Foliar Application of Silicon in Vitis vinifera: Targeted Metabolomics Analysis as a Tool to Investigate the Chemical Variations in Berries of Four Grapevine Cultivars
Abstract
1. Introduction
2. Results
2.1. Secondary Metabolite Fingerprinting of the Berries of the Four Grape Cultivars
2.2. Quali-Quantitative Fingerprint of Secondary Metabolites in Berries
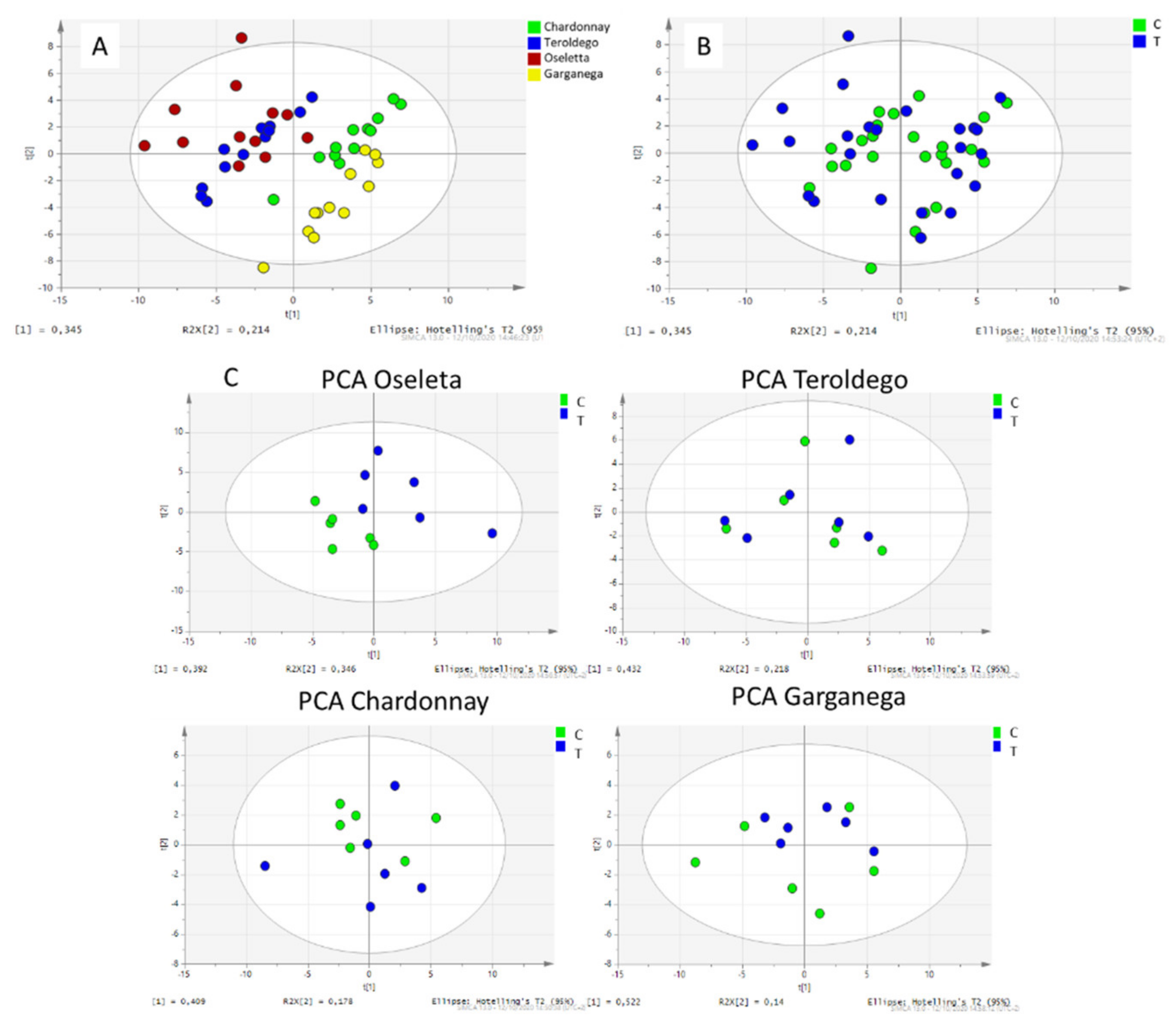
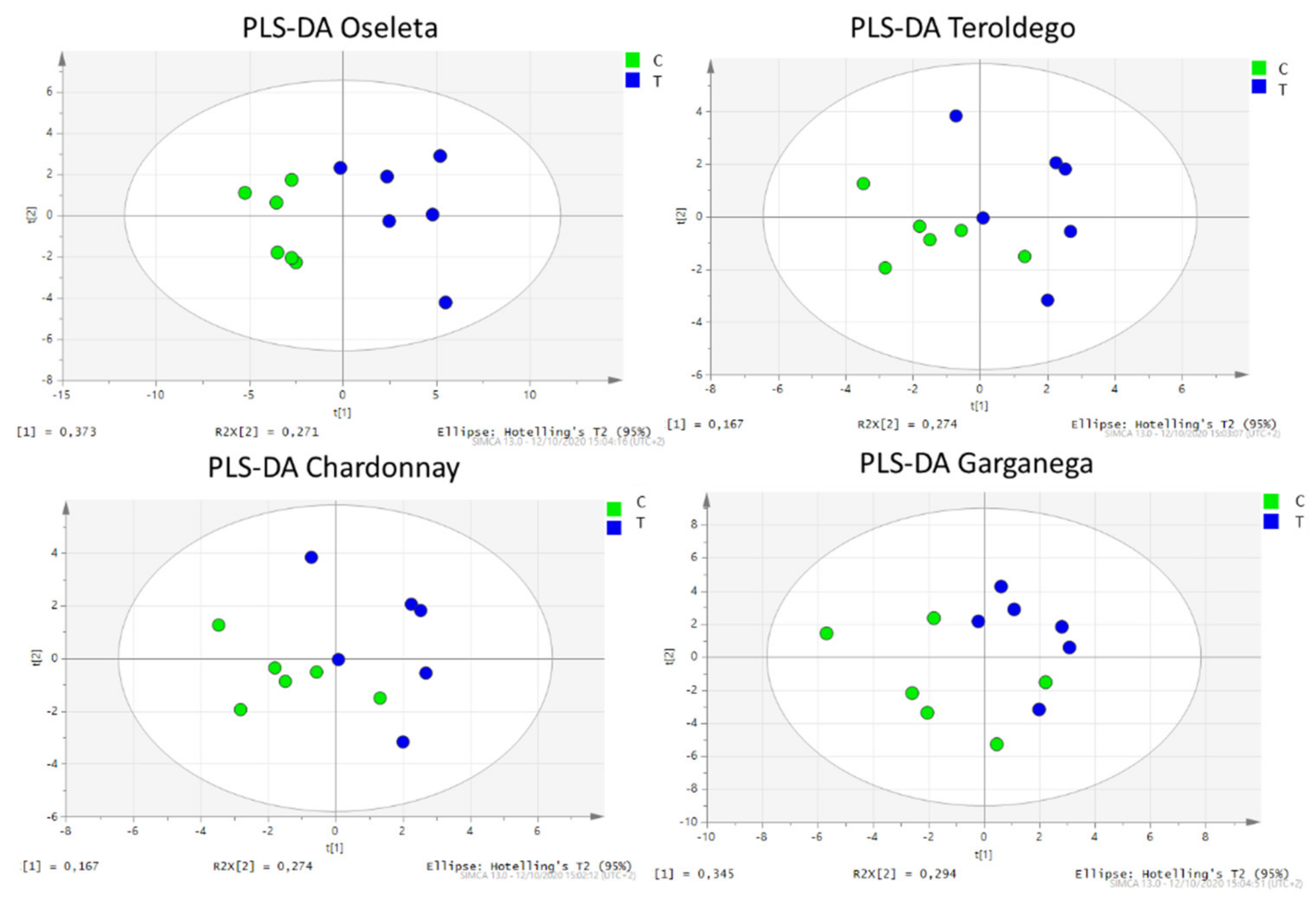
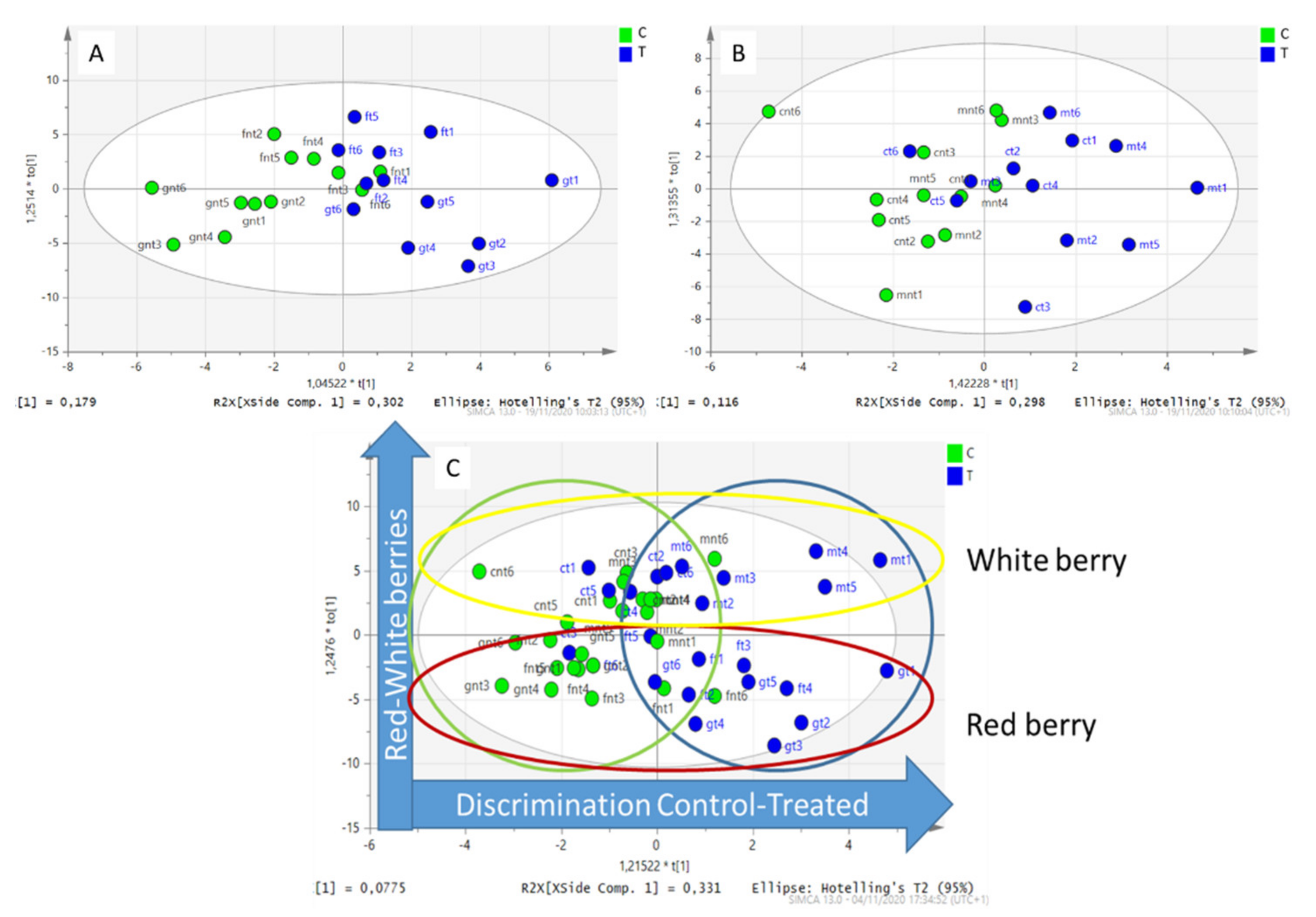
2.3. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Sites and Plant Samples
4.2. CNS Measurement
4.3. Sample Preparation for Secondary Metabolite Analysis
4.4. Secondary Metabolite Analysis
4.5. Amino Acid Analysis
4.6. Sugar Analysis and Tartaric Acid Quantification
4.7. Multivariate Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon With Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in us agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Yin, L.; Deng, X. How Does Silicon Mediate Plant Water Uptake and Loss Under Water Deficiency? Front. Plant Sci. 2018, 9, 281. [Google Scholar] [CrossRef]
- Van Bockhaven, J.; De Vleesschauwer, D.; Höfte, M. Towards establishing broad-spectrum disease resistance in plants: Silicon leads the way. J. Exp. Bot. 2013, 64, 1281–1293. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Cooke, J.; Leishman, M.R. Is plant ecology more siliceous than we realise? Trends Plant Sci. 2011, 16, 61–68. [Google Scholar] [CrossRef]
- Dos Santos Sarah, M.M.; de Mello Prado, R.; de Souza Júnior, J.P.; Teixeira, G.C.M.; dos Santos Duarte, J.C.; de Medeiros, R.L.S. Silicon supplied via foliar application and root to attenuate potassium deficiency in common bean plants. Sci. Rep. 2021, 11, 19690. [Google Scholar] [CrossRef]
- Artyszak, A. Effect of silicon fertilization on crop yield quantity and quality—A literature review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Resende, R.S.; Dallagnol, L.J.; Datnoff, L.E. Silicon Potentiates Host Defense Mechanisms Against Infection by Plant Pathogens; Rodrigues, A.F., Datnoff, L.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-22929-4. [Google Scholar]
