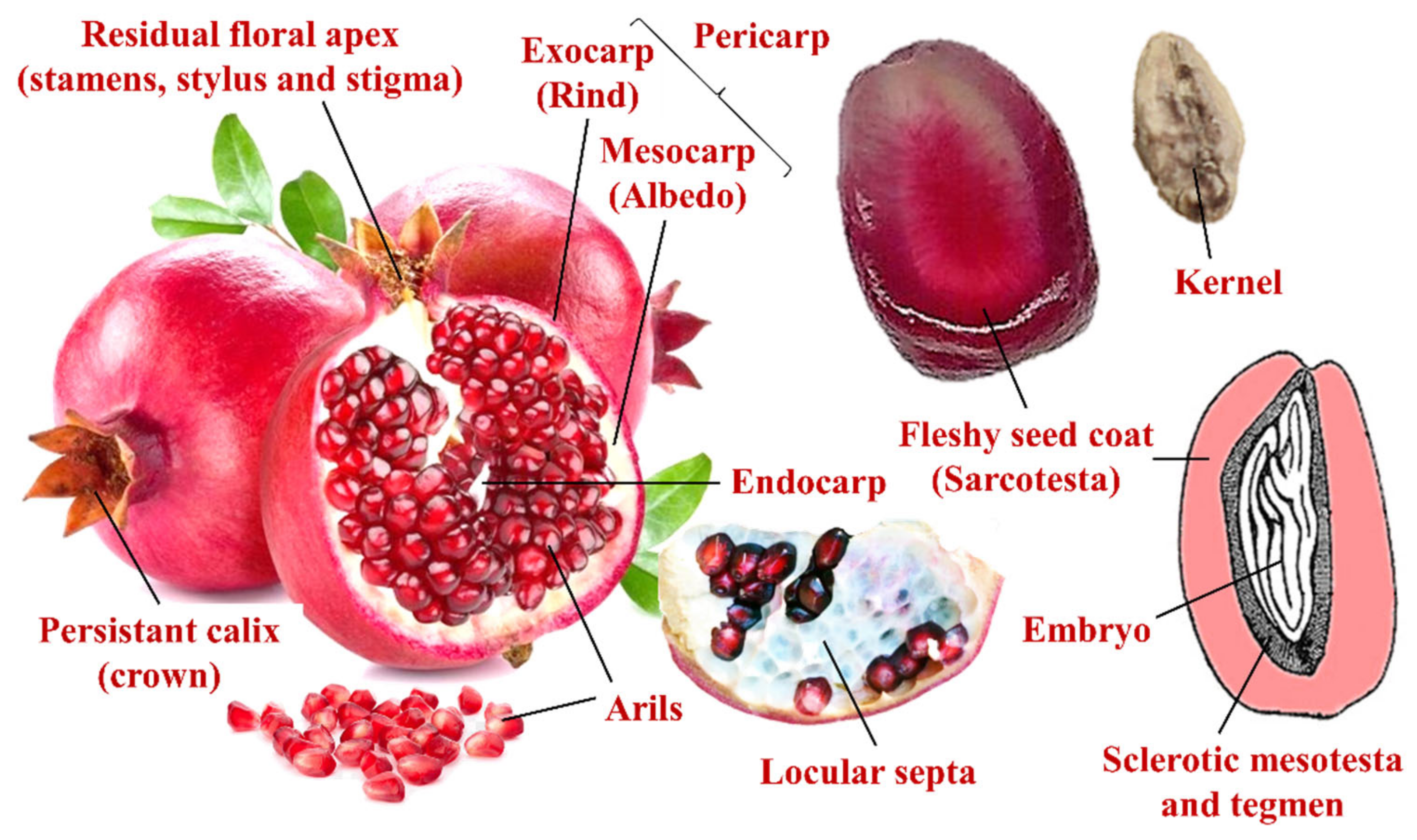
2.1. Pomological and Fruit Quality Attributes
Figure 2 reports the external and internal appearance of the ripe fruits of the four investigated Israeli pomegranate genotypes, the average fruit weight, and the relative proportion (weight percentage) of the isolated juice, kernel, and peel fruit parts.
