Identification of Renoprotective Phytosterols from Mulberry (Morus alba) Fruit against Cisplatin-Induced Cytotoxicity in LLC-PK1 Kidney Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
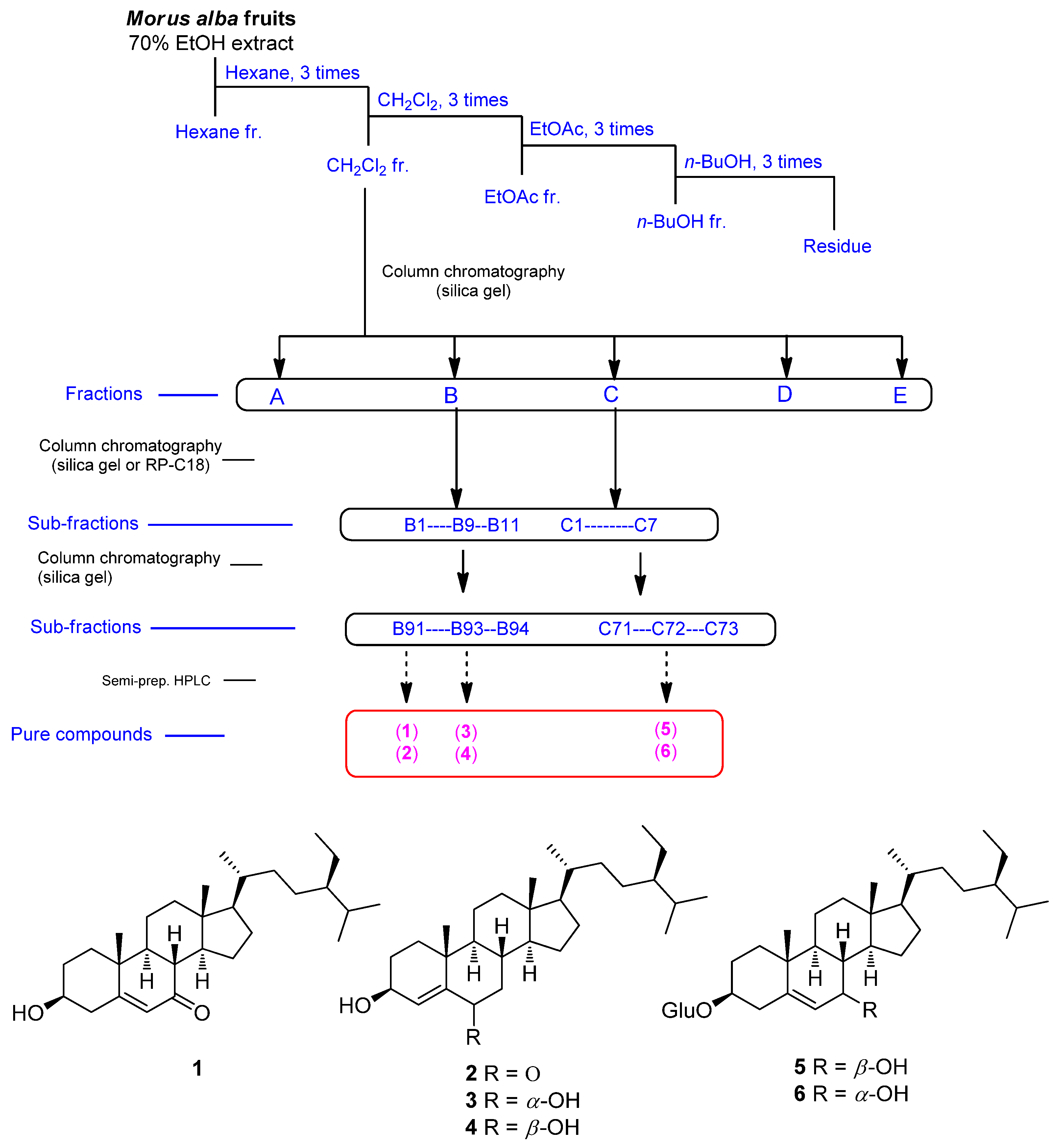
2.2. Plant Material, Extraction, and Isolation
2.3. Cell Culture and Cell Viability Assay
2.4. Image-Based Cytometric Assay
2.5. Western Blotting Analysis
2.6. Statistical Analysis
3. Results
3.1. Isolation and Identification of Compounds
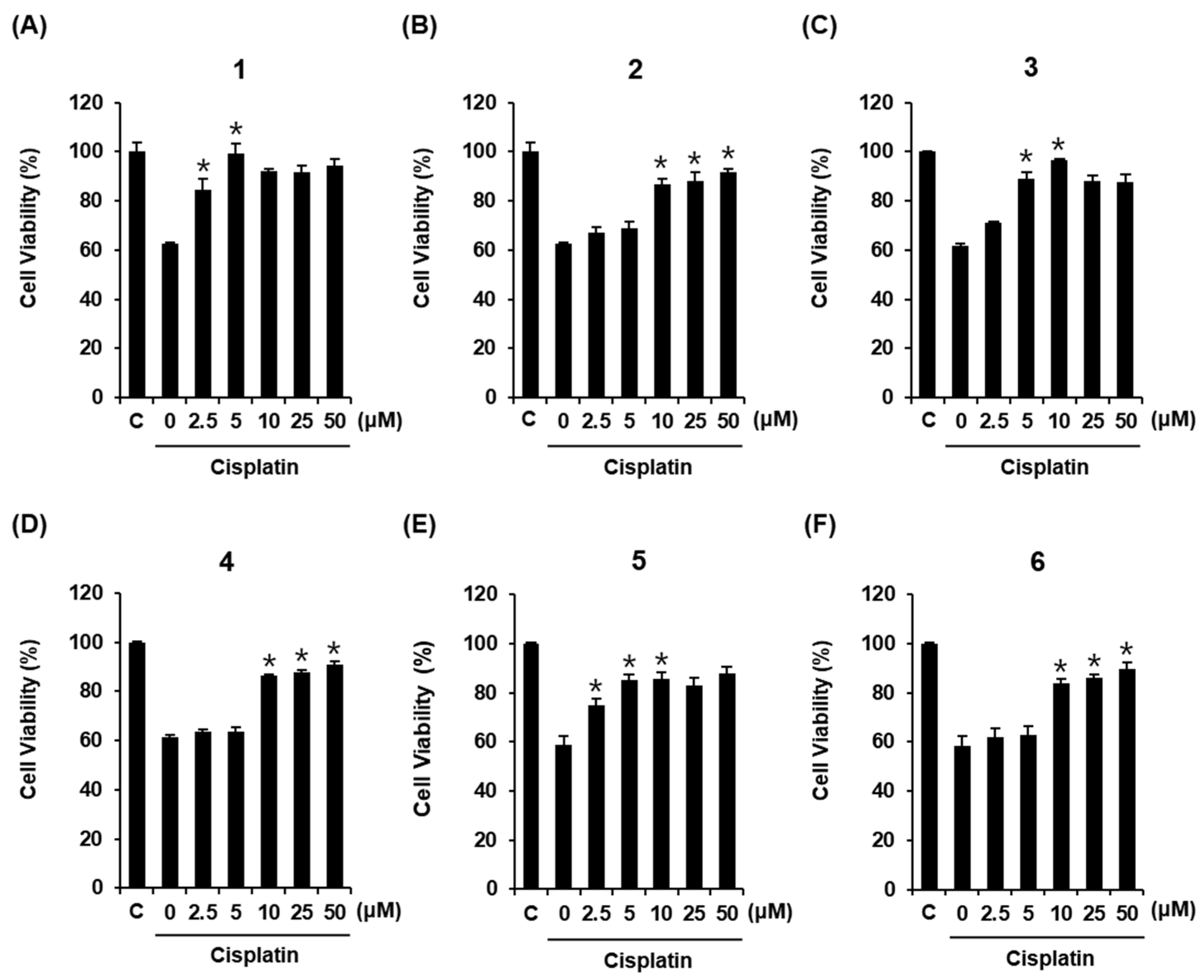
3.2. Compounds Isolated from M. alba Fruit Inhibit Cisplatin-Induced Death of LLC-PK1 Cells
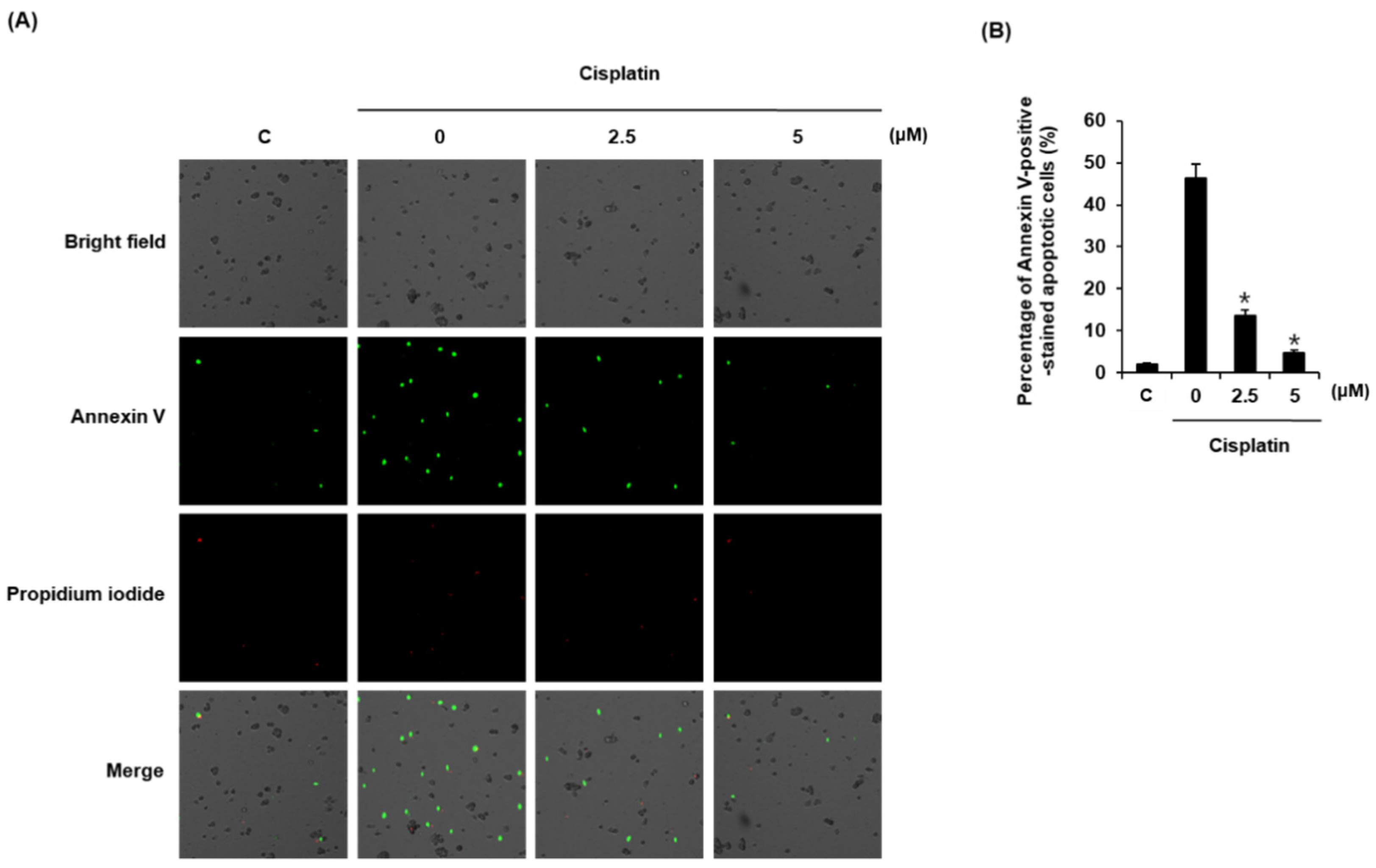
3.3. Compound 1 Inhibits Cisplatin-Induced Apoptosis in LLC-PK1 Cells
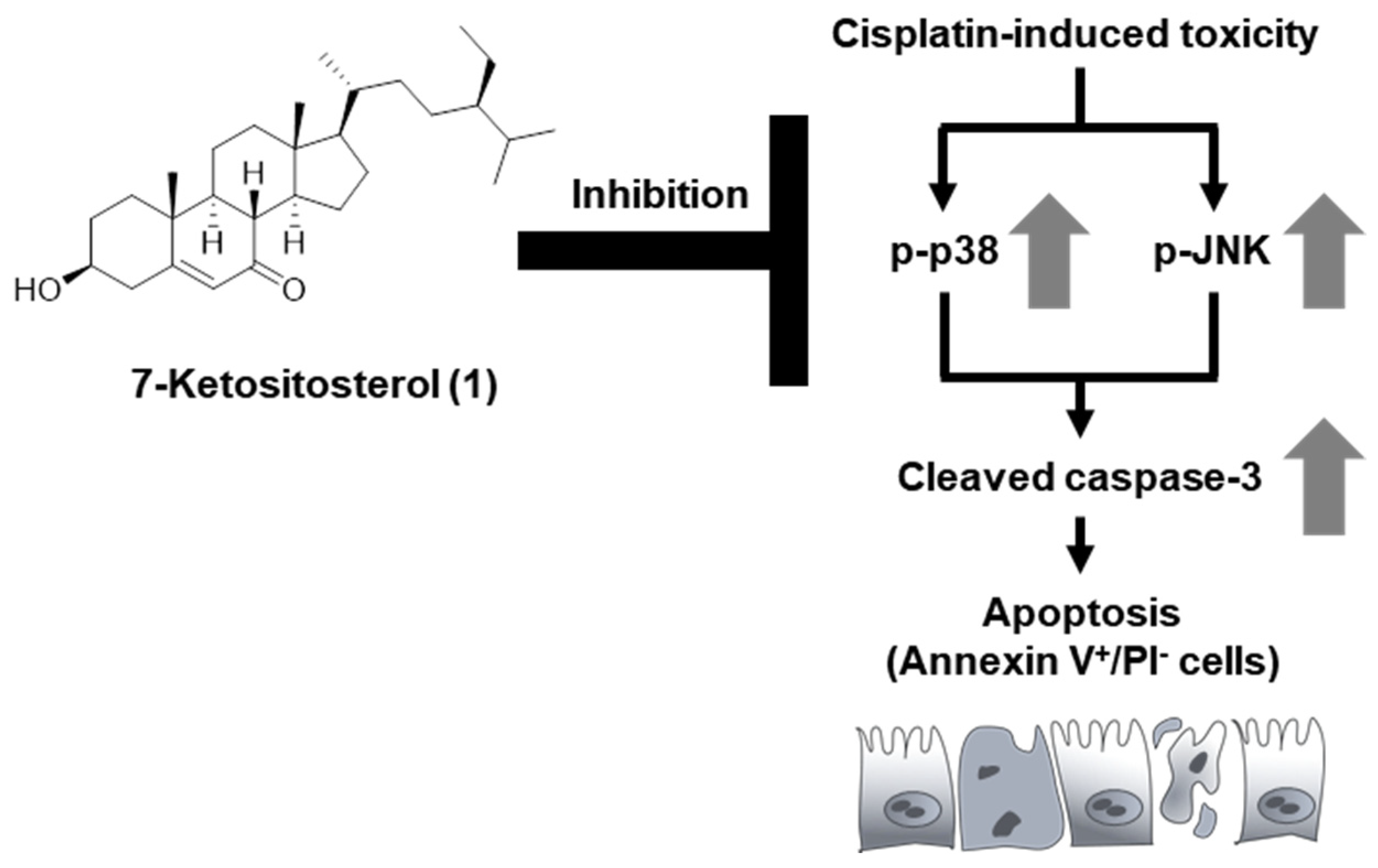
3.4. Compound 1 Inhibits Expression Levels of p38, JNK, and Cleaved Caspase-3 in Cisplatin-Treated LLC-PK1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astolfi, L.; Ghiselli, S.; Guaran, V.; Chicca, M.; Simoni, E.; Olivetto, E.; Lelli, G.; Martini, A. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: A retrospective evaluation. Oncol. Rep. 2013, 29, 1285–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volarevic, V.; Djokovic, B.; Jankovic, M.G.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N. Molecular mechanisms of cisplatin-induced nephrotoxicity: A balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019, 26, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. Oncologist 2017, 22, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridzuan, N.R.; Rashid, N.A.; Othman, F.; Budin, S.B.; Hussan, F.; Teoh, S.L. Protective role of natural products in cisplatin-induced nephrotoxicity. Mini Rev. Med. Chem. 2019, 19, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, M.E.; Quiroga, A.G.; Castro, J.; Ortiz, A.; Aller, P.; Mata, F. Inhibition of p38-MAPK potentiates cisplatin-induced apoptosis via GSH depletion and increases intracellular drug accumulation in growth-arrested kidney tubular epithelial cells. Toxicol. Sci. 2009, 111, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapiro, J.M.; Monks, T.J.; Lau, S.S. All-trans-retinoic acid-mediated cytoprotection in LLC-PK1 renal epithelial cells is coupled to p-ERK activation in a ROS-independent manner. Am. J. Physiol.-Ren. Physiol. 2017, 313, F1200–F1208. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of mulberry fruit (Morus alba L.) consumption on health outcomes: A mini-review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-S.; Jin, H.-G.; Lee, H.; Lee, D.-S.; Woo, E.-R. Phytochemical Constituents of the Root Bark from Morus alba and Their Il-6 Inhibitory Activity. Nat. Prod. Sci. 2019, 25, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Khalid, N.; Fawad, S.A.; Ahmed, I. Antimicrobial activity, phytochemical profile and trace minerals of black mulberry (Morus nigra L.) fresh juice. Pak. J. Bot. 2011, 43, 91–96. [Google Scholar]
- Kim, H.; Choi, P.; Kim, T.; Kim, Y.; Song, B.G.; Park, Y.-T.; Choi, S.-J.; Yoon, C.H.; Lim, W.-C.; Ko, H. Ginsenosides Rk1 and Rg5 inhibit transforming growth factor-β1-induced epithelial-mesenchymal transition and suppress migration, invasion, anoikis resistance, and development of stem-like features in lung cancer. J. Ginseng Res. 2021, 45, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Da Villa, G.; Ianiro, G.; Mangiola, F.; Del Toma, E.; Vitale, A.; Gasbarrini, A.; Gasbarrini, G. White mulberry supplementation as adjuvant treatment of obesity. J. Biol. Regul. Homeost. Agents 2014, 28, 141–145. [Google Scholar] [PubMed]
