The Reduced Longitudinal Growth Induced by Overexpression of pPLAIIIγ Is Regulated by Genes Encoding Microtubule-Associated Proteins
Abstract
1. Introduction
2. Results
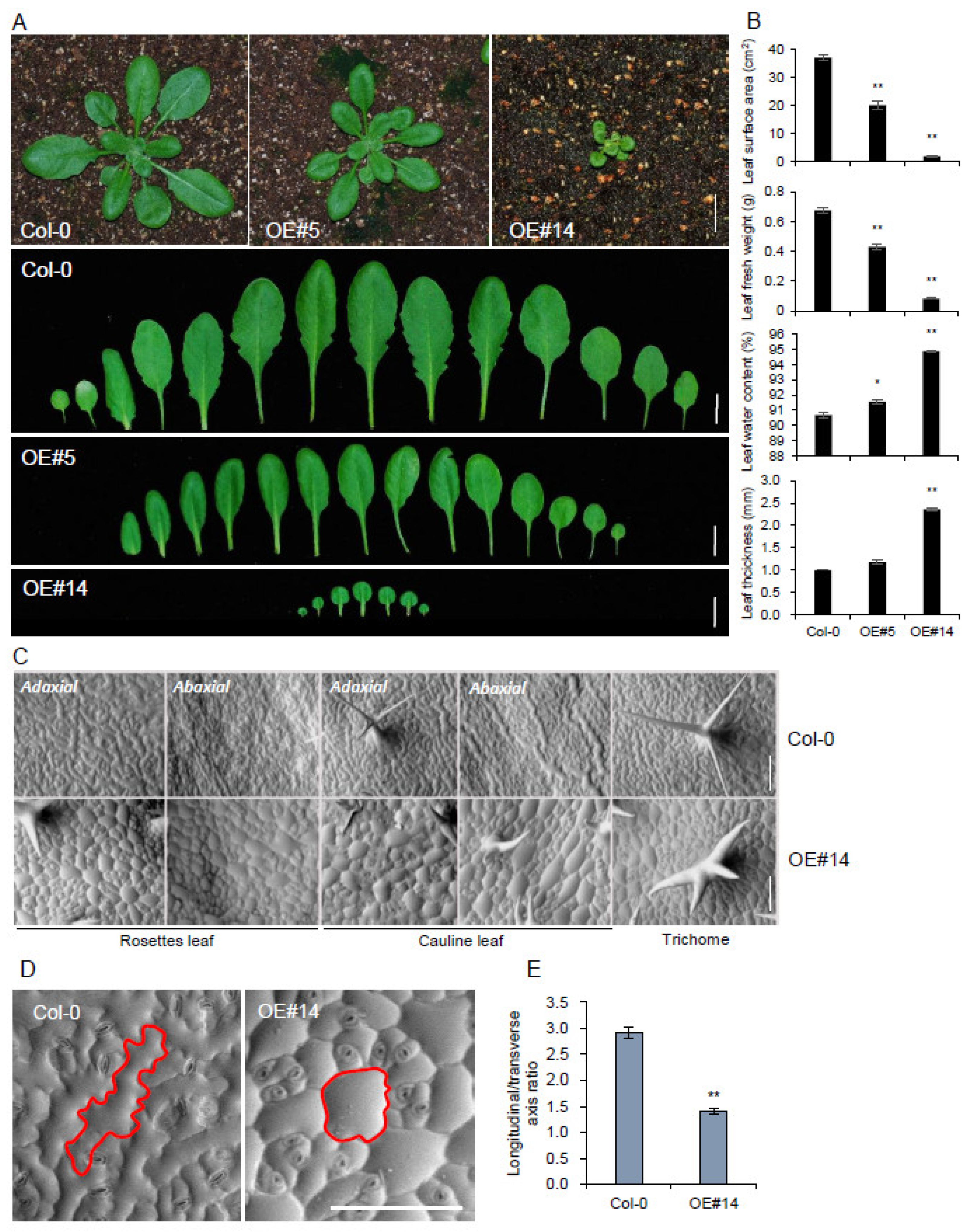
2.1. Overexpression of pPLAIIIγ Affects Anisotropic Cell Elongation
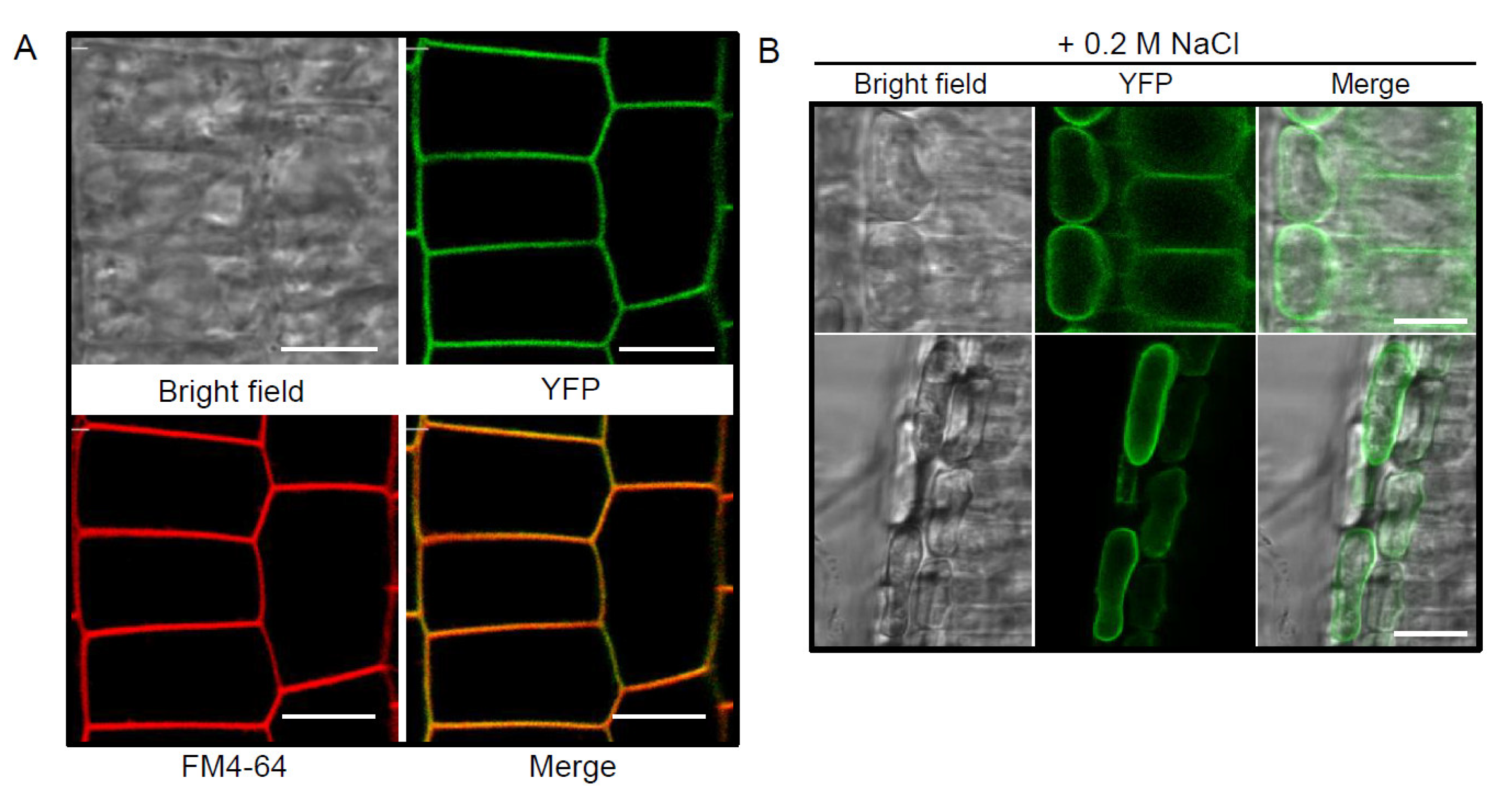
2.2. YFP-Tagged pPLAIIIγ Is Localized to the Plasma Membrane
2.3. pPLAIIIγOE Displayed Altered Anisotropic Cell Expansion in the Leaf and Trichome
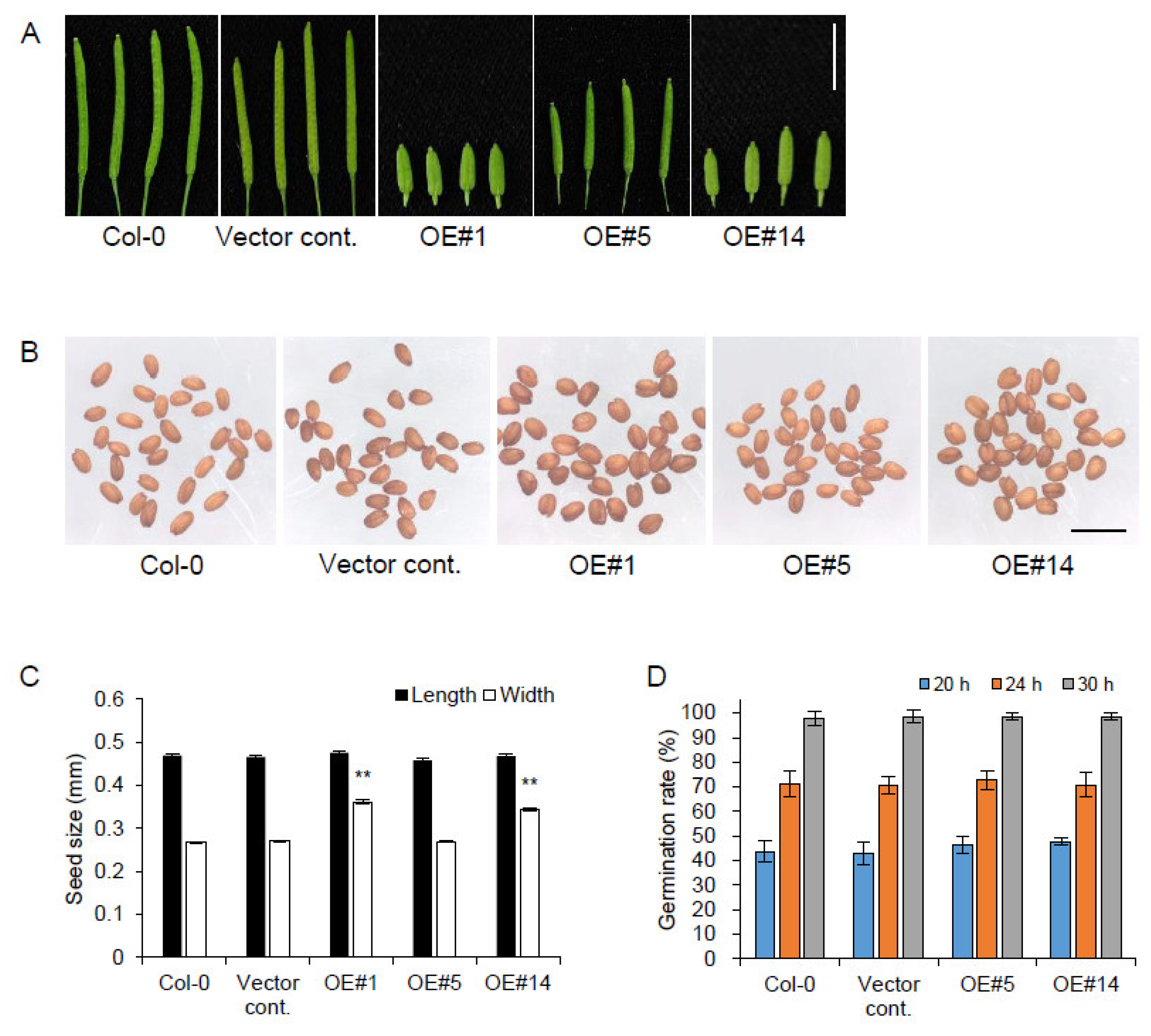
2.4. pPLAIIIγOE Increased Seed Size
2.5. PLD Genes Are Downregulated by pPLAIIIγOE
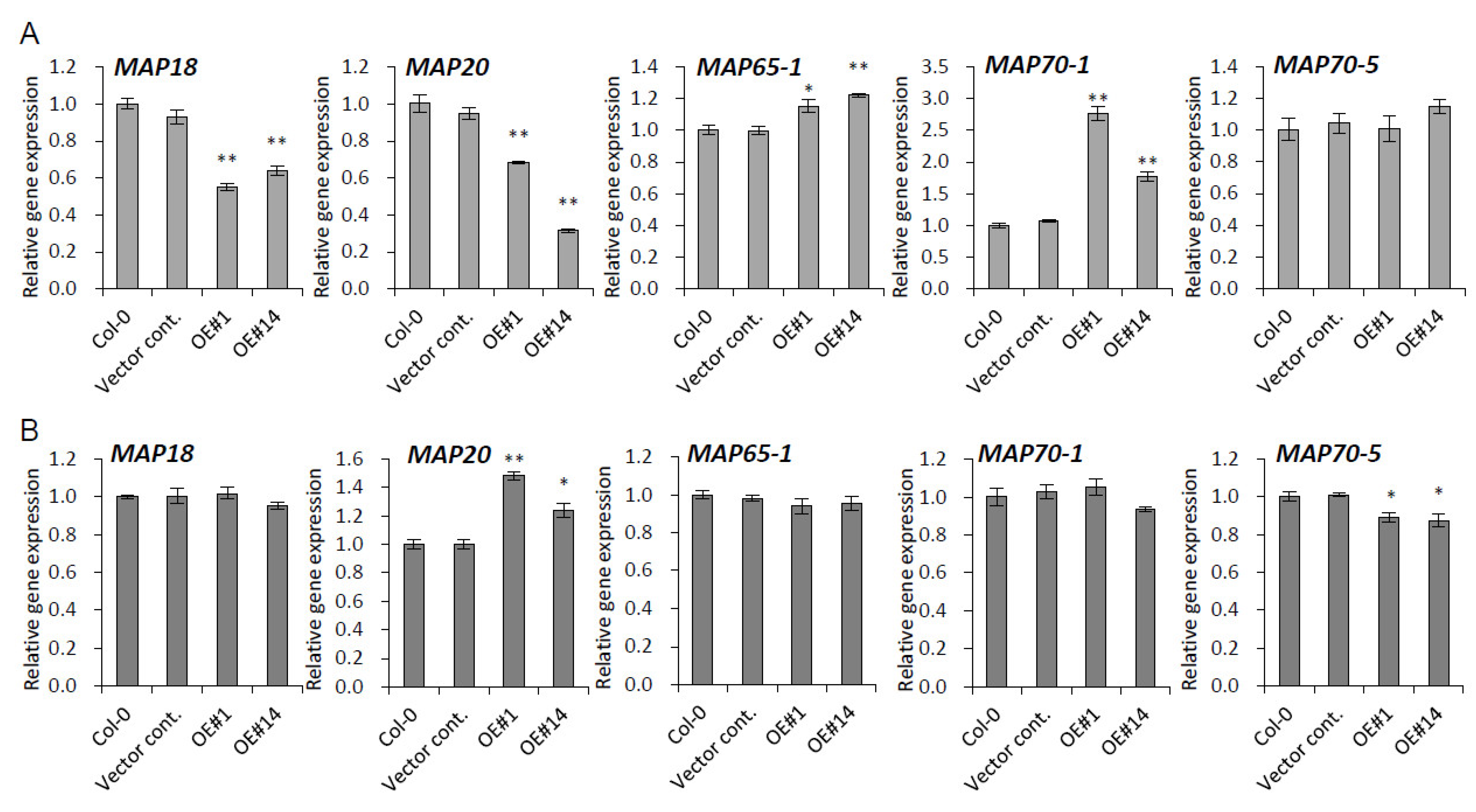
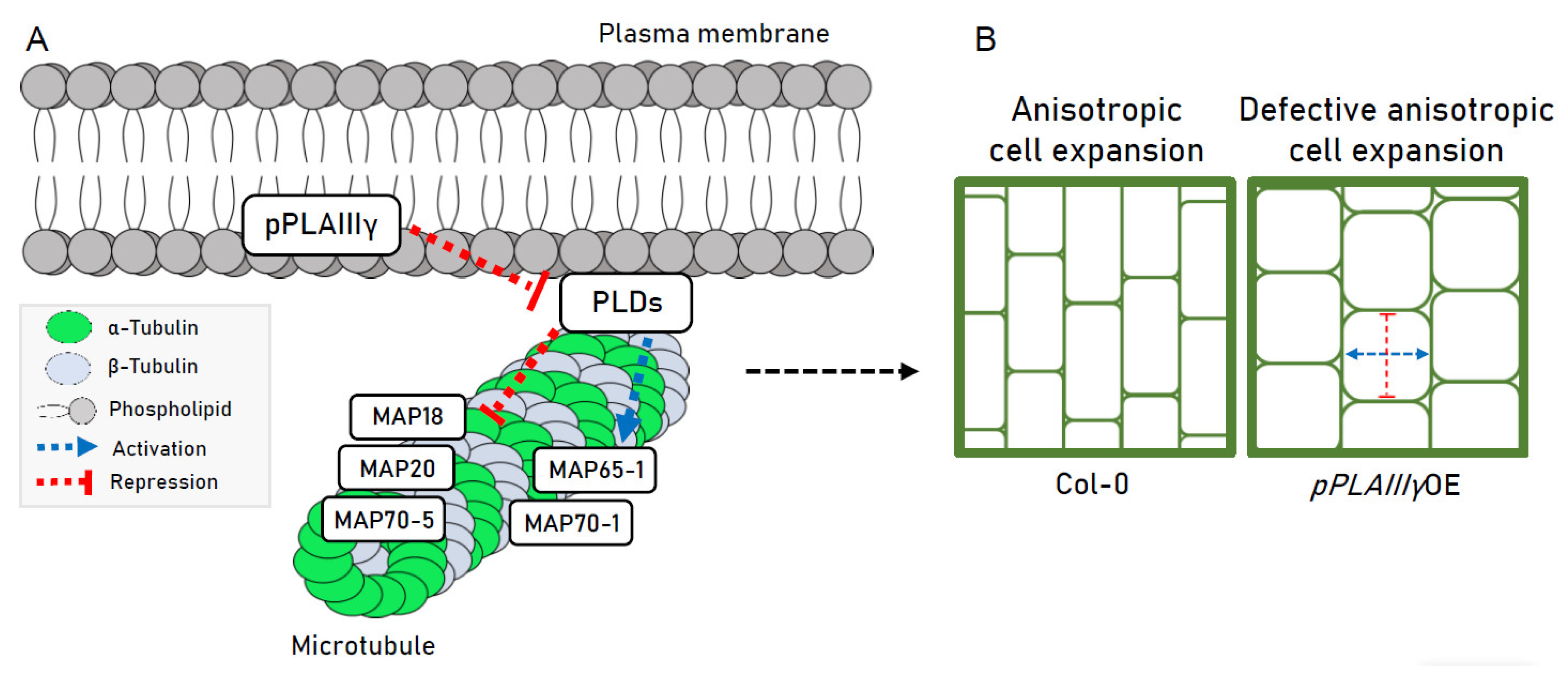
2.6. Genes Encoding Microtubule-Associated Proteins Are Modulated by pPLAIIIγOE
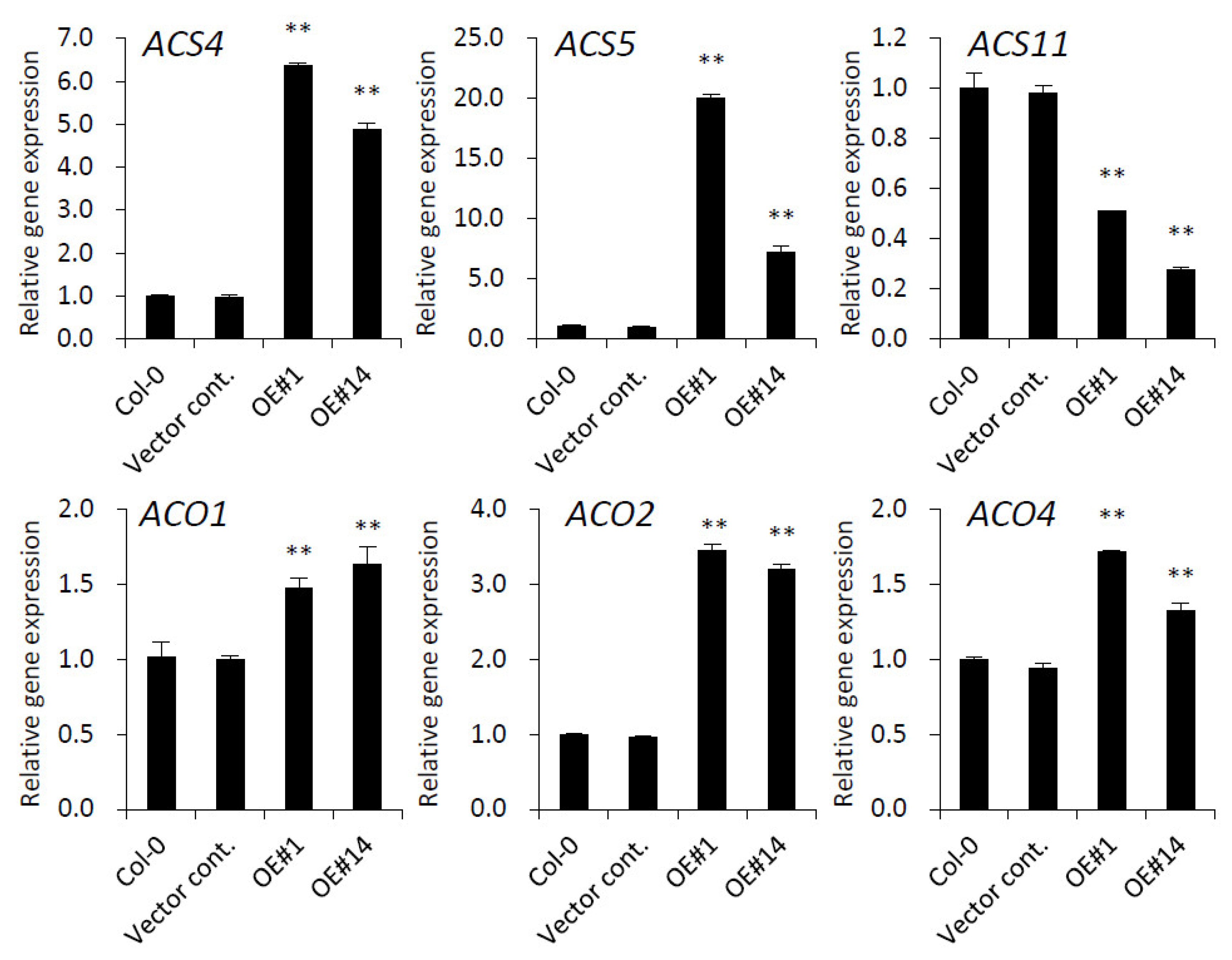
2.7. Ethylene Biosynthesis Genes Are Modulated by pPLAIIIγOE
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Transgenic Construct and Arabidopsis Transformation
4.3. qRT-PCR
4.4. Pollen Germination and Pollen Tube Length Measurement
4.5. Observation of Reporter Gene Expression
4.6. SEM Analysis
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crowell, E.; Gonneau, M.; Vernhettes, S.; Höfte, H. Regulation of anisotropic cell expansion in higher plants. Comptes Rendus Biol. 2010, 333, 320–324. [Google Scholar] [CrossRef]