- Schabl, P.; Gabler, C.; Kührer, E.; Wenzel, W. Effects of silicon amendments on grapevine, soil and wine. Plant Soil Environ. 2020, 66, 403–414. [Google Scholar] [CrossRef]
- Losada, M.M.; Hernández-Apaolaza, L.; Morata, A.; Revilla, E. Impact of the application of monosilicic acid to grapevine (Vitis vinifera L.) on the chemical composition of young red Mencia wines. Food Chem. 2022, 378, 132140. [Google Scholar] [CrossRef]
- Gomes, T.M.; Mazon, L.F.; Panceri, C.P.; Machado, B.D.; Brighenti, A.; Burin, V.M.; Bordignon-Luiz, M.T. Changes in vineyard productive attributes and phytochemical composition of sauvignon blanc grape and wine induced by the application of silicon and calcium. J. Sci. Food Agric. 2020, 100, 1547–1557. [Google Scholar] [CrossRef]
- Do Nascimento, C.W.A.; da Silva, F.B.V.; Lima, L.H.V.; Silva, J.R.; de Lima Veloso, V.; da Silva, F.L.; de Freitas, S.T.; dos Santos, L.F.; dos Santos, M.A. Silicon Application to Soil Increases the Yield and Quality of Table Grapes (Vitis vinifera L.) Grown in a Semiarid Climate of Brazil. Silicon 2022, preprint. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, Y.; Chu, G. Applying silicate fertilizer increases both yield and quality of table grape (Vitis vinifera L.) grown on calcareous grey desert soil. Sci. Hortic. 2017, 225, 757–763. [Google Scholar] [CrossRef]
- Pinu, F.R. Grape and wine metabolomics to develop new insights using untargeted and targeted approaches. Fermentation 2018, 4, 92. [Google Scholar] [CrossRef]
- Picone, G.; Trimigno, A.; Tessarin, P.; Donnini, S.; Rombolà, A.D.; Capozzi, F. 1H NMR foodomics reveals that the biodynamic and the organic cultivation managements produce different grape berries (Vitis vinifera L. cv. Sangiovese). Food Chem. 2016, 213, 187–195. [Google Scholar] [CrossRef]
