Barley Genotypes Vary in Stomatal Responsiveness to Light and CO2 Conditions
Abstract
1. Introduction
2. Results
2.1. Changes in Stomatal Density
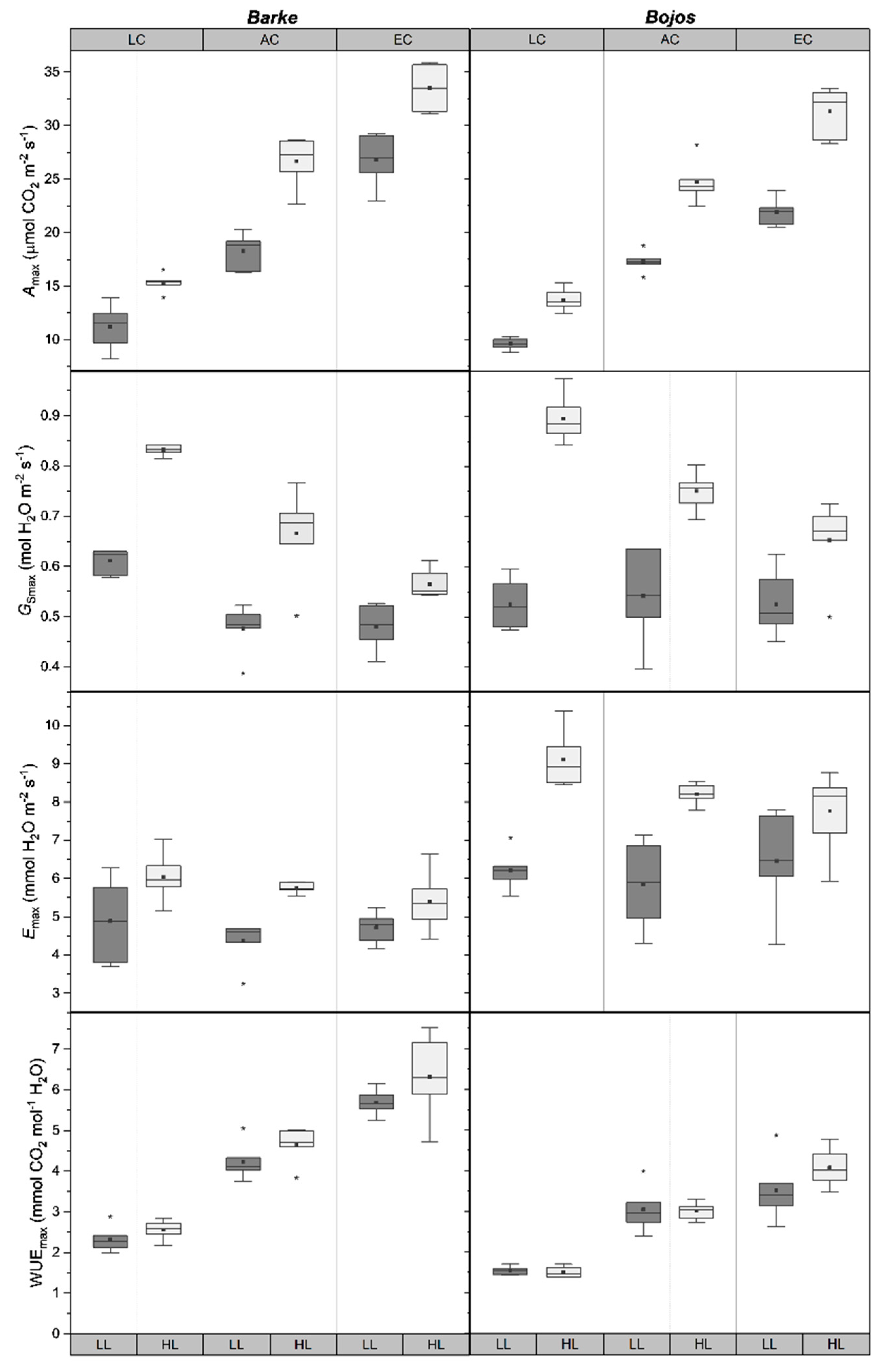
2.2. Stomatal Function
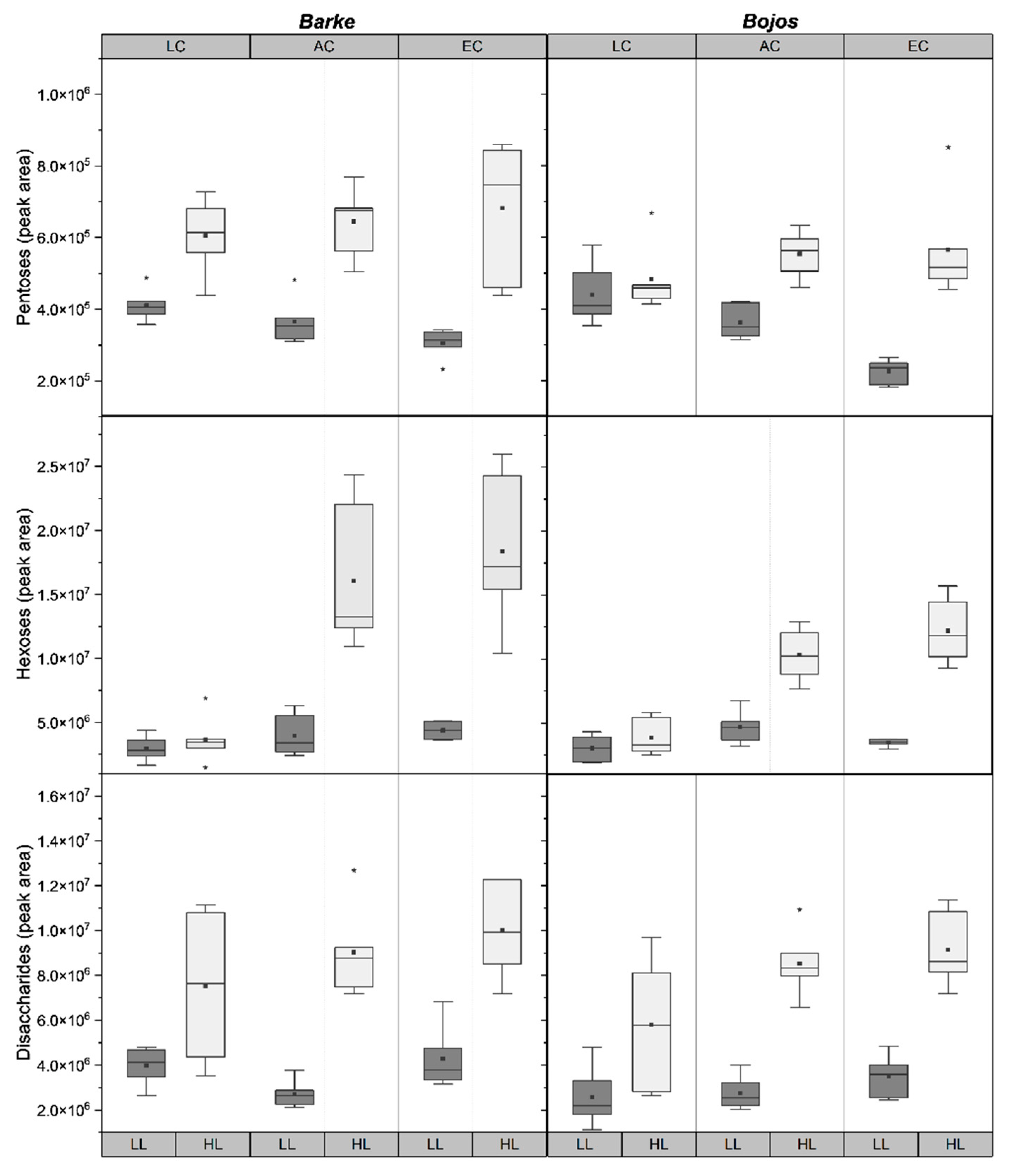
2.3. Sugar Metabolites
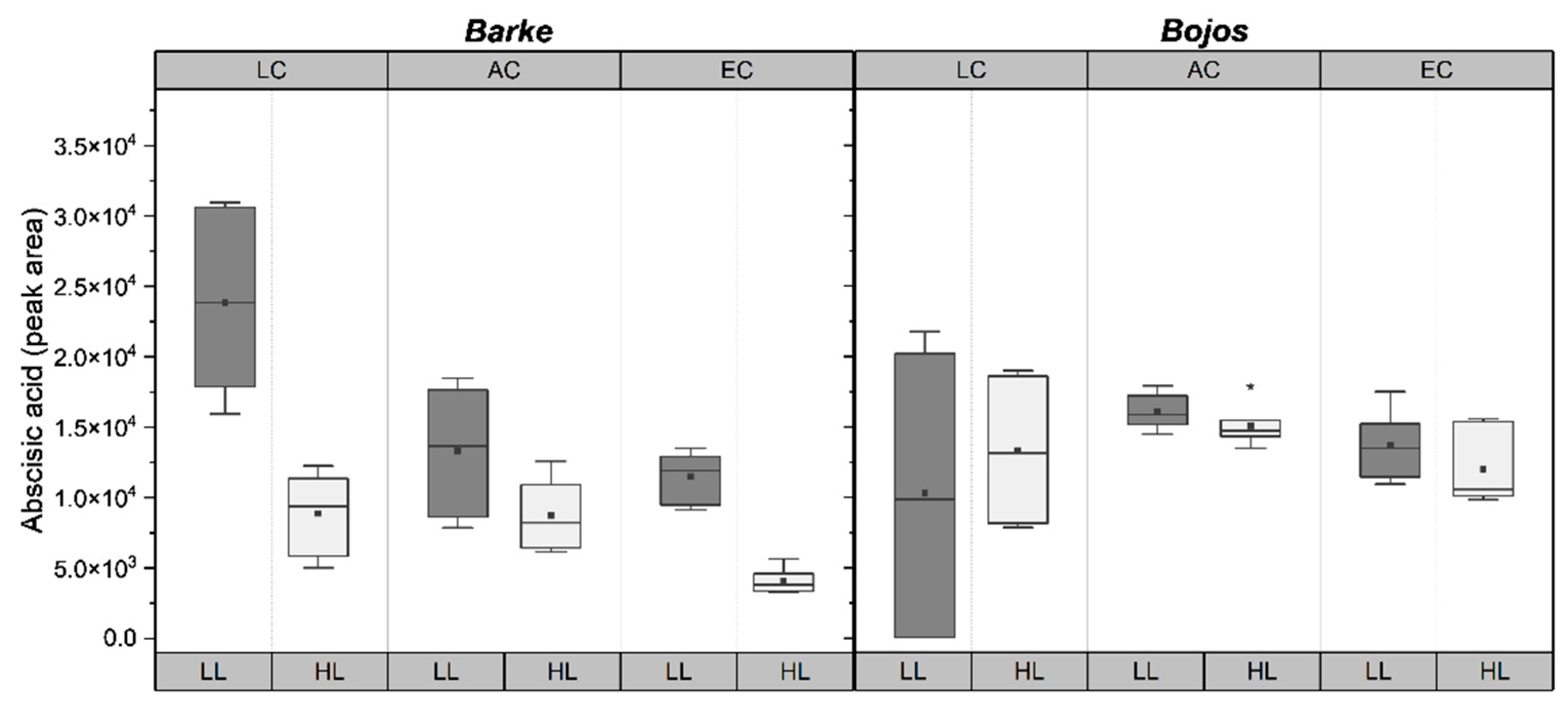
2.4. Abscisic Acid
2.5. Redundancy Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
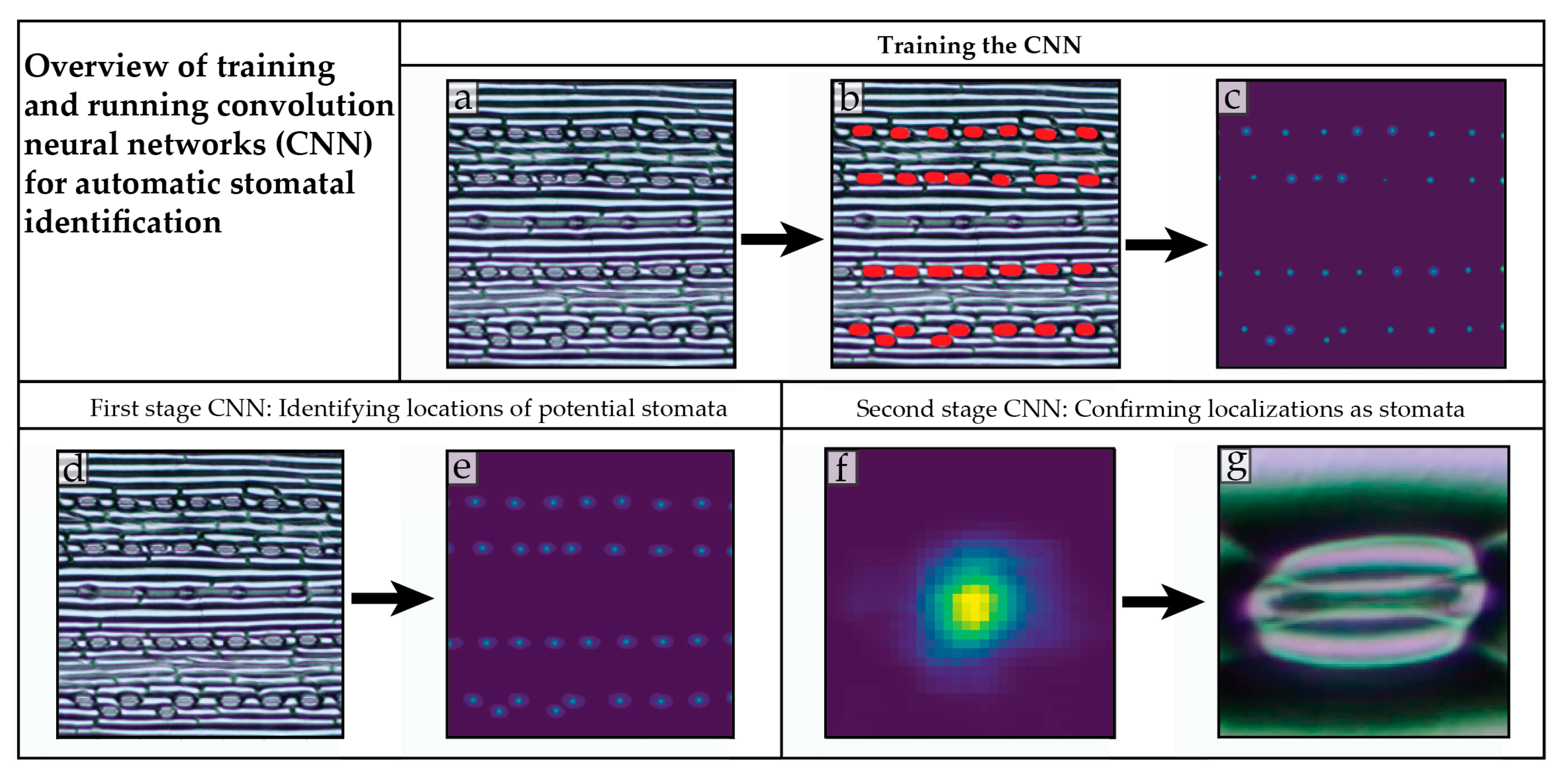
4.2. Training a Convolution Neural Network to Recognize Stomata
4.3. Gas Exchange Measurements
4.4. Identification of Sugar Metabolites and Phytohormones
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gray, A.; Liu, L.; Facette, M. Flanking Support: How Subsidiary Cells Contribute to Stomatal Form and Function. Front. Plant Sci. 2020, 11, 881. [Google Scholar] [CrossRef]
- Keenan, T.F.; Hollinger, D.Y.; Bohrer, G.; Dragoni, D.; Munger, J.W. Increase in forest water-use efficency as atmospheric carbon dioxide concentrations rise. Nature 2013, 499, 324–327. [Google Scholar] [CrossRef]
- Lake, J.; Quick, W.; Beerling, D.; Woodward, F. Plant development—Signals from mature to new leaves. Nature 2001, 411, 154. [Google Scholar] [CrossRef]
- De Boer, H.J.; Price, C.A.; Wagner-Cremer, F.; Dekker, S.; Franks, P.; Veneklaas, E.J. Optimal allocation of leaf epidermal area for gas exchange. New Phytol. 2016, 210, 1219–1228. [Google Scholar] [CrossRef]
- Vatén, A.; Bergmann, D.C. Mechanisms of stomatal development: An evolutionary view. EvoDevo 2012, 3, 11. [Google Scholar] [CrossRef]
- Driesen, E.; Ende, W.V.D.; De Proft, M.; Saeys, W. Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- Woodward, F.I.; Kelly, C.K. The influence of CO2 concentration on stomatal density. New Phytol. 1995, 131, 311–327. [Google Scholar] [CrossRef]
- Estiarte, M.; Peñuelas, J.; Kimball, B.; Idso, S.; Lamorte, R.; Pinter, J.P.; Wall, G.; Garcia, R. Elevated CO2 effects on stomatal density of wheat and sour orange trees. J. Exp. Bot. 1994, 45, 1665–1668. [Google Scholar] [CrossRef]
- Woodward, F.I. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 1987, 327, 617–618. [Google Scholar] [CrossRef]