- Gardiner, J.; Marc, J. Phospholipases may play multiple roles in anisotropic plant cell growth. Protoplasma 2013, 250, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.R.; Kim, S.J.; Kim, H.J.; Hong, J.K.; Ryu, S.B.; Lee, S.H.; Ganguly, A.; Cho, H.T. Phospholipase A2 is required for PIN-FORMED protein trafficking to the plasma membrane in the Arabidopsis root. Plant Cell 2010, 22, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Takáč, T.; Novák, D.; Šamaj, J. Recent Advances in the Cellular and Developmental Biology of Phospholipases in Plants. Front. Plant Sci. 2019, 10, 362. [Google Scholar] [CrossRef]
- Jang, J.H.; Nguyen, N.Q.; Légeret, B.; Beisson, F.; Kim, Y.J.; Sim, H.J.; Lee, O.R. Phospholipase pPLAIIIα increases germination rate and resistance to turnip crinkle virus when overexpressed. Plant Physiol. 2020, 184, 1482–1498. [Google Scholar] [CrossRef]
- Huang, S.; Cerny, R.E.; Bhat, D.S.; Brown, S.M. Cloning of an Arabidopsis Patatin-Like Gene, STURDY, by Activation T-DNA Tagging. Plant Physiol. 2001, 125, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bahn, S.C.; Guo, L.; Musgrave, W.; Berg, H.; Welti, R.; Wang, X. Patatin-related phospholipase pPLAIIIβ-induced changes in lipid metabolism alter cellulose content and cell elongation in Arabidopsis. Plant Cell 2011, 23, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chu, C.F.; Liu, P.H.; Lin, H.H.; Liang, S.C.; Hsu, W.E.; Lin, J.S.; Wang, H.M.; Chang, L.L.; Chien, C.T. Expression of an Oncidium gene encoding a patatin-like protein delays flowering in Arabidopsis by reducing gibberellin synthesis. Plant Cell Physiol. 2011, 52, 421–435. [Google Scholar] [CrossRef]