Fruit weight differed significantly among cultivars. Wonderful One had the highest average fruit weight (506 g), followed by Ako (438 g), Kamel (347 g), and Emek (329 g) (
Figure 2a). A similar ranking was obtained for fruits harvested from the same orchard in the years 2012 and 2013; however, compared to our findings, the average weight was higher for Wonderful One (622.3 g) and slightly lower for Ako (322.9 g) and Emek (309.8 g) [
22]. Much lower values were reported for Wonderful One (324–481 g) and Ako (151–350 g) cultivars grown in different Mediterranean countries, with a marked variability over the years [
3]. A similar average weight (522.7 g) was, instead, found in Wonderful One pomegranate fruits grown in a commercial orchard in the north of Apulia (Foggia, Italy), with a gradual decrease in reduced irrigation regimes (down to 332.5 g at 25% of crop evapotranspiration) [
23]. According to most authors, fruit weight is strongly affected by environmental conditions, cultivation practices, tree age, and total yield per tree.
Juice yield, one of the most important parameters from an industrial point of view, was between 37% (cultivar Kamel) and 46% (cultivar Ako), while kernels and peels contributed about 31–40% and 22–23% to the total fruit fw, respectively (
Figure 2b). Juice yields between 27 and 55% were reported depending on genotype, pedoclimatic conditions, cultivation practices, and applied processing parameters [
24,
25,
26,
27]. Moisture content varied significantly within fruit parts, but not among cultivars (
Table 1). On average, moisture was 86.8% in the juice, 31.1% in the kernels, and 76.1% in the peels. Fawole and Opara [
28] reported compatible moisture contents in ranges of 64.2–73.91%, 77.9–83.0%, and 74.9–82.0%, respectively, in the rind, mesocarp, and whole arils of seven pomegranate cultivars grown in South Africa.
