Optimization of Protoplast Isolation from Leaf Mesophylls of Chinese Cabbage (Brassica rapa ssp. pekinensis) and Subsequent Transfection with a Binary Vector
Abstract
:1. Introduction
2. Results
2.1. Optimization of Factors Influencing Protoplast Yield
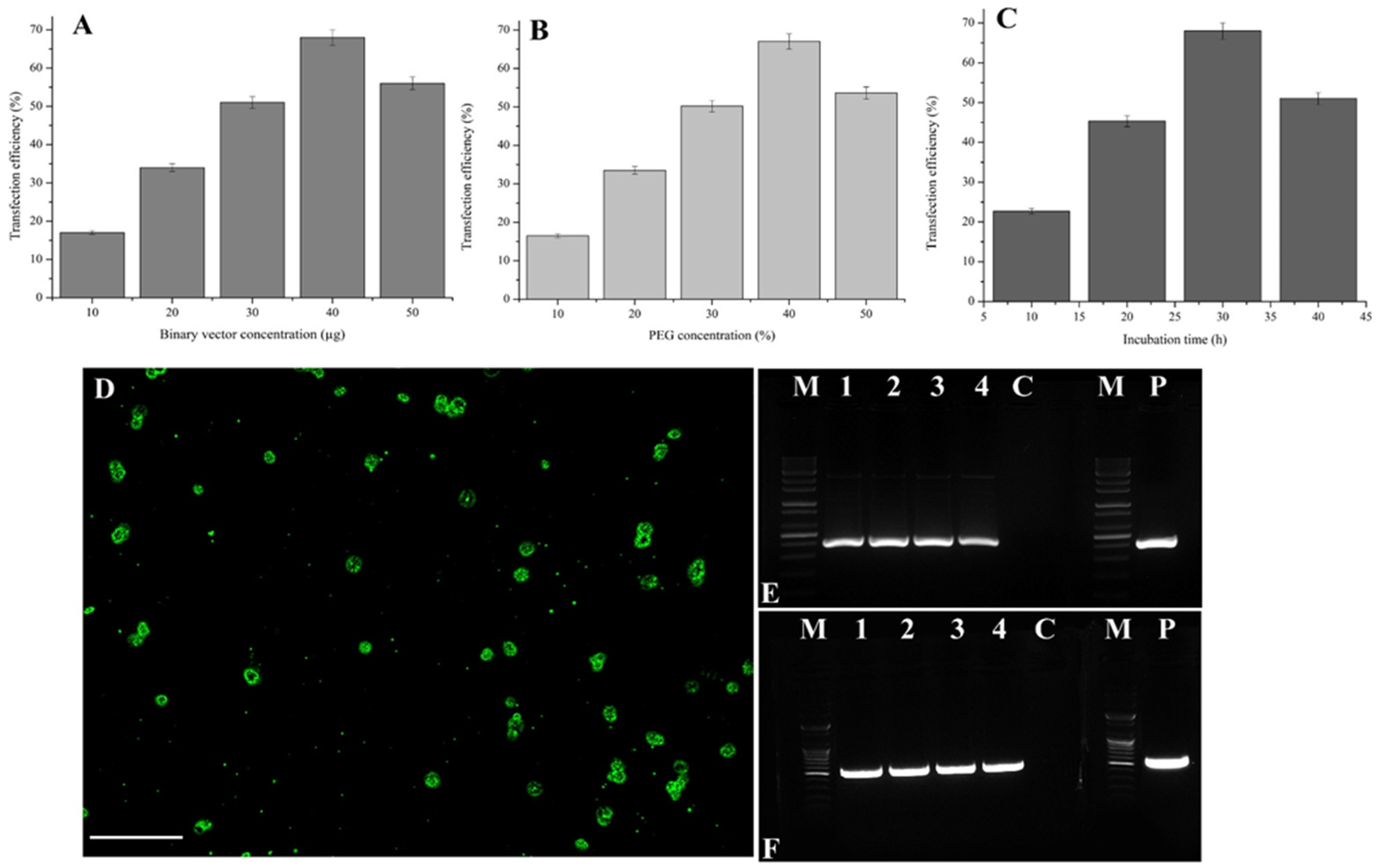
2.2. Optimization of Factors Influencing Transfection with a Binary Vector
2.3. Confirmation of Vector Integration by PCR
3. Discussion
4. Materials and Methods
4.1. Optimization of Factors Influencing Protoplast Yield
4.2. Analysis of Protoplast Viability
4.3. Optimization of Factors Influencing Protoplast Transfection with a Binary Vector
4.4. Confirmation of Vector Integration by PCR
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sivanandhan, G.; Choi, S.B.; Jiae, M.; Choi, S.R.; Kim, S.G.; Park, Y.D.; Lim, Y.P. High frequency in vitro regeneration of Chinese cabbage (cv. Kenshin) from hypocotyl and cotyledon explants. Hortic. Sci. Technol. 2019, 37, 640–650. [Google Scholar] [CrossRef]
- FAOSTAT. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 10 October 2021).