- Dong, Y.; Li, M.; Zhang, P.; Wang, X.; Fan, C.; Zhou, Y. Patatin-related phospholipase pPLAIIIδ influences auxin-responsive cell morphology and organ size in Arabidopsis and Brassica napus. BMC Plant Biol. 2014, 14, 332. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Lee, O.R. Overexpression of ginseng patatin-related phospholipase pPLAIIIβ alters the polarity of cell growth and decreases lignin content in Arabidopsis. J. Ginseng Res. 2020, 44, 321–331. [Google Scholar] [CrossRef]
- Qiao, Y.; Piao, R.; Shi, J.; Lee, S.I.; Jiang, W.; Kim, B.K.; Lee, J.H.; Han, L.; Ma, W.; Koh, H.J. Fine mapping and candidate gene analysis of dense and erect panicle 3, DEP3, which confers high grain yield in rice (Oryza sativa L.). Theor. Appl. Genet. 2011, 122, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, K.; Ai, J.; Deng, X.; Hong, Y.; Wang, X. Patatin-related phospholipase A, pPLAIIIα, modulates the longitudinal growth of vegetative tissues and seeds in rice. J. Exp. Bot. 2015, 66, 6945–6955. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, F.; Tawfall, A.; Tang, M.; Saettele, A.; Wang, X. Overexpression of patatin-related phospholipase AIIIδ altered plant growth and increased seed oil content in camelina. Plant Biotechnol. J. 2015, 13, 766–778. [Google Scholar] [CrossRef]