Similarly, a study conducted on pomegranate peels from Turkish varieties showed a dry matter content in the range of 24–34% [
29]. Unusually low water contents (from 24.12 mg∙g
−1 to 36.71 mg∙g
−1) with significant genotype differences were, instead, reported in the kernels of three cultivars (Tunisia soft seed, Taishanhong, and Qingpiruanzi) grown in China by Peng [
30]. The pH values and total soluble solids (TSS) of the juice did not vary significantly among cultivars and were, on average, 3.8 and 16.1° Brix, respectively (
Table 1). Similarly, peels showed a pH around 3.0–3.5 with no significant cultivar variation, in agreement with the 3.75 value of the peels from Pakistani cultivars [
31]. Significant genotypic differences were found, instead, for the contents of total ethanol-soluble carbohydrates and total organic acids in all assayed fruit parts (
Table 1). The fresh juices contained 75.2–112.9 mg∙g
−1 fw total soluble sugars and 15.6–28.8 mg∙g
−1 fw total organic acids. Meanwhile, the peels and kernels contained between 11.9–40.7 mg∙g
−1 fw and 46.9–90.0 mg∙g
−1 fw total ethanol-soluble sugars, respectively, within the ranges reported by other authors [
32,
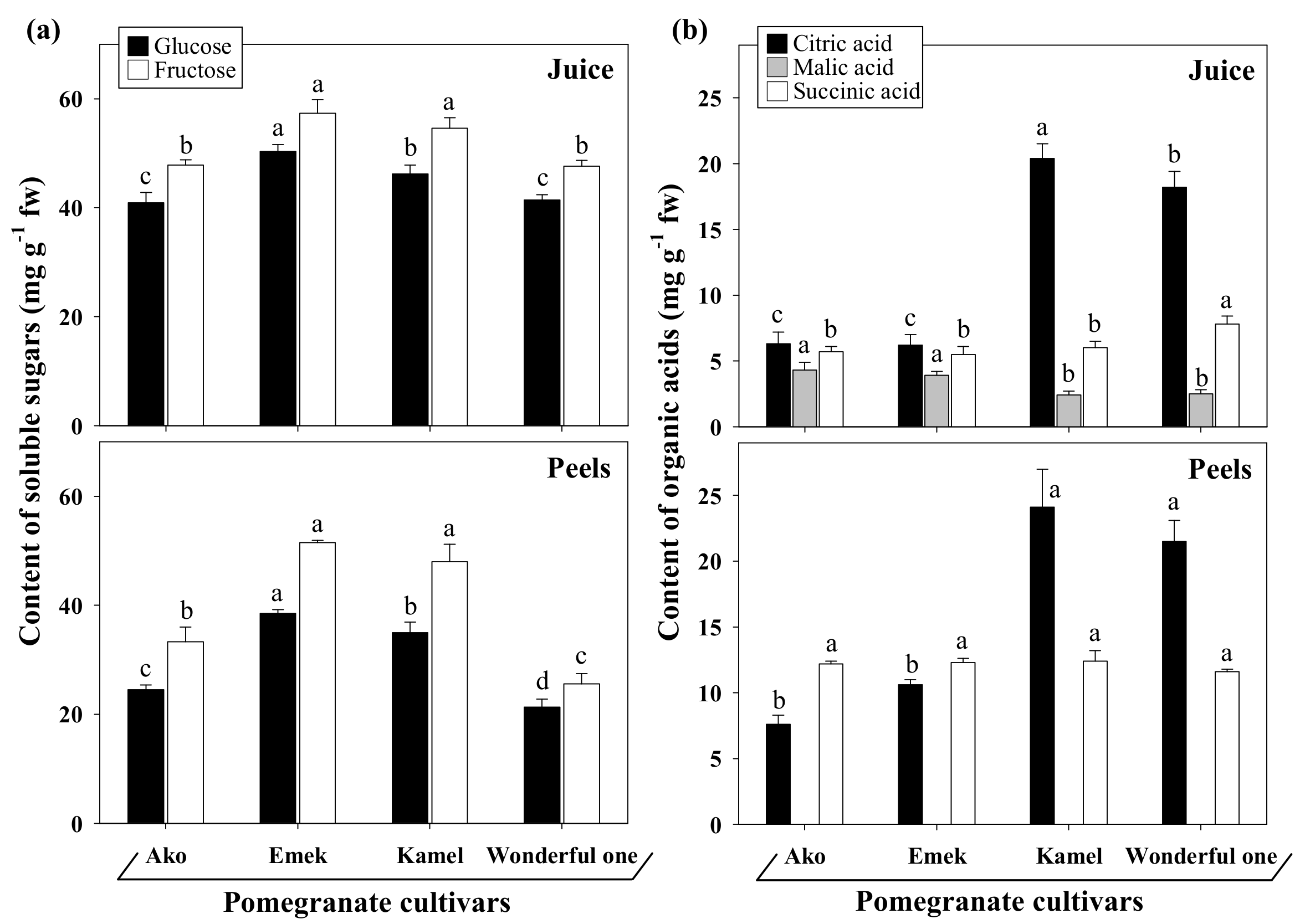
33]. In all cultivars, glucose and fructose were the principal monosaccharides identified, with fructose slightly exceeding glucose both in juices and peels (
Figure 3a). No sucrose and maltose were detected in any cultivars, in agreement with Fadavi et al. [
24]. Generally, pomegranate aril juices have equal amounts of fructose and glucose, with modest variations among cultivars. However, a slight prevalence of the first sugar over the other, and vice versa, was previously reported in the genotypes of different collections [
32].