- McElwain, J.C.; Chaloner, W.G. Stomatal Density and Index of Fossil Plants Track Atmospheric Carbon Dioxide in the Palaeozoic. Ann. Bot. 1995, 76, 389–395. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, H.; Wang, S.; Zhu, X.; Luo, C.; Li, Y.; Zhao, X. Gene or environment? Species-specific control of stomatal density and length. Ecol. Evol. 2012, 2, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Blatt, M.R. Cellular Signaling and Volume Control in Stomatal Movements in Plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 221–241. [Google Scholar] [CrossRef]
- Shimada, T.; Sugano, S.S.; Hara-Nishimura, I. Positive and negative peptide signals control stomatal density. Cell. Mol. Life Sci. 2011, 68, 2081–2088. [Google Scholar] [CrossRef]
- Chater, C.; Peng, K.; Movahedi, M.; Dunn, J.A.; Walker, H.J.; Liang, Y.-K.; McLachlan, D.H.; Casson, S.; Isner, J.-C.; Wilson, I.; et al. Elevated CO2 -Induced Responses in Stomata Require ABA and ABA Signaling. Curr. Biol. 2015, 25, 2709–2716. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising Atmospheric Carbon Dioxide: Plants FACE the Future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.; Moshelion, M.; David-Schwartz, R.; Halperin, O.; Wallach, R.; Attia, Z.; Belausov, E.; Granot, D. Hexokinase mediates stomatal closure. Plant J. 2013, 75, 977–988. [Google Scholar] [CrossRef]
- Talbott, L.D.; Zeiger, E. Central Roles for Potassium and Sucrose in Guard-Cell Osmoregulation. Plant Physiol. 1996, 111, 1051–1057. [Google Scholar] [CrossRef]
- Gotow, K.; Taylor, S.; Zeiger, E. Photosynthetic Carbon Fixation in Guard Cell Protoplasts of Vicia faba L.: Evidence from Radiolabel Experiments. Plant Physiol. 1988, 86, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015. [Google Scholar]
- Hunt, L.; Klem, K.; Lhotáková, Z.; Vosolsobě, S.; Oravec, M.; Urban, O.; Špunda, V.; Albrechtová, J. Light and CO2 Modulate the Accumulation and Localization of Phenolic Compounds in Barley Leaves. Antioxidants 2021, 10, 385. [Google Scholar] [CrossRef]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing Stomatal Density in Barley Improves Drought Tolerance without Impacting on Yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef]
