Abstract
The green seaweed Ulva spp. constitute major primary producers in marine coastal ecosystems. Some Ulva populations have declined in response to ocean warming, whereas others cause massive blooms as a floating form of large thalli mostly composed of uniform somatic cells even under high temperature conditions—a phenomenon called “green tide”. Such differences in population responses can be attributed to the fate of cells between alternative courses, somatic cell division (vegetative growth), and sporic cell division (spore production). In the present review, I attempt to link natural population dynamics to the findings of physiological in vitro research. Consequently, it is elucidated that the inhibition of biomass allocation to sporulation is an important key property for Ulva to cause a huge green tide.
Keywords:
biomass allocation; green tide; sporulation; Ulva ohnoi; Ulva prolifera; vegetative growth 1. Introduction
Ulva (Ulvophyceae, Chlorophyta) or sea lettuce is the most abundant green seaweed and is ubiquitous in tropical and temperate coastal ecosystems around the world. The genus Ulva currently includes at least 85 taxonomically accepted species [1]. The thallus body is uniformly sheet-like, being two cells thick or tubular with a single cell layer, except for a very small holdfast part (Figure 1A,B). The generation time of Ulva is short, and its spores can develop into a thallus having the potential ability to produce spores again in 2–3 weeks. Spore formation occurs in the somatic cells which directly transform into sporangia (Figure 1C,D). The sporulation first occurs at the tip of the algal body and then sequentially occurs toward the bottom, several tens of spores per sporangia are released, and the empty sporangium are spontaneously detached from the thallus.
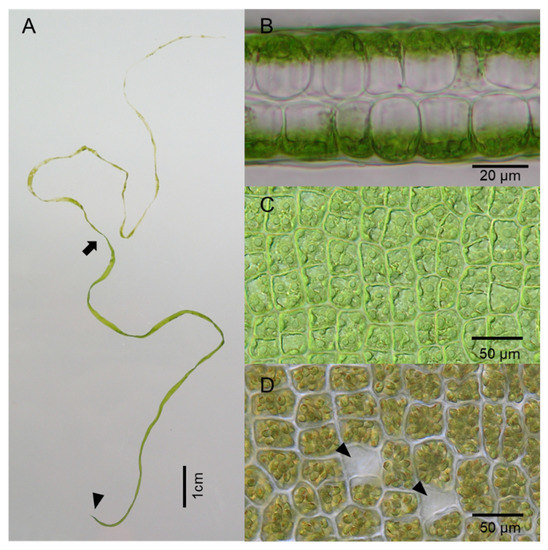
Figure 1.
A living Ulva specimen (U. aragoënsis). (A) The developed thallus with sporulation in the upper part. Above the part indicated by the arrow, all the cells formed spores and some of them released spores. Arrowhead indicates a small holdfast; (B) Cross-section of the middle part of the thallus having a two-cell layered structure; (C) Surface view of somatic or blade cells in the vegetative state in the middle part of the thallus; (D) Surface view of the cells forming spores. Arrowheads indicate empty cells after spores were released.
A few Ulva species cause massive kilometer-scale blooms termed as green tides, and these have been recorded mainly around the industrialized coastlines of Europe, North America, and east Asia [2]. The world’s most extensive Ulva green tides have repeatedly occurred in the Yellow Sea since 2007 [3] or 2008 [4]. The causative green tide species can substantially increase their biomass in a free-floating state by increasing the size of the thalli and their fragments. Smetacek and Zingone [5] pointed out that this is crucial because it is the unattached forms that, by invading new space, are able to increase their nutrient supply, free themselves from competition for limited hard substrates, and avoid their many benthic grazers. As a result, the unattached forms can build up a large biomass, forming massive green tides. In general, most Ulva spp. grow when their motile spores settle on the substrate. The attached populations in temperate regions are regularly present in early spring, increase to a maximum in late spring, and rapidly decrease through summer, showing a unimodal pattern of biomass fluctuation (Figure 2A) [6,7,8,9]. The summer decline of the attached populations occurs markedly at 20–25 °C. However, green tide species peak in biomass in the summer [10] and occasionally continue to grow [11]. Such a temporal difference of thallus growth pattern between attached populations and floating green tide populations has not received much attention in the literature. I consider that the species-specific differences of ecophysiological characteristics are a crucial key in determining if an algal species causes an excessive bloom or not.
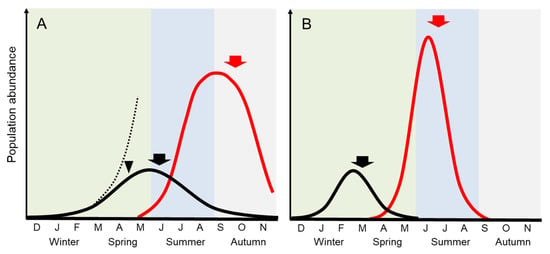
Figure 2.