Paradoxically, the sweet cultivar Ako had the lowest total ethanol-soluble carbohydrate content. Accordingly, higher sugar contents in sour cultivars than in sweet ones were reported by Hasnaoui et al. [
34] while assaying twelve Tunisian autochthonous genotypes. The same authors described a close correlation between sourness and concentrations of organic acids, especially citric acid. Indeed, total organic acid concentrations in Kamel and Wonderful one Cultivars, which are sweet-sour and sour, respectively, were 73–85% higher than in the sweet ones, with citric acid strongly predominant. Instead, Ako and Emek were characterized by a more balanced organic acid profile (
Figure 3b). Differently, malic acid, followed by citric acid, was found as the most abundant organic acid of the juices from the autochthonous wild pomegranates of Montenegro [
27]; this discrepancy between wild and cultivated genotypes is likely the result of selective breeding to meet consumer taste preferences. A strong correlation was reported between sourness and citric acid content, which is assumed to be the major factor contributing to pomegranate fruit sourness [
34]. Contrary to the juices, the peels showed no detectable amounts of malic acid in all cultivars, while the prevalence of citric acid of sweet-sour and sour cultivars was confirmed. The amount of total ethanol-insoluble carbohydrates (
Table 1), mainly comprising starch and dietary fibers (cell wall polysaccharides), was particularly high in kernels (65.1–80.6 mg∙g
−1 fw), followed by peels (27.9–42.3 mg∙g
−1 fw) and juice (32.8–35.8 mg∙g
−1 fw), with no significant differences among cultivars, except for peels. At this regard, it is worth recalling that the exploitation of the polysaccharide fraction potential in the preparation of plant-based products for human health demands deep investigation. Indeed, polysaccharides extracted from pomegranate peels have been proposed to exert immunomodulatory effects and antiproliferative activity on several cancer cell lines [
35,
36].
2.2. Functional Quality Attributes
The total content of phenols, flavonoids, anthocyanins, AsA, and DAsA in the juices, kernels, and peels isolated from the ripe fruits of the four investigated pomegranate cultivars is reported in
Table 2. It is worth noticing that, in plants, phenols occur in both soluble and insoluble forms. Soluble phenols are mainly sequestered within vacuoles and are easily extracted from tissues with diluted alcohols (i.e., methanol or ethanol). Insoluble phenols, instead, are covalently bound (primarily esterified) to the polymeric constituents of cell walls, including structural polysaccharides and proteins, and are, thus, resistant to extraction, unless a preliminary hydrolysis step is performed [
37].
Though the literature abounds with studies on the phenolic composition of pomegranate arils, juice, and peels, most just focus on soluble phenols, ignoring the insoluble-bound form, often underestimating the total content of phenols in the fruit parts and their health potential. Effectively, in all juice and peel samples, soluble phenols largely exceeded the bound forms, contributing between 97.2 and 99.7% to the total, while they ranged between 66.6 and 76.8% in the kernels. Significant differences were observed among cultivars in both juices and kernels, within a range of 4.6–9.7 and 4.4–6.6 mg gallic acid equivalents (GAE)∙g
−1 fw, respectively, but not in peels, where the highest concentrations were registered (51.7–61.9 mg GAE∙g
−1 fw), in agreement with Orak et al. [
29]. Similarly, the phenolic content of the peels of pomegranates from three different provinces of Iran (Natanz, Shahreza, and Doorak) was 3.8–11.6- and 5.7–33.3-fold higher than in kernels and juice, respectively [
38], and 10-fold higher than that of aril juices from 28 assayed Chinese cultivars [
15,
39].
Of all phenolic compounds, flavanols, ellagitannins and derivatives of ellagic acid had the highest concentrations in pomegranate juices [
27], while hydrolysable tannins have been proposed as the predominant class of phenols in peels, with very high amounts of ellagitannins of the gallagyl ester group, such as punicalins and punicalagins [
40]. Hence, obtaining juices from whole fruits (peel and arils), as in commercial production, enhances the content and profile of polyphenol compounds and the antioxidant activity of the product [
41].