- Dunn, J.; Hunt, L.; Afsharinafar, M.; Al Meselmani, M.; Mitchell, A.; Howells, R.; Wallington, E.; Fleming, A.J.; Gray, J.E. Reduced stomatal density in bread wheat leads to increased water-use efficiency. J. Exp. Bot. 2019, 70, 4737–4748. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Shabala, L.; Zhou, M.; Brodribb, T.J.; Corkrey, R.; Shabala, S. Factors determining stomatal and non-stomatal (residual) transpiration and their contribution towards salinity tolerance in contrasting barley genotypes. Environ. Exp. Bot. 2018, 153, 10–20. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Doheny-Adams, T.W.; Britton-Harper, Z.J.; Gray, J.E. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015, 207, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Fetter, K.C.; Eberhardt, S.; Barclay, R.S.; Wing, S.; Keller, S.R. StomataCounter: A neural network for automatic stomata identification and counting. New Phytol. 2019, 223, 1671–1681. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Influence of different light intensities on specific leaf weight, stomatal density photosynthesis and seed yield in soybean. Plant Physiol. Rep. 2020, 25, 277–283. [Google Scholar] [CrossRef]
- Takahashi, S.; Monda, K.; Negi, J.; Konishi, F.; Ishikawa, S.; Hashimoto-Sugimoto, M.; Goto, N.; Iba, K. Natural Variation in Stomatal Responses to Environmental Changes among Arabidopsis thaliana Ecotypes. PLoS ONE 2015, 10, e0117449. [Google Scholar] [CrossRef]
- Miskin, E.; Rasmusson, D.C. Frequency and distribution of stomata in barley. Crop Sci. 1970, 10, 575–578. [Google Scholar] [CrossRef]
- Kubinova, L. Stomata and Mesophyll Characteristics of Barley Leaf as Affected by Light: Stereological Analysis. J. Exp. Bot. 1991, 42, 995–1001. [Google Scholar] [CrossRef]
- Wild, A.; Wolf, G. The Effect of Different Light Intensities on the Frequency and Size of Stomata, the Size of Cells, the Number, Size and Chlorophyll Content of Chloroplasts in the Mesophyll and the Guard Cells during the Ontogeny of Primary Leaves of Sinapis alba. Z. Pflanzenphysiol. 1980, 97, 325–342. [Google Scholar] [CrossRef]