- Jang, J.H.; Bae, E.K.; Choi, Y.I.; Lee, O.R. Ginseng-derived patatin-related phospholipase PgpPLAIIIβ alters plant growth and lignification of xylem in hybrid poplars. Plant Sci. 2019, 288, 110224. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, O.R. Patatin-related phospholipase AtpPLAIIIα affects lignification of xylem in Arabidopsis and hybrid poplars. Plants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, M.; Wnag, X. Proteomic insight into reduced cell elongation resulting from overexpression of patatin-related phospholipase pPLAIIIδ in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e28519-2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gertel, E.T.; Green, P.B. Cell Growth Pattern and Wall Microfibrillar Arrangement: Experiments with Nitella. Plant Physiol. 1977, 60, 247–254. [Google Scholar] [CrossRef]
- Giddings, T.H.; Staehelin, L.A. 1 Microtubule-mediated control of microfibril deposition: A re-examination of the hypothesis. In The Cytoskeletal Basis of Plant Growth and Form; Lloyd, C.W., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 85–100. [Google Scholar]
- Gardiner, J.C.; Harper, J.D.I.; Weerakoon, N.D.; Collings, D.A.; Ritchie, S.; Gilroy, S.; Cyr, R.J.; Macr, J. A 90-kD Phospholipase D from Tobacco Binds to Microtubules and the Plasma Membrane. Plant Cell 2001, 13, 2143–2158. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, T.; Zheng, Y.; Cosgrove, D.J.; Anderson, C.T. Xyloglucan deficiency disrupts microtubule stability and cellulose biosynthesis in Arabidopsis, altering cell growth and morphogenesis. Plant Physiol. 2016, 170, 234–249. [Google Scholar] [CrossRef]