Regarding the juices, our results on total soluble phenolic compounds are generally higher than those reported in the literature, but very close to the contents found for wild Montenegro genotypes (4.4–8.5 mg∙g
−1 fw) [
27,
39,
42,
43]. Lower levels of total soluble phenolic compounds were also reported by Ferrara et al. [
22] for juices extracted from Ako (1.77 mg∙g
−1 fw), Emek (1.50 mg∙g
−1 fw), and Wonderful One (2.34 mg∙g
−1 fw) cultivars grown in the same orchard as this study, but in previous years. This suggests that tree age is a factor affecting the level of fruit antioxidants, as was reported for mandarin juice [
44]. Reduced levels of total phenolic compounds were also detected by Pande and Akoh [
45] and Jing et al. [
46] for kernels from Georgia- (0.85–0.91 mg GAE∙g
−1 fw) and China-grown (1.29–2.17 mg GAE∙g
−1 fw) pomegranate cultivars, possibly due to different growing conditions, genotypes, and extraction methods. In particular, soluble-conjugated and insoluble-bound phenolic acids were found to significantly contribute to the total phenolic content of kernels, in agreement with Ambigaipalan et al. [
47] and Jing et al. [
46]. The authors found 1.38, 1.39, and 0.62 mg GAE g
−1 fw as free, esterified, and insoluble-bound phenolic compounds, respectively, in pomegranate kernels, with 3,4-Dihydroxybenzoic acid predominantly in the soluble-conjugate form, ferulic acid primarily as insoluble-bound form, and both vanillic and syringic acids almost equally distributed between the soluble and insoluble forms. For peels, the levels we found were comparable to the literature data [
39]. Kernels, instead, was the fraction most abundant in bound phenols (1.97 to 2.21 mg GAE∙g
−1 fw), followed by peels (0.19 to 0.23 mg GAE∙g
−1 fw) and juice (0.07–0.13 mg GAE∙g
−1 fw), with no statistically significant differences among cultivars.
Pomegranate fruit is particularly rich in flavonoids in all its parts, hence the interest for an efficient recovery of these bioactive molecules from the industrial by-products [
20,
48]. Indeed, regardless of genotype, kernels ranked first, also, for total flavonoids (4.2–5.6 mg CE∙g
−1 fw), followed by peels (4.0–5.1 mg CE∙g
−1 fw) and juice (0.88–1.44 mg CE∙g
−1 fw). In agreement, He et al. [
49] highlighted the value of a pomegranate seed-residue by-product as a source of several phenolic compounds, including phenolic acid derivatives, flavan-3-ols, flavonoid glycosides, and hydrolysable tannins, and obtained extracts with a total phenolic content up to 2.4 g catechin equivalents (CE)/100 g dry weight (dw) and high antioxidant potential.
A wide variability of the content of phenols in pomegranate has been reported in the literature, and is strictly related to the cultivar analyzed [
50]. According to Li et al. [