- Poveda, J.; Francisco, M.; Cartea, M.E.; Velasco, P. Development of transgenic Brassica crops against biotic stresses caused by pathogens and arthropod pests. Plants 2020, 9, 1664. [Google Scholar] [CrossRef]
- Svitashev, S.; Schwartz, C.; Lenderts, B.; Young, J.K.; Mark Cigan, A. Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat. Commun. 2016, 16, 13274–13281. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chen, J.C.; Fang, S.C. A protoplast transient expression system to enable molecular, cellular, and functional studies in Phalaenopsis orchids. Front. Plant Sci. 2018, 9, 843. [Google Scholar] [CrossRef]
- He, F.; Chen, S.; Ning, Y.; Wang, G.L. Rice (Oryza sativa) protoplast isolation and its application for transient expression analysis. Curr. Protoc. Plant Biol. 2016, 1, 373–383. [Google Scholar] [CrossRef]
- Xiong, L.; Li, C.; Li, H.; Lyu, X.; Zhao, T.; Liu, J.; Zao, Z.; Liu, B. A transient expression system in soybean mesophyll protoplasts reveals the formation of cytoplasmic GmCRY1 photobody-like structures. Sci. China Life Sci. 2019, 62, 1070–1077. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, G.; Chen, Z.; Han, J.; Hu, Y.; Wang, K. Optimization of protoplast isolation, transformation and its application in sugarcane (Saccharum spontaneum L). Crop J. 2021, 9, 133–142. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Cohen, S.S. Subcellular Localization of Spermidine Synthase in the Protoplasts of Chinese Cabbage Leaves. Plant Physiol. 1984, 76, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Balint, R.; Cohen, S.S. The incorporation of radiolabeled polyamines and methionine into turnip yellow mosaic virus in protoplasts from infected plants. Virology 1985, 144, 181–193. [Google Scholar] [CrossRef]
- Balint, R.; Cohen, S.S. The effects of dicyclohexylamine on polyamine biosynthesis and incorporation into turnip yellow mosaic virus in Chinese cabbage protoplasts infected in vitro. Virology 1985, 144, 194–203. [Google Scholar] [CrossRef]
- Boyer, J.C.; Zaccomer, B.; Haenni, A.L. Electrotransfection of turnip yellow mosaic virus RNA into Brassica leaf protoplasts and detection of viral RNA products with a non-radioactive probe. J. Gen. Virol. 1993, 74, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ryschka, U.; Marthe, F.; Klocke, E.; Schumann, G.; Zhao, H. Culture and fusion of pollen protoplasts of Brassica oleracea L. var. italica with haploid mesophyll protoplasts of B. rapa L. ssp. pekinensis. Protoplasma 2007, 231, 89–97. [Google Scholar] [CrossRef]
- Lian, Y.J.; Zhao, X.M.; Lin, G.Z.; Lim, H.T. Protoplast isolation and culture for somatic hybridisation of rapid cycling Brassica rapa with “Anand” CMS and Brassica juncea. Plant Cell Tissue Organ Cult. 2012, 109, 565–572. [Google Scholar] [CrossRef]
- Murovec, J.; Guček, K.; Bohanec, B.; Avbelj, M.; Jerala, R. DNA-free genome editing of Brassica oleracea and B. rapa protoplasts using CRISPR-cas9 ribonucleoprotein complexes. Front. Plant Sci. 2018, 871, 1594. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Ahn, H.; Ryu, J.; Oh, Y.; Sivanandhan, G.; Won, K.H.; Park, Y.D.; Kim, J.S.; Kim, H.; Lim, Y.P.; et al. Generation of early-flowering Chinese cabbage (Brassica rapa spp. pekinensis) through CRISPR/Cas9-mediated genome editing. Plant Biotechnol. Rep. 2019, 13, 491–499. [Google Scholar] [CrossRef]
- Montecillo, J.A.V.; Chu, L.L.; Bae, H. CRISPR-Cas9 system for plant genome editing: Current approaches and emerging developments. Agronomy 2020, 10, 1033. [Google Scholar] [CrossRef]