- Laghi, L.; Versari, A.; Marcolini, E.; Parpinello, G.P. Metabonomic Investigation by 1H-NMR to Discriminate between Red Wines from Organic and Biodynamic Grapes. Food Nutr. Sci. 2014, 5, 52–59. [Google Scholar] [CrossRef]
- Botelho, R.V.; Roberti, R.; Tessarin, P.; Garcia-Mina, J.M.; Rombolà, A.D. Physiological responses of grapevines to biodynamic management. Renew. Agric. Food Syst. 2016, 31, 402–413. [Google Scholar] [CrossRef]
- Malagoli, M.; Sut, S.; Kumar, G.; Dall’Acqua, S. Variations of elements, pigments, amino acids and secondary metabolites in Vitis vinifera (L.) cv Garganega after 501 biodynamic treatment. Chem. Biol. Technol. Agric. 2022, 9, 36. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Bhat, J.A.; Siddiqui, M.H.; Rinklebe, J.; Ahmad, P. Integration of silicon and secondary metabolites in plants: A significant association in stress tolerance. J. Exp. Bot. 2020, 71, 6758–6774. [Google Scholar] [CrossRef]
- Parpinello, G.P.; Ricci, A.; Rombolà, A.D.; Nigro, G.; Versari, A. Comparison of Sangiovese wines obtained from stabilized organic and biodynamic vineyard management systems. Food Chem. 2019, 283, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Afonso, J.; Nogueira, M.; Oliveira, A.A.; Cosme, F.; Falco, V. Silicates of potassium and aluminium (Kaolin); comparative foliar mitigation treatments and biochemical insight on grape berry quality in Vitis vinifera L. (cv. touriga national and touriga franca). Biology 2020, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef]
- Springob, K.; Nakajima, J.; Yamazaki, M.; Saito, K. Recent advances in the biosynthesis and accumulation of anthocyanins. Nat. Prod. Rep. 2003, 20, 288–303. [Google Scholar] [CrossRef]
- Rashad, Y.M.; El-Sharkawy, H.H.A.; Belal, B.E.A.; Abdel Razik, E.S.; Galilah, D.A. Silica Nanoparticles as a Probable Anti-Oomycete Compound Against Downy Mildew, and Yield and Quality Enhancer in Grapevines: Field Evaluation, Molecular, Physiological, Ultrastructural, and Toxicity Investigations. Front. Plant Sci. 2021, 12, 763365. [Google Scholar] [CrossRef]