- Sun, J.; Ma, Q.; Mao, T. Ethylene regulates the Arabidopsis microtubule-associated protein WAVE-DAMPENED2-LIKE5 in etiolated hypocotyl elongation. Plant Physiol. 2015, 169, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, Y.; Fu, Y.; Guo, H. Ethylene-induced microtubule reorientation is essential for fast inhibition of root elongation in Arabidopsis. J. Integr. Plant Biol. 2018, 60, 864–877. [Google Scholar] [CrossRef]
- Holk, A.; Rietz, S.; Zahn, M.; Quader, H.; Scherer, G.F.E. Molecular identification of cytosolic, patatin-related phospholipases A from Arabidopsis with potential functions in plant signal transduction. Plant Physiol. 2002, 130, 90–101. [Google Scholar] [CrossRef]
- Scherer, G.F.; Ryu, S.B.; Wang, X.; Matos, A.R.; Heitz, T. Patatin-related phospholipase A: Nomenclature, subfamilies and functions in plants. Trends Plant Sci. 2010, 15, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bahn, S.C.; Fan, C.; Li, J.; Phan, T.; Ortiz, M.; Roth, M.R.; Welti, R.; Jaworski, J.; Wang, X. Patatin-related phospholipase pPLAIIIδ increases seed oil content with long-chain fatty acids in Arabidopsis. Plant Physiol. 2013, 162, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Yao, S.; Cai, G.; Wang, X. Patatin-related phospholipase pPLAIIIγ involved in osmotic and salt tolerance in Arabidopsis. Plants 2020, 9, 650. [Google Scholar]