- Tichá, I. Photosynthetic Characteristics during Ontogenesis of Leaves. VII: Stomata Denity and Sizes; Czechoslovak Academy of Science, Institute of Experimental Botany: Prague, Czech Republic, 1982. [Google Scholar]
- Hronková, M.; Wiesnerová, D.; Šimková, M.; Skůpa, P.; Dewitte, W.; Vráblová, M.; Zažímalová, E.; Šantrůček, J. Light-induced STOMAGEN-mediated stomatal development in Arabidopsis leaves. J. Exp. Bot. 2015, 66, 4621–4630. [Google Scholar] [CrossRef] [PubMed]
- Hepworth, C.; Caine, R.; Harrison, E.; Sloan, J.; Gray, J.E. Stomatal development: Focusing on the grasses. Curr. Opin. Plant Biol. 2018, 41, 1–7. [Google Scholar] [CrossRef]
- Arenas-Corraliza, M.G.; Rolo, V.; López-Díaz, M.L.; Moreno, G. Wheat and barley can increase grain yield in shade through acclimation of physiological and morphological traits in Mediterranean conditions. Sci. Rep. 2019, 9, 9547. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, Y.; Liu, Y.; Shi, P. Simulation of the Stomatal Conductance of Winter Wheat in Response to Light, Temperature and CO2 Changes. Ann. Bot. 2004, 93, 435–441. [Google Scholar] [CrossRef]
- Morison, J.I.L. Sensitivity of stomata and water use efficiency to high CO2. Plant, Cell Environ. 1985, 8, 467–474. [Google Scholar] [CrossRef]
- Xu, M. The optimal atmospheric CO2 concentration for the growth of winter wheat (Triticum aestivum). J. Plant Physiol. 2015, 184, 89–97. [Google Scholar] [CrossRef]
- Gray, J.E.; Holroyd, G.H.; Van Der Lee, F.M.; Bahrami, A.R.; Sijmons, P.C.; Woodward, F.I.; Schuch, W.; Hetherington, A.M. The HIC signalling pathway links CO2 perception to stomatal development. Nature 2000, 408, 713–716. [Google Scholar] [CrossRef]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, A.T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; et al. Global Climate Projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; pp. 747–845. [Google Scholar]