- Hossain, M.A.; Wani, S.H.; Bhattacharjee, S.; Burritt, D.J.; Tran, L.-S.P. Drought Stress Tolerance in Plants, Volume 1, 1st ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Stines, A.P.; Grubb, J.; Gockowiak, H.; Henschke, P.A.; Høj, P.B.; Van Heeswijck, R. Proline and arginine accumulation in developing berries of Vitis vinifera L. in Australian vineyards: Influence of vine cultivar, berry maturity and tissue type. Aust. J. Grape Wine Res. 2000, 6, 150–158. [Google Scholar] [CrossRef]
- Kennedy, J.; Hayasaka, Y.; Vidal, S.; Waters, E.; Jones, G. Composition of Grape Skin Proanthocyanidins at Different Stages of Berry Development. J. Agric. Food Chem. 2001, 49, 5348–5355. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Mostafa, A.I.; Lee, C.B. Effect of silicon application on roselle (Hibiscus sabdariffa L.) grown in a Vertisol in Egypt. J. Soil Sci. Environ. Manag. 2016, 7, 45–52. [Google Scholar] [CrossRef]
- Ravazzolo, L.; Trevisan, S.; Forestan, C.; Varotto, S.; Sut, S.; Dall’acqua, S.; Malagoli, M.; Quaggiotti, S. Nitrate and ammonium affect the overall maize response to nitrogen availability by triggering specific and common transcriptional signatures in roots. Int. J. Mol. Sci. 2020, 21, 686. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.M.; da Silva Padilha, C.V.; Miskinis, G.A.; de Sá, A.G.B.; Pereira, G.E.; de Azevêdo, L.C.; dos Santos Lima, M. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: Method validation and characterization of products from northeast Brazil. J. Food Compos. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef]
| RT | Ion | Fragments | Identification | Oseleta | Teroldego | Garganega | Chardonnay |
|---|---|---|---|---|---|---|---|
| [M + H]+ | Anthocyanidins | ||||||
| 25.9 | 465 | 303 | delphinidin-3-O-glucoside | 0.10 ± 0.01 | 0.114 ± 0.01 | - | - |
| 29.8 | 448 | 287 | cyanidin-3-O-glucoside | 0.03 ± 0.010 | 0.233 ± 0.001 | - | - |
| 31.5 | 479 | 317 | petunidin-3-O-glucoside | 0.083 ± 0.005 | 0.118 ± 0.005 | - | - |
| 32.9 | 463 | 301 | peonidin-3-O-glucoside | 0.066 ± 0.001 | 0.454 ± 0.001 | - | - |