- Jang, J.H.; Seo, H.S.; Lee, O.R. Overexpression of patatin-related phospholipase pPLAIIIγ in xylem of stem reduces lignification by regulating peroxidases. Front. Plant Sci. 2021. submitted. [Google Scholar]
- Li, G.; Xue, H.W. Arabidopsis PLDζ2 regulates vesicle trafficking and is required for auxin response. Plant Cell 2007, 19, 281–295. [Google Scholar] [CrossRef]
- Krtková, J.; Benáková, M.; Schwarzerová, K. Multifunctional microtubule-associated proteins in plants. Front. Plant Sci. 2016, 7, 474. [Google Scholar] [CrossRef]
- Atalla, R.H.; Agarwal, U.P. Raman microprobe optimization, and sampling technique for studies of plant cell walls. In Microbeam Analysis; Romig, A.D., Goldstein, J.I., Eds.; San Francisco Press, Inc.: San Francisco, CA, USA, 1984; pp. 125–126. [Google Scholar]
- Wang, C.; Wang, X. A novel phospholipase D of Arabidopsis that is activated by oleic acid and associated with the plasma membrane. Plant Physiol. 2001, 127, 1102–1112. [Google Scholar] [CrossRef]
- Paredez, A.R.; Somerville, C.R.; Ehrhardt, D.W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 2006, 312, 1491–1495. [Google Scholar] [CrossRef]
- Kirik, A.; Mudgett, M.B. SOBER1 phospholipase activity suppresses phosphatidic acid accumulation and plant immunity in response to bacterial effector AvrBsT. Proc. Natl. Acad. Sci. USA 2009, 106, 20532–20537. [Google Scholar] [CrossRef]