- Mahboubi, M. Morus alba (mulberry), a natural potent compound in management of obesity. Pharmacol. Res. 2019, 146, 104341. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.Y.; Kim, S.B.; Lee, M.K.; Park, H.; Kim, S.Y. Improved chemotherapeutic activity by Morus alba fruits through immune response of toll-like receptor 4. Int. J. Mol. Sci. 2015, 16, 24139–24158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butkhup, L.; Samappito, W.; Samappito, S. Phenolic composition and antioxidant activity of white mulberry (Morus alba L.) fruits. Int. J. Food Sci. 2013, 48, 934–940. [Google Scholar] [CrossRef]
- Gungor, N.; Sengul, M. Antioxidant activity, total phenolic content and selected physicochemical properties of white mulberry (Morus alba L.) fruits. Int. J. Food Prop. 2008, 11, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Mohamad Razali, U.H.; Saikim, F.H.; Mahyudin, A.; Mohd Noor, N.Q.I. Morus alba L. Plant: Bioactive Compounds and Potential as a Functional Food Ingredient. Foods 2021, 10, 689. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, K.H.; Jeon, M.S.; Lee, Y.H.; Ko, Y.J.; Pang, C.; Kim, B.; Chung, K.H.; Kim, K.H. Hepatoprotective potency of chrysophanol 8-O-glucoside from Rheum palmatum L. against hepatic fibrosis via regulation of the STAT3 signaling pathway. Int. J. Mol. Sci. 2020, 21, 9044. [Google Scholar] [CrossRef]
- Yu, J.S.; Sahar, N.E.; Bi, Y.R.; Jung, K.; Pang, C.; Huh, J.Y.; Kim, K.H. The effects of triterpenoid saponins from the seeds of Momordica cochinchinensis on adipocyte differentiation and mature adipocyte inflammation. Plants 2020, 9, 984. [Google Scholar] [CrossRef]
