
- Torcato, I.M.; Huang, Y.H.; Franquelim, H.G.; Gaspar, D.; Craik, D.J.; Castanho, M.A.R.B.; Troeira, H.S. Design and characterization of novel antimicrobial peptides, R-BP100 and RW-BP100, with activity against Gram-negative and Gram-positive bacteria. BBA Biomembr. 2013, 1828, 944–955. [Google Scholar] [CrossRef] [Green Version]
- Ciandrini, E.; Morroni, G.; Arzeni, D.; Kamysz, W.; Neubauer, D.; Kamysz, E.; Cirioni, O.; Brescini, L.; Baffone, W.; Campana, R. Antimicrobial activity of different antimicrobial peptides (AMPs) against clinical methicillin-resistant Staphylococcus aureus (MRSA). Curr. Top. Med. Chem. 2018, 18, 2116–2126. [Google Scholar] [CrossRef] [PubMed]
- Badosa, E.; Ferre, R.; Franceâs, J.; Bardajõâ, E.; Feliu, L.; Planas, M.; Montesinos, E. Sporicidal activity of synthetic antifungal undecapeptides and control of Penicillium rot of apples. Appl. Environ. Microbiol. 2009, 75, 5563–5569. [Google Scholar] [CrossRef] [Green Version]
- Camó, C.; Torné, M.; Besalú, E.; Rosés, C.; Cirac, A.D.; Moiset, G.; Badosa, E.; Bardaji, E.; Montesinos, E.; Planas, M.; et al. Tryptophan-containing cyclic decapeptides with activity against plant pathogenic bacteria. Molecules 2017, 22, 1817. [Google Scholar] [CrossRef] [Green Version]
- Chahardoli, M.; Fazeli, A.; Ghabooli, M. Antimicrobial activity of LFchimera synthetic peptide against plant pathogenic bacteria. Arch. Phytopathol. Phytopatol. Plant Prot. 2017, 50, 1008–1018. [Google Scholar] [CrossRef]
- Cirac, A.D.; Torné, M.; Badosa, E.; Montesinos, E.; Salvador, P.; Feliu, L.; Planas, M. Rational design of cyclic antimicrobial peptides based on BPC194 and BPC198. Molecules 2017, 22, 1054. [Google Scholar] [CrossRef] [Green Version]
- De Zotti, M.; Sella, L.; Bolzonello, A.; Gabbatore, L.; Peggion, C.; Bortolotto, A.; Elmaghraby, I.; Tundo, S.; Favaron, F. Targeted amino acid substitutions in a trichoderma peptaibol confer activity against fungal plant pathogens and protect host tissues from Botrytis cinerea infection. Int. J. Mol. Sci. 2020, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cabrefiga, J.; Montesinos, E. Lysozyme enhances the bactericidal effect of BP100 peptide against Erwinia amylovora, the causal agent of fire blight of rosaceous plants. BMC Microbiol. 2017, 17, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheard, D.E.; O’Brien-Simpson, N.M.; Wade, J.D.; Separovic, F. Combating bacterial resistance by combination of antibiotics with antimicrobial peptides. Pure Appl. Chem. 2019, 91, 199–209. [Google Scholar] [CrossRef]
- Ilyas, H.; Kim, J.; Lee, D.; Malmsten, M.; Bhunia, A. Structural insights into the combinatorial effects of antimicrobial peptides reveal a role of aromatic-aromatic interactions in antibacterial synergism. J. Biol. Chem. 2019, 294, 14615–14633. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, R.; White, P.; Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22, 4–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Ramasamy, R.P. Current and prospective methods for plant disease detection. Biosensors 2015, 5, 537–561. [Google Scholar] [CrossRef] [Green Version]
- Oliveras, À.; Baró, A.; Montesinos, L.; Badosa, E.; Montesinos, E.; Feliu, L.; Planas, M. Antimicrobial activity of linear lipopeptides derived from BP100 towards plant pathogens. PLoS ONE 2018, 13, e0201571. [Google Scholar] [CrossRef] [PubMed]
- Glossop, H.D.; Zoysa, G.H.; Pilkington, L.I.; Barker, D.; Sarojini, V. Fluorinated O-phenylserine residues enhance the broad-spectrum antimicrobial activity of ultrashort cationic lipopeptides. J. Fluor. Chem. 2021, 241, 109685. [Google Scholar] [CrossRef]
- Li, J.; Koh, J.J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane active antimicrobial peptides: Translating mechanistic insights to design. Front. Neurosci. Switz. 2017, 11, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.W.; Lin, Y.M.; Wang, C.F.; Liaos, Y.D. Outer membrane lipoprotein Lpp is gram-negative bacterial cell surface receptor for cationic antimicrobial peptides. J. Biol. Chem. 2012, 287, 418–428. [Google Scholar] [CrossRef] [Green Version]
- Kurowska, M.; Eickenscheidt, A.; Guevara-Solarte, D.L.; Widyaya, V.T.; Marx, F.; Al-Ahmad, A.; Lienkamp, K. A simultaneously antimicrobial, protein-repellent, and cell-compatible polyzwitterion network. Biomacromolecules 2017, 18, 1373–1386. [Google Scholar] [CrossRef]