| 33.4 | 493 | 331 | malvidin-3-O-glucoside | 0.284 ± 0.003 | 0.315 ± 0.002 | - | - |
| 34.4 | 507 | 303 | delphinidin-3-O-(6″-acetyl-glucoside) | 0.018 ± 0.002 | 0.003 ± 0.002 | - | - |
| 35.5 | 491 | 287 | cyanidin-3-O-(6″-acetyl-glucoside) | 0.007 ± 0.005 | 0.002 ± 0.001 | - | - |
| 35.9 | 521 | 317 | petunidin-3-O-(6″-acetyl-glucoside) | 0.021 ± 0.003 | 0.001 ± 0.001 | - | - |
| 37 | 505 | 301 | peonidin-3-O-(6″-acetyl-glucoside) | 0.118 ± 0.001 | 0.009 ± 0.001 | - | - |
| 37.3 | 611 | 303 | delphinidin-3-O-(6-p-coumaroyl)glucoside | ||||
| 37.7 | 535 | 331 | malvidin-3-O-(6″-acetyl-glucoside) | ||||
| 38 | 655 | 331 | malvidin-3-O-(6″-caffeoyl-glucoside) | 0.011 ± 0.005 | 0.009 ± 0.001 | - | - |
| 38.1 | 595 | 287 | cyanidin-3-O-(6-p-coumaroyl)glucoside | 0.017 ± 0.003 | 0.005 ± 0.001 | - | - |
| 38.3 | 625 | 317 | petunidin-3-O-(6-p-coumaroyl)glucoside | ||||
| 39 | 639 | 331 | malvidin-3-O-(6-p-coumaroyl)glucoside | 0.097 ± 0.001 | 0.043 ± 0.002 | - | - |
| 39.1 | 609 | 301 | peonidin-3-O-(6-p-coumaroyl)glucoside | ||||
| total amount | 0.852 | 1.306 | |||||
| [M - H]- | Flavonoids | ||||||
| 34.9 | 463 | 301 | quercetin-7-O-glucoside | - | - | 0.008 ± 0.001 | 0.004 ± 0.001 |
| 35.2 | 477 | 301 | quercetin-O-glucuronide | - | - | 0.01 ± 0.001 | 0.005 ± 0.001 |
| 35.3 | 463 | 301 | quercetin-3-O-glucoside | - | - | 0.033 ± 0.001 | 0.002 ± 0.001 |
| 37 | 477 | 285 | kaempferol-O-hexoside | - | - | 0.006 ± 0.001 | - |
| total amount | 0.057 | 0.011 | |||||
| [M - H]- | PAC derivatives | ||||||
| 16.2 | 577 | 425 407 | pac dimer isomer 1 | 0.01 ± 0.001 | 0.013 ± 0.001 | 0.019 ± 0.001 | 0.027 ± 0.001 |
| 17.1 | 289 | 245 205 | catechin | 0.02 ± 0.005 | 0.043 ± 0.005 | 0.013 ± 0.002 | 0.054 ± 0.005 |
| 19.8 | 865 | 695 577 | pac trimer isomer 1 | 0.029 ± 0.002 | 0.043 ± 0.002 | 0.027 ± 0.005 | 0.021 ± 0.001 |
| 21.4 | 443 | - | unknown | - | - | 0.002 ± 0.001 | - |
| 23.2 | 577 | 425 407 | pac dimer isomer 2 | 0.020 ± 0.006 | 0.025 ± 0.002 | 0.013 ± 0.001 | 0.010 ± 0.001 |