- Delmas, R.J.; Ascencio, J.-M.; Legrand, M. Polar ice evidence that atmospheric CO2 20,000 yr BP was 50% of present. Nature 1980, 284, 155–157. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Cerling, T.E. Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants. Tree Physiol. 1995, 15, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Faralli, M.; Cockram, J.; Ober, E.; Wall, S.; Galle, A.; Van Rie, J.; Raines, C.; Lawson, T. Genotypic, Developmental and Environmental Effects on the Rapidity of gs in Wheat: Impacts on Carbon Gain and Water-Use Efficiency. Front. Plant Sci. 2019, 10, 492. [Google Scholar] [CrossRef]
- Franks, P.J.; Beerling, D. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef] [PubMed]
- Büssis, D.; von Groll, U.; Fisahn, J.; Altman, T. Stomatal aperture can compensate altered stomatal density. Funct. Plant Biol. 2006, 33, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-X.; Chang, J.; Wang, G.-X. Stomatal density and gas exchange in six wheat cultivars. Cereal Res. Commun. 2005, 33, 719–726. [Google Scholar] [CrossRef]
- Schuler, M.L.; Sedelnikova, O.V.; Walker, B.J.; Westhoff, P.; Langdale, J.A. Shortroot-Mediated Increase in Stomatal Density Has No Impact on Photosynthetic Efficiency. Plant Physiol. 2018, 176, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Urban, O.; Klem, K.; Holišova, P.; Šigut, L.; Šprtová, M.; Teslová-Navrátilová, P.; Zitová, M.; Špunda, V.; Marek, M.V.; Grace, J. Impact of elevated CO2 concentration on dynamics of leaf photosynthesis in Fagus sylvatica is modulated by sky conditions. Environ. Pollut. 2014, 185, 271–280. [Google Scholar] [CrossRef]
- Misra, B.B.; Acharya, B.R.; Granot, D.; Assmann, S.M.; Chen, S. The guard cell metabolome: Functions in stomatal movement and global food security. Front. Plant Sci. 2015, 6, 334. [Google Scholar] [CrossRef]
- Gago, J.; De Menezes Daloso, D.; Figueroa, C.M.; Flexas, J.; Fernie, A.R.; Nikoloski, Z. Relationships of Leaf Net Photosynthesis, Stomatal Conductance, and Mesophyll Conductance to Primary Metabolism: A Multispecies Meta-Analysis Approach. Plant Physiol. 2016, 171, 265–279. [Google Scholar] [CrossRef]