- Baek, S.C.; Lee, B.S.; Yi, S.A.; Lee, J.; Kim, K.H. Carthamusuchuric acid, an enolic glucoside of phenylpyruvic acid from the florets of Carthamus tinctorius and anti-adipogenic phenolic compounds. Tetrahedron Lett. 2020, 61, 152237. [Google Scholar] [CrossRef]
- Yu, J.S.; Park, M.; Pang, C.; Rashan, L.; Jung, W.H.; Kim, K.H. Antifungal phenols from Woodfordia uniflora collected in Oman. J. Nat. Prod. 2020, 83, 2261–2268. [Google Scholar] [CrossRef]
- Lee, D.; Lee, D.S.; Jung, K.; Hwang, G.S.; Lee, H.L.; Yamabe, N.; Lee, H.J.; Eom, D.W.; Kim, K.H.; Kang, K.S. Protective effect of ginsenoside Rb1 against tacrolimus-induced nephrotoxicity in renal proximal tubular LLC-PK1 cells. J. Ginseng Res. 2018, 42, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Lee, D.; Lee, H.J.; Noh, H.J.; Jung, K.; Kang, K.S.; Kim, K.H. Renoprotective chemical constituents from an edible mushroom, Pleurotus cornucopiae in cisplatin-induced nephrotoxicity. Bioorg. Chem. 2017, 71, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.R.; Lee, D.; Eom, H.J.; Lee, S.R.; Lee, K.R.; Kang, K.S.; Kim, K.H. Identification and mechanism of action of renoprotective constituents from peat moss Sphagnum palustre in cisplatin-induced nephrotoxicity. J. Funct. Foods 2016, 20, 358–368. [Google Scholar] [CrossRef]
- Lee, D.; Yu, J.S.; Lee, S.R.; Hwang, G.S.; Kang, K.S.; Park, J.G.; Kim, H.Y.; Kim, K.H.; Yamabe, N. Beneficial effects of bioactive compounds in mulberry fruits against anticancer drug-induced nephrotoxicity. Int. J. Mol. Sci. 2018, 19, 1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Julien-David, D.; Miesch, M.; Geoffroy, P.; Raul, F.; Roussi, S.; Aoude-Werner, D.; Marchioni, E. Identification and quantitative analysis of β-sitosterol oxides in vegetable oils by capillary gas chromatography-mass spectrometry. Steroids 2005, 70, 896–906. [Google Scholar] [CrossRef]