- Dhonukshe, P.; Laxalt, A.M.; Goedhart, J.; Gadella, T.W.; Munnik, T. Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell 2003, 15, 2666–2679. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, L.; Liu, B.; Wang, C.; Jin, L.; Zhao, Q.; Yuan, M. Arabidopsis Microtubule-Associated Protein18 functions in directional cell growth by destabilizing cortical microtubules. Plant Cell 2007, 19, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Korolev, A.V.; Chan, J.; Naldrett, M.J.; Doonan, J.H.; Lloyd, C.W. Identification of a novel family of 70 kDa microtubule-associated proteins in Arabidopsis cells. Plant J. 2005, 42, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.R.; Courtney, S.; Hassfurder, M.; Dhingra, S.; Bryant, A.; Shaw, S.L. Microtubule-associated proteins MAP65-1 and MAP65-2 positively regulate axial cell growth in etiolated Arabidopsis hypocotyls. Plant Cell 2011, 23, 1889–1903. [Google Scholar] [CrossRef]
- Rajangam, A.S.; Kumar, M.; Aspeborg, H.; Guerriero, G.; Arvestad, L.; Pansri, P.; Brown, C.; Hober, S.; Blomqvist, K.; Divne, C.; et al. MAP20, a microtubule-associated protein in the secondary cell walls of hybrid aspen, is a target of the cellulose synthesis inhibitor 2, 6-dichlorobenzonitrile. Plant Physiol. 2008, 148, 1283–1294. [Google Scholar] [CrossRef]
- Gómez-Lim, M.A.; Valdés-López, V.; Cruz-Hernandez, A.; Saucedo-Arias, L.J. Isolation and characterization of a gene involved in ethylene biosynthesis from Arabidopsis thaliana. Gene 1993, 134, 217–221. [Google Scholar] [CrossRef]