- Zeiger, E.; Talbott, L.D.; Frechilla, S.; Srivastava, A.; Zhu, J. The guard cell chloroplast: A perspective for the twenty-first century. New Phytol. 2002, 153, 415–424. [Google Scholar] [CrossRef]
- Medeiros, D.B.; Barros, J.A.; Fernie, A.R.; Araújo, W.L. Eating Away at ROS to Regulate Stomatal Opening. Trends Plant Sci. 2020, 25, 220–223. [Google Scholar] [CrossRef]
- Watkins, J.M.; Hechler, P.J.; Muday, G.K. Ethylene-induced flavonol accumulation in guard cells supressed reactive oxygen species and moderates stomatal aperture. Plant Physiol. 2014, 164, 1707–1717. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Postiglione, A.E.; Muday, G.K. The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling. Front. Plant Sci. 2020, 11, 968. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Hills, A.; Lim, C.K.; Blatt, M.R. Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J. 2010, 61, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.; Hagiwara, S. Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc. Natl. Acad. Sci. USA 1990, 87, 9305–9309. [Google Scholar] [CrossRef] [PubMed]
- Gietler, M.; Fidler, J.; Labudda, M.; Nykiel, M. Abscisic Acid—Enemy or Savior in the Response of Cereals to Abiotic and Biotic Stresses? Int. J. Mol. Sci. 2020, 21, 4607. [Google Scholar] [CrossRef]
- Cuvelier, M.-E.; Richard, H.; Berset, C. Comparison of the Antioxidative Activity of Some Acid-phenols: Structure-Activity Relationship. Biosci. Biotechnol. Biochem. 2014, 56, 324–325. [Google Scholar] [CrossRef]
- Plumbe, A.M.; Willmer, C.M. Phytoalexins, Water-stress and Stomata. Iii. The effects of some phenolics, fatty acids and some other compounds on stomatal responses. New Phytol. 1986, 103, 17–22. [Google Scholar] [CrossRef]
- Ouzounis, T.; Parjikolaei, B.R.; Frettã, X.; Rosenqvist, E.; Ottosen, C.-O. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Front. Plant Sci. 2015, 6, 19. [Google Scholar] [CrossRef]
- Finelstein, R.R.; Gibson, S.I. ABA and sugar interactions regulating development: Cross-talk or voices in a crowd? Curr. Opin. Plant Biol. 2002, 5, 26–32. [Google Scholar] [CrossRef]