- Rodriguez, J.; Nunez, L.; Peixinho, S.; Jimenez, C. Isolation and synthesis of the first natural 6-hydroximino-4-en-3-one- steroids from the sponges Cinachyrella spp. Tetrahedron Lett. 1997, 38, 1833–1836. [Google Scholar] [CrossRef]
- Zhao, C.C.; Shao, J.H.; Li, X.; Xu, J.; Zhang, P. Antimicrobial constituents from fruits of Ailanthus altissima SWINGLE. Arch. Pharm. Res. 2005, 28, 1147–1151. [Google Scholar] [CrossRef]
- Kimura, Y.; Akihisa, T.; Yasukawa, K.; Takido, M.; Tamura, Y. Structures of five hydroxylated sterols from the seeds of Trichosanthes kirilowii Maxim. Chem. Pharm. Bull. 1995, 43, 1813–1817. [Google Scholar] [CrossRef] [Green Version]
- Chaurasia, N.; Wichtl, M. Sterols and steryl glycosides from Urtica dioica. J. Nat. Prod. 1987, 50, 881–885. [Google Scholar] [CrossRef]
- Kim, S.Y.; Moon, A. Drug-induced nephrotoxicity and its biomarkers. Biomol. Ther. 2012, 20, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; You, J.; Wang, K.; Li, Y.; Zhang, Y.; Wei, H.; Liang, X.; Liu, Y. N-acetylcysteine attenuates cisplatin-induced acute kidney injury by inhibiting the C5a receptor. Biomed. Res. Int. 2019, 2019, 4805853. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Feinfeld, D.A. N-acetylcysteine as salvage therapy in cisplatin nephrotoxicity. Ren. Fail. 2002, 24, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.-Y.; Lou, D.-Y.; Zhou, L.-Q.; Wang, J.-C.; Yang, B.; He, Q.-J.; Wang, J.-J.; Weng, Q.-J. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021, 1–19. [Google Scholar] [CrossRef]