| 26.5 | 289 | 245 205 | epicatechin | 0.020 ± 0.003 | 0.040 ± 0.008 | 0.020 ± 0.006 | 0.059 ± 0.003 |
| 27 | 729 | 577 407 | procyanidin dimer monogallate | 0.014 ± 0.001 | 0.005 ± 0.001 | 0.003 ± 0.001 | - |
| 31.2 | 865 | 695 577 | pac trimer isomer 2 | 0.003 ± 0.001 | 0.023 ± 0.002 | 0.019 ± 0.001 | 0.005 ± 0.001 |
| 32 | 1153 | 865 575 | pac tetramer | - | 0.007 ± 0.001 | 0.004 ± 0.001 | - |
| 33 | 729 | 577 407 | procyanidin dimer monogallate | - | - | - | 0.001 ± 0.001 |
| 33.7 | 441 | catechin gallate | - | 0.017 ± 0.001 | 0.018 ± 0.001 | - | |
| 35.3 | 865 | 695 577 | pac trimer isomer 3 | - | 0.033 ± 0.002 | - | - |
| total amount | 0.116 | 0.249 | 0.138 | 0.177 |
| Oseleta | Teroldego | Chardonnay | Garganega | |
|---|---|---|---|---|
| Tot PAC C | 0.09 ± 0.03 | 0.26 ± 0.07 | 0.22 ± 0.05 | 0.13 ± 0.06 |
| Tot PAC T | 0.14 ± 0.04 | 0.24 ± 0.08 | 0.17 ± 0.05 | 0.15 ± 0.04 |
| anthocyanin C | 0.48 ± 0.23 * | 1.31 ± 0.19 | - | - |
| anthocyanin T | 1.25 ± 0.33 * | 1.34 ± 0.18 | - | - |
| flavonoid C | - | - | 0.010 ± 0.004 | 0.055 ± 0.018 |
| flavonoid T | - | - | 0.012 ± 0.006 | 0.060 ± 0.015 |
| amino acids C | 467 ± 51 | 398 ± 51 * | 443 ± 52 | 394 ± 98 |
| amino acids T | 444 ± 48 | 590 ± 42 * | 467 ± 90 | 429 ± 87 |
| tartaric acid C | 0.93 ± 0.4 * | 2.5 ± 0.5 | 0.93 ± 0.35 * | 1.09 ± 0.16 |
| tartaric acid T | 1.21 ± 0.2 * | 2.6 ± 0.3 | 0.66 ± 0.13 * | 1.09 ± 0.19 |
| glucose C | 117.7 ± 9.6 | 103 ± 4.7 | 115.4 ± 8.0 | 91.5 ± 14.9 |
| glucose T | 121.2 ± 4.4 | 98.4 ± 5.4 | 111.7 ± 10.5 | 84.5 ± 9.2 |
| fructose C | 98.6 ± 7.4 | 95.4 ± 3.7 * | 98.7 ± 7.0 | 75.7 ± 9.6 |
| fructose T | 103.5 ± 4.7 | 88.4 ± 4.4 * | 94.5 ± 7.1 | 71.6 ± 8.8 |
| Samples | Metabolites | T vs. C (%) |
|---|---|---|
| Oseleta | valine | −54.7 * |
| arginine | −81.3 * | |
| phenyl alanine | −31.2 * | |
| asparagine | −16.3 * | |
| malvidin-3-O-glucoside | +119.9 * | |
| peonidine-3-O-glucoside | +318.3 * | |
| petunidin-3-O-(6″-acetyl-glucoside) | +176.6 * | |
| cyanidin-3-O-(6-p-coumaroyl)glucoside/petunidin-3-O-(6-p-coumaroyl)glucoside | +141.2 * | |