- Lu, P.; Outlaw, W.H., Jr.; Smith, B.G.; Freed, G.A. A New Mechanism for the Regulation of Stomatal Aperture Size in Intact Leaves (Accumulation of Mesophyll-Derived Sucrose in the Guard-Cell Wall of Vicia faba). Plant Physiol. 1997, 114, 109–118. [Google Scholar] [CrossRef]
- Outlaw, W.H.; De Vlieghere-He, X. Transpiration Rate. An Important Factor Controlling the Sucrose Content of the Guard Cell Apoplast of Broad Bean. Plant Physiol. 2001, 126, 1716–1724. [Google Scholar] [CrossRef]
- Ruan, Y.-L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Granot, D. Potting plant hexokinases in their proper place. Phytochemistry 2008, 69, 2649–2654. [Google Scholar] [CrossRef]
- Asad, M.A.U.; Wang, F.; Ye, Y.; Guan, X.; Zhou, L.; Han, Z.; Pan, G.; Cheng, F. Contribution of ABA metabolism and ROS generation to sugar starvation-induced senescence of rice leaves. Plant Growth Regul. 2021, 95, 241–257. [Google Scholar] [CrossRef]
- Dai, N.; Schaffer, A.; Petreikov, M.; Shahak, Y.; Giller, Y.; Ratner, K.; Levine, A.; Granot, D. Overexpression of Arabidopsis Hexokinase in Tomato Plants Inhibits Growth, Reduces Photosynthesis, and Induces Rapid Senescence. Plant Cell 1999, 11, 1253. [Google Scholar] [CrossRef]
- Wu, Y.; Tiedemann, A. Light-dependent oxidative stress determines physiological lead spot formation in barley. Phytopathology 2004, 94, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Klem, K.; Ač, A.; Holub, P.; Kováč, D.; Špunda, V.; Robson, T.M.; Urban, O. Interactive effects of PAR and UV radiation on the physiology, morphology and leaf optical properties of two barley varieties. Environ. Exp. Bot. 2012, 75, 52–64. [Google Scholar] [CrossRef]
- Agrární Komora České Republiky. Ústrědní Kontrolní a Zkušební Ústav Zemědělsý: Obilniny 2018; Národní Odrůový Úřad: Brno, Czech Republic, 2018. [Google Scholar]
- Le Cun, Y.; Bottou, L.; Bengio, Y.; Haffner, P. Gradient-Based Learning Appliedto Document Recognition. Proc. IEEE 1998, 86, 2278–2324. [Google Scholar] [CrossRef]
- Chollet, F.E.A. Keras. 2015. Available online: https://github.com/fchollet/keras (accessed on 4 March 2019).