15], the average total phenolic and flavonoid contents of peels were 249.4 mg tannic acid equivalents (TAE)∙g
−1 dw and 59.1 mg rutin equivalents (RE)∙g
−1 dw, respectively. Much lower concentrations, up to 1.22 and 0.13 mg RE∙g
−1 dw, were, instead, reported by Fellah et al. [
51] in the peels of the Nabli and Gabsi Tunisian cultivars, respectively. Our values, expressed on a dw basis after correcting for moisture content (218.1–261.2 mg GAE∙g
−1 dw and 16.9–21.1 mg CE∙g
−1 dw, respectively), fall in the upper part of these wide ranges.
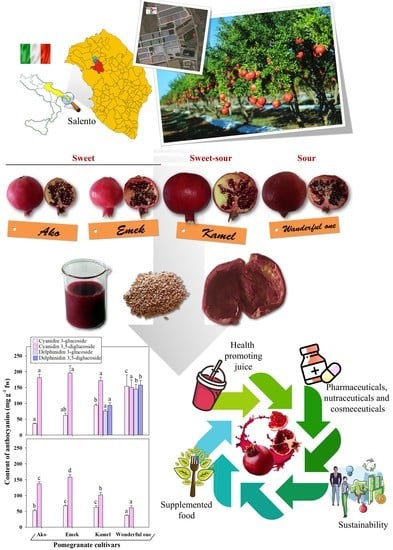
The highest anthocyanin concentrations were registered in the juices (217–608 µg cyanidin-3-glucoside equivalents (CGE)∙g
−1 fw), followed by the peels (104.8–232.9 µg CGE∙g
−1 fw) and the kernels (61.2–240 µg CGE∙g
−1 fw). A significant cultivar-dependent variability was found for flavonoids and anthocyanins in all fruit parts. Correspondingly, Elfalleh et al. [
52] and Fellah et al. [
51] reported significant variation in the total amount of polyphenols, flavonoids, anthocyanins, and hydrolysable tannins among different plant parts (seeds, leaves, flowers, and peels). It has been shown that the bright color of the pomegranate fruit depends mostly on anthocyanin concentration; that, however, can be strongly influenced by several parameters, including pre-harvest treatments (e.g., fertirrigation, methyl jasmonate application), the time of harvesting, and post-harvest storage conditions (temperature, humidity) [
52,
53,
54]. The juice of Wonderful One had levels of anthocyanins considerably higher than those of the other cultivars, presenting, in fact, with an intense deep-red color. Noda et al. [
55] reported that three major anthocyanidins found in pomegranate juice were delphinidin, cyanidin, and pelargonidin. Cyanidin 3,5-diglucoside, pelargonidin 3,5-diglucoside, delphinidin 3,5-diglucoside, cyanidin 3-glucoside, pelargonidin 3-glucoside, and delphinidin 3-glucoside were identified in the juices of six old Italian pomegranate varieties, with cyanidin 3-glucoside, followed by pelargonidin 3,5-diglucoside, as the most representative, while anthocyanins were below the limit of detection in the peel extract samples [
56]. Only small amounts of anthocyanins (0.37–0.070 mg CGE∙g
−1 fw) were detected in the kernels of California pomegranates, presumably as contaminants from those remaining from the juiced arils [
47].
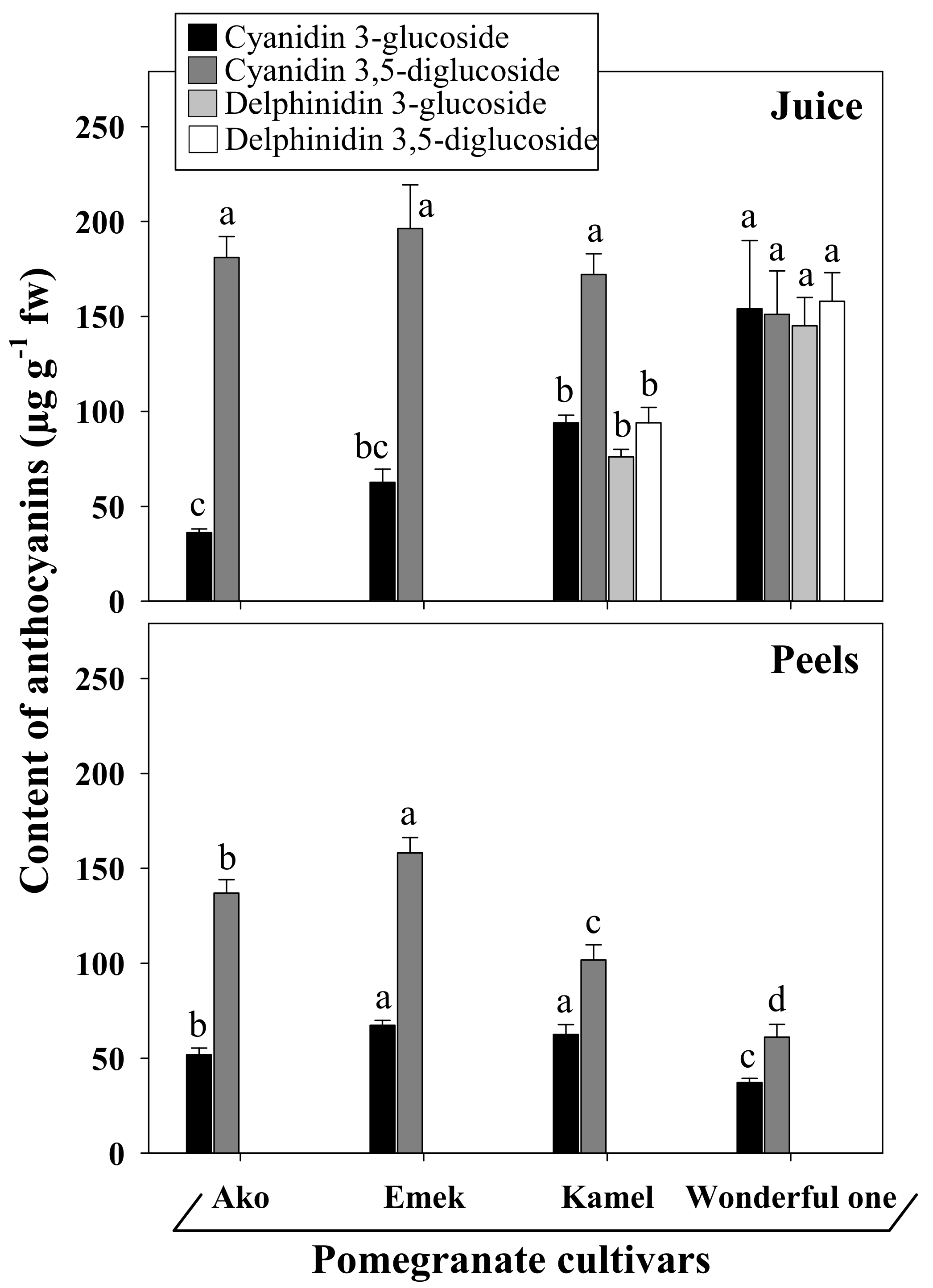
Our results show evident differences in the profiles of the main anthocyanins detected in the juices and peels of the four assayed cultivars (
Figure 4). In particular, cyanidin 3- and 3,5-(di)glucosides were present in all samples, although in different quantities and ratios, while delphinidin glucosides were exclusively detected in the juices of the cultivars Kamel and Wonderful One.