- Huo, A.; Chen, Z.; Wang, P.; Yang, L.; Wang, G.; Wang, D.; Liao, S.; Cheng, T.; Cheng, J.; Shi, J. Establishment of transient gene expression systems in protoplasts from Liriodendron hybrid mesophyll cells. PLoS ONE 2017, 12, e0172475. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.H.; Naing, A.H.; Kim, C.K. Protoplast isolation and shoot regeneration from protoplast-derived callus of Petunia hybrida cv. Mirage rose. Biology 2020, 9, 228. [Google Scholar] [CrossRef]
- Adedeji, O.S.; Naing, A.H.; Kim, C.K. Protoplast isolation and shoot regeneration from protoplast-derived calli of Chrysanthemum cv. White ND. Plant Cell. Tissue Organ Cult. 2020, 141, 571–581. [Google Scholar] [CrossRef]
- Wu, J.Z.; Liu, Q.; Geng, X.S.; Li, K.M.; Luo, L.J.; Liu, J.P. Highly efficient mesophyll protoplast isolation and PEG-mediated transient gene expression for rapid and large-scale gene characterization in cassava (Manihot esculenta Crantz). BMC Biotechnol. 2017, 17, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Yuan, Q.; Zheng, H.; Liang, S.; Jiang, M.; Wang, M.M.; Chen, Q.; Li, M.Y.; Zhang, Y.; Luo, Y.; et al. An efficient and economical protocol for isolating, purifying and PEG-mediated transient gene expression of Chinese kale hypocotyl protoplasts. Plants 2019, 8, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Wang, Z.; Cheng, J.; Zhao, W.; Li, X.; Wang, H.; Zang, Z.; Sui, X. An efficient cucumber (Cucumis sativus L.) protoplast isolation and transient expression system. Sci. Hortic. 2013, 150, 206–212. [Google Scholar] [CrossRef]
- Schmoll, M. Regulation of plant cell wall degradation by light in Trichoderma. Fungal Biol. Biotechnol. 2018, 5, 10. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Zhang, F.; Xiao, N.; Jiang, M.; Yuan, Q.; Xue, S.; Miao, H.; Chen, Q.; Li, M.; Wang, X.; et al. An efficient mesophyll protoplast isolation, purification and PEG-mediated transient gene expression for subcellular localization in Chinese kale. Sci. Hortic. 2018, 241, 187–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J.; Duan, S.; Ao, Y.; Dai, J.; Liu, J.; Wang, P.; Li, Y.; Liu, B.; Feng, D.; et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 2011, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Burris, K.; Dlugosz, E.; Collins, G.; Stewart, N.; Lenaghan, S. Development of a rapid, low-cost protoplast transfection system for switchgrass (Panicum virgatum L.). Plant Cell Rep. 2016, 35, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Liao, X.; Gan, Z.; Peng, X.; Wang, P.; Li, S.; Li, T. Protoplast isolation and development of a transient expression system for sweet cherry (Prunus avium L.). Sci. Hortic. 2016, 209, 14–21. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivanandhan, G.; Bae, S.; Sung, C.; Choi, S.-R.; Lee, G.-J.; Lim, Y.-P. Optimization of Protoplast Isolation from Leaf Mesophylls of Chinese Cabbage (Brassica rapa ssp. pekinensis) and Subsequent Transfection with a Binary Vector. Plants 2021, 10, 2636. https://doi.org/10.3390/plants10122636
Sivanandhan G, Bae S, Sung C, Choi S-R, Lee G-J, Lim Y-P. Optimization of Protoplast Isolation from Leaf Mesophylls of Chinese Cabbage (Brassica rapa ssp. pekinensis) and Subsequent Transfection with a Binary Vector. Plants. 2021; 10(12):2636. https://doi.org/10.3390/plants10122636
Chicago/Turabian StyleSivanandhan, Ganeshan, Solhee Bae, Chaemin Sung, Su-Ryun Choi, Geung-Joo Lee, and Yong-Pyo Lim. 2021. "Optimization of Protoplast Isolation from Leaf Mesophylls of Chinese Cabbage (Brassica rapa ssp. pekinensis) and Subsequent Transfection with a Binary Vector" Plants 10, no. 12: 2636. https://doi.org/10.3390/plants10122636
APA StyleSivanandhan, G., Bae, S., Sung, C., Choi, S.-R., Lee, G.-J., & Lim, Y.-P. (2021). Optimization of Protoplast Isolation from Leaf Mesophylls of Chinese Cabbage (Brassica rapa ssp. pekinensis) and Subsequent Transfection with a Binary Vector. Plants, 10(12), 2636. https://doi.org/10.3390/plants10122636