- Steen, D.A.; Chadwick, A.V. Ethylene effects in pea stem tissue: Evidence of microtubule mediation. Plant Physiol. 1981, 67, 460–466. [Google Scholar] [CrossRef]
- Sonesson, A.; Berglund, M.; Staxen, I.; Widell, S. The characterization of plasma membrane-bound tubulin of cauliflower using Triton X-114 fractionation. Plant Physiol. 1997, 115, 1001–1007. [Google Scholar] [CrossRef]
- Boavida, L.C.; McCormick, S. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 2007, 52, 570–582. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.H.; Seo, H.S.; Lee, O.R. The Reduced Longitudinal Growth Induced by Overexpression of pPLAIIIγ Is Regulated by Genes Encoding Microtubule-Associated Proteins. Plants 2021, 10, 2615. https://doi.org/10.3390/plants10122615
Jang JH, Seo HS, Lee OR. The Reduced Longitudinal Growth Induced by Overexpression of pPLAIIIγ Is Regulated by Genes Encoding Microtubule-Associated Proteins. Plants. 2021; 10(12):2615. https://doi.org/10.3390/plants10122615
Chicago/Turabian StyleJang, Jin Hoon, Hae Seong Seo, and Ok Ran Lee. 2021. "The Reduced Longitudinal Growth Induced by Overexpression of pPLAIIIγ Is Regulated by Genes Encoding Microtubule-Associated Proteins" Plants 10, no. 12: 2615. https://doi.org/10.3390/plants10122615
APA StyleJang, J. H., Seo, H. S., & Lee, O. R. (2021). The Reduced Longitudinal Growth Induced by Overexpression of pPLAIIIγ Is Regulated by Genes Encoding Microtubule-Associated Proteins. Plants, 10(12), 2615. https://doi.org/10.3390/plants10122615