With regard to the three fruit parts, the differences observed in the accumulation of phenolics and flavonoids are essentially due to fruit tissue specialization. Peels preferentially accumulate phenolic compounds acting as protective screens against high-energy wavelengths to reduce light-induced oxidative stress and fruit sunburn, as well as those with toxic effects or protein precipitation capacities, as a line of defense against pathogens and predators. The cells of the juicy sarcotesta preferentially synthesize anthocyanins at the cytosolic surface of the endoplasmic reticulum and transport them across the tonoplast through specific carriers (ATP-binding cassette transporter and multidrug and toxic compound extrusion proteins) within large vacuoles. Vacuolar anthocyanins contribute to the brilliant bright-red color of the arils that is fundamental for attracting seed dispersers. Kernels are characterized, instead, by high levels of insoluble-bound phenols likely involved in hardening the thick wall of sclerotesta cells. Phenols (both soluble and insoluble) play, also, vital roles in the protection of the zygotic and reserve tissues from biotic aggression and abiotic stresses favoring seed survival, as well as in seed development, maturation, dormancy, and germination. An increase in the ratio of insoluble-bound to soluble phenolic compounds was observed in lentil seeds during germination, suggesting that phenol secretion to the cell wall, followed by insolubilization, may exert a pivotal action in regulating the process [
37,
57].
AsA and DAsA contents of most fruit parts were significantly affected by genotype. Peels revealed the highest concentration of both AsA (2.6–4.4 mg∙g
−1 fw) and DAsA (0.3–2.4 mg∙g
−1 fw), followed by juices (0.42–0.95 and 0.06–0.70 mg∙g
−1 fw) and kernels (0.22–0.37 and 0.03–0.16 mg∙g
−1 fw) (
Table 2). In agreement with our results, Opara et. al. [
58] reported that vitamin C content in the pomegranate peel was significantly higher than in the aril, with differences ranging from 24.4% to 97.0%, depending on variety. Compared to our results, much lower levels of ascorbic acid were found in pomegranate juices from India (0.198 mg g
−1 fw), Iran (0.09–0.40 mg g
−1 fw), Poland (0.091–0.268 mg g
−1 fw), and South Africa (0.168 mg g
−1 fw) [
23,
40,
59,
60]. This discrepancy is possibly due to different agricultural practices and/or to the peculiar pedoclimatic conditions of southern Italy. In particular, the mild weather, high sun irradiation, and the medium-textured lime-rich soils of the Salento peninsula make for the perfect terroir for the development and ripening of some fruits with improved levels of primary and secondary metabolites, including grapes, tomatoes, and olives [
61,
62,
63].