- Tsuruya, K.; Ninomiya, T.; Tokumoto, M.; Hirakawa, M.; Masutani, K.; Taniguchi, M.; Fukuda, K.; Kanai, H.; Kishihara, K.; Hirakata, H. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 2003, 63, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Choi, S.; Yamabe, N.; Kim, K.H.; Kang, K.S. Recent Findings on the Mechanism of Cisplatin-Induced Renal Cytotoxicity and Therapeutic Potential of Natural Compounds. Nat. Prod. Sci. 2020, 26, 28–49. [Google Scholar]
- Lee, D.; Kim, K.H.; Lee, W.Y.; Kim, C.-E.; Sung, S.H.; Kang, K.B.; Kang, K.S. Multiple targets of 3-dehydroxyceanothetric acid 2-methyl ester to protect against cisplatin-induced cytotoxicity in kidney epithelial LLC-PK1 cells. Molecules 2019, 24, 878. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lee, D.; Ryoo, R.; Kim, J.C.; Park, H.B.; Kang, K.S.; Kim, K.H. Calvatianone, a Sterol Possessing a 6/5/6/5-Fused Ring System with a Contracted Tetrahydrofuran B-Ring, from the Fruiting Bodies of Calvatia nipponica. J. Nat. Prod. 2020, 83, 2737–2742. [Google Scholar] [CrossRef]
- Lee, S.R.; Kang, H.S.; Yoo, M.J.; Yi, S.A.; Beemelmanns, C.; Lee, J.C.; Kim, K.H. Anti-adipogenic Pregnane Steroid from a Hydractinia-associated Fungus, Cladosporium sphaerospermum SW67. Nat. Prod. Sci. 2020, 26, 230–235. [Google Scholar]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.H.; Kim, S.H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a hallucinogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharm. Res. 2020, 43, 214–223. [Google Scholar] [CrossRef]