| Teroldego | fructose | −7.6 * |
| glucose | −5.1 | |
| procyanidin trimer isomer 1 | −16.9 | |
| cyanidin-3-O-(6″-acetyl-glucoside) | −34.0 * | |
| asparagine | +29.4 | |
| procyanidin trimer isomer 2 | +28.6 | |
| malvidin-3-O-(6-p-coumaroyl)glucoside/peonidin-3-O-(6-p-coumaroyl)glucoside | +15 | |
| delphinidin-3-O-(6″-acetyl-glucoside) | +11.1 | |
| Garganega | kaemferol O hexoside | −13.8 |
| glucose | −7.6 | |
| fructose | −5.4 | |
| arginine | −9 | |
| proline | +105.1 * | |
| procyanidin trimer isomer 2 | +26.6 | |
| procyanidin tetramer | +132.8 | |
| catechin gallate | +62.1 | |
| Chardonnay | procyanidin trimer isomer 2 | −27.7 |
| catechin | −32 | |
| tartaric acid | −28.8 * | |
| epicatechin | −26 | |
| histidine | +3.02 | |
| tyrosine | +1.37 | |
| serine | +1.83 | |
| leucine | +0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sut, S.; Malagoli, M.; Dall’Acqua, S. Foliar Application of Silicon in Vitis vinifera: Targeted Metabolomics Analysis as a Tool to Investigate the Chemical Variations in Berries of Four Grapevine Cultivars. Plants 2022, 11, 2998. https://doi.org/10.3390/plants11212998
Sut S, Malagoli M, Dall’Acqua S. Foliar Application of Silicon in Vitis vinifera: Targeted Metabolomics Analysis as a Tool to Investigate the Chemical Variations in Berries of Four Grapevine Cultivars. Plants. 2022; 11(21):2998. https://doi.org/10.3390/plants11212998
Chicago/Turabian StyleSut, Stefania, Mario Malagoli, and Stefano Dall’Acqua. 2022. "Foliar Application of Silicon in Vitis vinifera: Targeted Metabolomics Analysis as a Tool to Investigate the Chemical Variations in Berries of Four Grapevine Cultivars" Plants 11, no. 21: 2998. https://doi.org/10.3390/plants11212998
APA StyleSut, S., Malagoli, M., & Dall’Acqua, S. (2022). Foliar Application of Silicon in Vitis vinifera: Targeted Metabolomics Analysis as a Tool to Investigate the Chemical Variations in Berries of Four Grapevine Cultivars. Plants, 11(21), 2998. https://doi.org/10.3390/plants11212998