- Ioffe, S.; Szegedy, C. Batch normalization: Accelerating deep network training by reducing internal covariate shift. arXiv 2015, arXiv:1502.03167. [Google Scholar]
- Srivastava, N.; Hinton, G.; Krizhevsky, A.; Sutskever, I.; Salakhutdinov, R. Dropout: A simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 2014, 15, 1929–1958. [Google Scholar]
- Nair, V.; Hinton, G.E. Rectified linear units improve restricted boltzmann machines. In Proceedings of the 27th International Conference on Machine Learning (ICML-10), Haifa, Israel, 21–24 June 2010; pp. 807–814. [Google Scholar]
- Gunderson, H.J.G. Notes on estimation of numerical density of arbitrary profiles: The edge effect. J. Microsc. 1997, 111, 219–223. [Google Scholar] [CrossRef]
- Kubinova, L. Recent stereological methods for measuring leaf anatomical characteristics: Estimation of the number and sizes of stomata and mesophyll cells. J. Exp. Bot. 1994, 45, 119–127. [Google Scholar] [CrossRef]
- Albrechtova, J.; Kubinova, L. Quantitative Analysis of the Structure of Etiolated Barley Leaf Using Stereological Methods. J. Exp. Bot. 1991, 42, 1311–1314. [Google Scholar] [CrossRef]
- Kubínová, L.; Radichivá, B.; Lhotáková, Z.; Kubínová, Z.; Albrechtová, J. Stereology, an unbiased methodological approach to study plant anatomy and cytology: Past, present and future. Image Anal. Stereol. 2017, 36, 187–205. [Google Scholar] [CrossRef][Green Version]
- Večeřová, K.; Večeřa, Z.; Mikuška, P.; Coufalík, P.; Oravec, M.; Dočekal, B.; Novotná, K.; Veselá, B.; Pompeiano, A.; Urban, O. Temperature alters susceptibility of Picea abies seedlings to airborne pollutants: The case of CdO nanoparticles. Environ. Pollut. 2019, 253, 646–654. [Google Scholar] [CrossRef]
- Smilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]


| Treatment | Stomatal Density | Emax | Amax | Gsmax | WUEmax | Pentoses | Hexoses | Disacch-Arides | ABA |
|---|---|---|---|---|---|---|---|---|---|
| Genotype (G) | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 | 0.005 | 0.003 | 0.054 | 0.002 |
| CO2 | <0.001 | 0.046 | <0.001 | <0.001 | <0.001 | 0.268 | <0.001 | 0.007 | <0.001 |
| Light (L) | <0.001 | <0.001 | <0.001 | <0.001 | 0.013 | <0.001 | <0.001 | <0.001 | <0.001 |
| G × CO2 | 0.068 | 0.869 | 0.073 | 0.018 | <0.001 | 0.550 | 0.060 | 0.474 | 0.272 |
| G × L | 0.084 | 0.004 | 0.472 | 0.010 | 0.253 | 0.041 | 0.004 | 0.715 | <0.001 |
| CO2 × L | 0.119 | 0.059 | <0.001 | <0.001 | 0.171 | <0.001 | <0.001 | 0.037 | 0.017 |
| G × CO2 × L | 0.273 | 0.464 | 0.166 | 0.120 | 0.802 | 0.576 | 0.091 | 0.980 | 0.021 |
| Stomata Density (Pores mm−2) | |||||
|---|---|---|---|---|---|
| All Genotypes | Barke | Bojos | |||
| Average | 35 ± 7 | Average | 37 ± 8 | Average | 33 ± 7 |
| HL | 37 ± 8 | HL | 40 ± 8 | HL | 35 ± 7 |
| LL | 33 ± 7 | LL | 35 ± 7 | LL | 32 ± 6 |
| LC | 37 ± 8 | LC | 40 ± 8 | LC | 35 ± 6 |
| AC | 34 ± 7 | AC | 35 ± 7 | AC | 33 ± 7 |
| EC | 35 ± 7 | EC | 37 ± 7 | EC | 33 ± 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunt, L.; Fuksa, M.; Klem, K.; Lhotáková, Z.; Oravec, M.; Urban, O.; Albrechtová, J. Barley Genotypes Vary in Stomatal Responsiveness to Light and CO2 Conditions. Plants 2021, 10, 2533. https://doi.org/10.3390/plants10112533
Hunt L, Fuksa M, Klem K, Lhotáková Z, Oravec M, Urban O, Albrechtová J. Barley Genotypes Vary in Stomatal Responsiveness to Light and CO2 Conditions. Plants. 2021; 10(11):2533. https://doi.org/10.3390/plants10112533
Chicago/Turabian StyleHunt, Lena, Michal Fuksa, Karel Klem, Zuzana Lhotáková, Michal Oravec, Otmar Urban, and Jana Albrechtová. 2021. "Barley Genotypes Vary in Stomatal Responsiveness to Light and CO2 Conditions" Plants 10, no. 11: 2533. https://doi.org/10.3390/plants10112533
APA StyleHunt, L., Fuksa, M., Klem, K., Lhotáková, Z., Oravec, M., Urban, O., & Albrechtová, J. (2021). Barley Genotypes Vary in Stomatal Responsiveness to Light and CO2 Conditions. Plants, 10(11), 2533. https://doi.org/10.3390/plants10112533