Interestingly, pomegranate peels had, on average, 12-fold higher AsA concentrations than the peels of oranges, limes, and mandarins, fruits notoriously rich in vitamin C, while the AsA contents of the juices were similar or even higher than in the pulp of citrus fruits, as well as of pineapple, kiwi, and other pomegranate cultivars [
64,
65]. Nevertheless, it is important to reiterate that vitamin C is very unstable, being sensitive to light, heat, and air, rapidly deteriorating in the food during transport, processing, denting, and cutting [
66].
Therefore, the obtained results suggest the need to raise the consumer’s awareness about the importance of pomegranate as a source of vitamin C that widely exceeds other consumed fresh products.
2.5. Element Composition
Table 6 summarizes the elemental concentrations of the different fruit parts isolated from ripe fruits of the four pomegranate cultivars under investigation. The amount of most elements was significantly different among either cultivars or fruit parts. Overall, potassium (K) ranked first for concentration, followed by magnesium (Mg) and sodium (Na). The relative order of the other assayed elements was, instead, variable within samples. Boron (B), iron (Fe), and copper (Cu) were the main microelements in the juices, kernels, and peels, respectively. Fe, Cu, Na, Mg, manganese (Mn), vanadium (V), and zinc (Zn) concentrations were significantly lower in juices than in peels and, largely, in kernels, where the highest concentrations were registered on a fw basis regardless of cultivar. Similarly, aluminum (Al) content was low in the juices of all cultivars, but much higher in the other fruit parts, with kernels or peels showing the highest concentrations depending on cultivar. Silver (Ag) and molybdenum (Mo) were, instead, only detected in the peels of Ako and Kamel, respectively, and in the kernels of all cultivars, suggesting a selective sequestration of these elements in the different fruit tissues.
Ag, bismuth (Bi), cadmium (Cd), cobalt (Co), lithium (Li), and thallium (Tl) were, instead, below the detection limit (0.001 µg∙g
−1 fw) in all samples. Most of our findings are consistent with the results of other studies on the mineral composition of pomegranates grown in different countries. Indeed, many authors reported that the main elements found in the juice of pomegranates were, roughly in decreasing order, nitrogen (N), K, Na, phosphorous (P), Mg, calcium (Ca), Fe, Zn, Cu and Mn, within concentration ranges that were largely affected by genotype and ripening stage, as well as by pedological, climatic, and agronomical factors [
27,
74,
75,
76,
77,
78]. Recently, Loukhmas et al. [
79] found iodine (I) as the most abundant macroelement in pomegranate juice obtained from ten Moroccan pomegranate cultivars, followed by phosphorus (P) and sulfur (S).
According to Mirdehghan and Rahemi [
80], the relative order of concentration of macroelements both in the arils and peels was K > N > Ca > P > Mg > Na, while the relative order of concentration of microelements was B > Fe > Zn > Cu > Mn in the arils and B > Fe > Zn = Mn > Cu in the peels of freshly harvested pomegranate fruits. A similar order (Na > > K >>Fe > Mn-Zn) was reported by Ullah et al. in the peels of an unspecified pomegranate cultivar grown in Pakistan [
30]. Fawole and Opara [
27] found considerable differences in trace element concentrations in the fruit parts (rind, mesocarp, and entire arils) and among cultivars in seven pomegranate genotypes (Arakta, Bhagwa, Ganesh, Herskawitz, Mollar de Elche, Ruby, and Wonderful) grown in South Africa. The authors reported relatively high amounts of Mn, Fe, Cu, Zn, B, Nickel (Ni), Selenium (Se), Al, and Strontium (Sr) in most cultivars, while the amounts of Ni, Co, Pb, Cd, As, Li, Ti, and V in the fruit parts were low or below the detection level. They also found that Na was particularly abundant in the cultivar Wonderful; accordingly, we found that this element was much more concentrated in the juices and peels of the related genotype Wonderful One than in all other cultivars. Further, Okatan et al. [
78] reported a statistically significant positive correlation between N and Cu contents, as well as between phosphorus, AsA, TAA, and total anthocyanins.