- Jo, M.S.; Lee, S.; Yu, J.S.; Baek, S.C.; Cho, Y.-C.; Kim, K.H. Megastigmane Derivatives from the Cladodes of Opuntia humifusa and Their Nitric Oxide Inhibitory Activities in Macrophages. J. Nat. Prod. 2020, 83, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Li, C.; Kwon, M.; Oh, T.; Lee, T.H.; Kim, D.H.; Ahn, J.S.; Ko, S.K.; Kim, C.S.; Cao, S. Herqueilenone a, a unique rearranged benzoquinone-chromanone from the hawaiian volcanic soil-associated fungal strain Penicillium herquei FT729. Bioorg. Chem. 2020, 105, 104397. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Kim, J.; Kim, H.; Jang, W.; Kim, K.H. Mushrooms: An Important Source of Natural Bioactive Compounds. Nat. Prod. Sci. 2020, 26, 118–131. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Lee, S.R.; Park, B.J.; Song, J.H.; Kim, J.K.; Ko, Y.; Kang, K.S.; Kim, K.H. Identification of Renoprotective Phytosterols from Mulberry (Morus alba) Fruit against Cisplatin-Induced Cytotoxicity in LLC-PK1 Kidney Cells. Plants 2021, 10, 2481. https://doi.org/10.3390/plants10112481
Lee D, Lee SR, Park BJ, Song JH, Kim JK, Ko Y, Kang KS, Kim KH. Identification of Renoprotective Phytosterols from Mulberry (Morus alba) Fruit against Cisplatin-Induced Cytotoxicity in LLC-PK1 Kidney Cells. Plants. 2021; 10(11):2481. https://doi.org/10.3390/plants10112481
Chicago/Turabian StyleLee, Dahae, Seoung Rak Lee, Bang Ju Park, Ji Hoon Song, Jung Kyu Kim, Yuri Ko, Ki Sung Kang, and Ki Hyun Kim. 2021. "Identification of Renoprotective Phytosterols from Mulberry (Morus alba) Fruit against Cisplatin-Induced Cytotoxicity in LLC-PK1 Kidney Cells" Plants 10, no. 11: 2481. https://doi.org/10.3390/plants10112481
APA StyleLee, D., Lee, S. R., Park, B. J., Song, J. H., Kim, J. K., Ko, Y., Kang, K. S., & Kim, K. H. (2021). Identification of Renoprotective Phytosterols from Mulberry (Morus alba) Fruit against Cisplatin-Induced Cytotoxicity in LLC-PK1 Kidney Cells. Plants, 10(11), 2481. https://doi.org/10.3390/plants10112481