- Taute, H.; Bester, M.J.; Gaspar, A.R.M. The dual functionality of antimicrobial peptides Os and Os-C in human leukocytes. J. Pept. Sci. 2019, 25, e3156. [Google Scholar] [CrossRef]
- Qutb, A.M.; Wei, F.; Dong, W. Prediction and characterization of cationic arginine-rich plant antimicrobial peptide sm-985 from teosinte (Zea mays ssp. mexicana). Front. Microbiol. 2020, 11, 1353. [Google Scholar] [CrossRef]
- Pacor, S.; Benincasa, M.; Musso, M.V.; Krce, L.; Aviani, I.; Pallavicini, A.; Scocchi, M.; Gerdol, M.; Mardirossian, M. The proline-rich myticalins from Mytilus galloprovincialis display a membrane-permeabilizing antimicrobial mode of action. Peptides 2021, 143, 170594. [Google Scholar] [CrossRef]
- Mehring, A.; Erdmann, N.; Walther, J.; Stiefelmaier, J.; Strieth, D.; Ulber, R. A simple and low-cost resazurin assay for vitality assessment across species. J. Biotechnol. 2021, 333, 63–66. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Xue, Z.; Jia, Y.; Li, R.; He, C.; Chen, H. The structure-mechanism relationship and mode of actions of antimicrobial peptides: A review. Trends Food Sci. Tech. 2021, 109, 103–115. [Google Scholar] [CrossRef]
- Piqué, N.; Miñana-Galbis, D.; Merino, S.; Tomás, J.M. Virulence factors of Erwinia amylovora: A review. Int. J. Mol. Sci. 2015, 16, 12836–12854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, P.; Glossop, H.; Meikle, T.G.; Aburto-Medina, A.; Conn, C.E.; Sarojini, V.; Valery, C. Molecular engineering of antimicrobial peptides: Microbial targets, peptide motifs and translation opportunities. Biophys. Rev. 2021, 13, 35–69. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.Z.; Li, Y.R.; Zou, H.S.; Zou, L.F.; Zhang, B.; Chen, G.Y. A novel antimicrobial protein for plant protection consisting of a Xanthomonas oryzae harpin and active domains of cecropin A and melittin. Microb. Biotechnol. 2011, 4, 777–793. [Google Scholar] [CrossRef] [Green Version]
- Badosa, E.; Moiset, G.; Montesinos, L.; Talleda, M.; Bardají, E.; Feliu, L.; Planas, M.; Montesinos, E. Derivatives of the antimicrobial peptide BP100 for expression in plant systems. PLoS ONE 2013, 8, e85515. [Google Scholar] [CrossRef] [Green Version]
- Dobson, A.J.; Purves, J.; Kamysz, W.; Rolff, J. Comparing selection on S. aureus between antimicrobial peptides and common antibiotics. PLoS ONE 2013, 8, e76521. [Google Scholar] [CrossRef] [Green Version]


| Peptide | Sequence | Net Charge a | MW (Da) b |
|---|---|---|---|
| BP100 | KKLFKKILKYL-NH2 | +6 | 1419.9 |
| RW-BP100 | RRLFRRILRWL-NH2 | +6 | 1583.0 |
| CA-M | KWKLFKKIGAVLKVL-NH2 | +6 | 1769.2 |
| AMP | Strain | MIC (μM) | MBC (μM) |
|---|---|---|---|
| BP100:RW-BP100 | LMG 2024 | 2.5 | 2.5 |
| Ea 630 | 2.5 | 2.5 | |
| Ea 680 | 2.5 | 2.5 | |
| BP100:CA-M | LMG 2024 | 2.5 | 3.25 |
| Ea 630 | 4 | 4 | |
| Ea 680 | 2.5 | 3.25 | |
| RW-BP100:CA-M | LMG 2024 | 4 | 4 |
| Ea 630 | 3.25 | 3.25 | |
| Ea 680 | 2.5 | 4 |
| Strain | Host | Isolated From | Geographic Origin | Year | |
|---|---|---|---|---|---|
| Species | Cultivar | ||||
| LMG 2024 | Pear | N/D | N/D | UK | 1959 |
| Ea 630 | Apple | ‘Gala’ | Branch | Cadaval, Portugal | 2015 |
| Ea 680 | Pear | ‘Rocha’ | Branch | Cadaval, Portugal | 2015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, R.J.; Sario, S.; Luz, J.P.; Tassi, N.; Teixeira, C.; Gomes, P.; Tavares, F.; Santos, C. Evaluation of Three Antimicrobial Peptides Mixtures to Control the Phytopathogen Responsible for Fire Blight Disease. Plants 2021, 10, 2637. https://doi.org/10.3390/plants10122637
Mendes RJ, Sario S, Luz JP, Tassi N, Teixeira C, Gomes P, Tavares F, Santos C. Evaluation of Three Antimicrobial Peptides Mixtures to Control the Phytopathogen Responsible for Fire Blight Disease. Plants. 2021; 10(12):2637. https://doi.org/10.3390/plants10122637
Chicago/Turabian StyleMendes, Rafael J., Sara Sario, João Pedro Luz, Natália Tassi, Cátia Teixeira, Paula Gomes, Fernando Tavares, and Conceição Santos. 2021. "Evaluation of Three Antimicrobial Peptides Mixtures to Control the Phytopathogen Responsible for Fire Blight Disease" Plants 10, no. 12: 2637. https://doi.org/10.3390/plants10122637
APA StyleMendes, R. J., Sario, S., Luz, J. P., Tassi, N., Teixeira, C., Gomes, P., Tavares, F., & Santos, C. (2021). Evaluation of Three Antimicrobial Peptides Mixtures to Control the Phytopathogen Responsible for Fire Blight Disease. Plants, 10(12), 2637. https://doi.org/10.3390/plants10122637