Comparison of seasonal abundance change between the attached populations and the green tides of Ulva in the temperate region. Arrowhead indicates the period when biomass increase is suppressed mainly by light limitation. Arrows show the period when allocation of biomass to sporulation begins to be greater than vegetative growth. (A) The attached U. australis modified from [9,12] (black line). Dotted line is a growth curve predicted in case of no light limitation. Ulva ohnoi green tide modified from [11] (red line); (B) Attached U. prolifera in brackish water modified from [13,14] (black line). The U. prolifera green tide in the Yellow Sea modified from [4,15] (red line).
In various previous publications, it has been explained that Ulva green tides are a symptom resulting from eutrophication [16]. Indeed, the supply of dissolved inorganic nitrogen (N) and phosphorus (P) is required to sustain Ulva thallus growth. However, as an example of green tides in Tokyo Bay, the scale of Ulva blooms has expanded despite a significant decrease in N and P concentrations year by year [17]. In this case, a subtropical species, U. ohnoi, unintentionally introduced and excessively grew as unattached form without the summer decline. In the Yellow Sea, free-floating Ulva populations during the early stage of green tides in spring include four or more Ulva species, but only one species, U. prolifera, continuously expand, resulting in monospecific spectacular blooms in summer [18,19]. All the other species do not seem to be able to continue vegetative growth while enduring the high temperatures from spring to summer, even though they sympatrically experience the same eutrophic conditions in the Yellow Sea. These examples of U. ohnoi and U. prolifera indicate that when specific species, having a continuous growth ability in high temperatures, encounter the minimum nutrient conditions for sustaining its vegetative growth, a huge bloom can occur.
In this review, I compare the Ulva biomass fluctuation patterns between attached populations and green tide populations and explain which ecophysiological properties of a species or strain is essential for causing green tides. In addition, I will focus on the mechanism of switching between the vegetative growth and sporulation of Ulva cells. By relating field observations and laboratory experiments, I attempt to more comprehensively examine the relationships between the cells, individuals, and populations underlying the mechanism of the development of green tides.
2. Attached Population Dynamics
2.1. Population Fluctuation Follows Individual Size Fluctuation
In the temperate coastal zone, the biomass of attached Ulva populations fluctuates seasonally according to the periodic fluctuation of the water temperature. Many temperate species such as U. rigida, U. lactuca, and U. australis (syn. U. pertusa) show a regular fluctuation pattern in which the attached biomass increases from winter to spring and declines during high temperatures from summer to autumn, as described in Figure 2A [6,7,8,9,12,20,21]. The biomass peaks shift to later in the year in cold regions at high latitudes [22]. This review will progress the story about the temperate populations. There has been detailed demographic research study on the attached population of U. australis over a period of three consecutive years [9]. It demonstrated that the seasonal fluctuations of the Ulva population synchronize with those of the thallus size rather than with changes in the density of thallus individuals. This indicates that biomass fluctuations of Ulva population are attributed to that of well-developed thallus individuals.
2.2. Increase Phase
Ulva with a simple multicellular body perform ‘diffuse growth’ in which cell divisions can occur more or less throughout the tissues of the organism [23]. The somatic cells divide synchronously in standardized conditions once a day [24]. Therefore, the Ulva thalli are capable of exponential growth, displaying extremely high growth rates. In fact, a daily rate of over fourfold in U. meridionalis in the culture experiment has been reported as the highest growth rate ever reported for multicellular autotrophic plants. In the same paper, a strain isolated from the attached U. prolifera population was also revealed to display two-fold growth rate per day [25]. The high exponential growth of Ulva spp. generally occurs in high temperatures of 20–30 °C in suitable light and nutrient conditions, as described below in Section 3. If such high growth continues in the sea from spring to summer with the optimum high temperatures, a bloom would occur explosively. However, in the attached population, the rapid biomass increase is suppressed mainly by light limitation caused by self-shading as density increases (Figure 2A). Although light limitation is an inevitable suppression factor, the population is also negatively affected by some irregular factors of low salinities by precipitation and herbivory by benthic organisms such as snails and sea hares. Their inhibitory effects occur because the population is attached to the substrate.
2.3. Decline Phase
As the water temperature rises over 20 °C, Ulva thalli are highly promoted to produce and release spores. The allocation to sporulation in thalli of the attached Ulva populations has been observed to be significantly larger during warmer months [12,20]. Niesenbaum [20] described “the seasonality of reproduction, and changes in the abundance of total biomass and reproductive biomass during the reproductive season could have been a function of temperature. The sharp decline of total biomass in early August, and its low rate of recovery through August and September were probably due to temperature effects on growth and reproduction”. Furthermore as “When temperature reached seasonal highs, allocation of biomass to the formation and release of swarmers (spores) was greatest, while the rate of vegetative replacement diminished as temperatures first became suboptimal and then inhibitory for growth. This could explain the increases in percent reproductive tissue during August and September”. These findings are essential for understanding the Ulva biomass fluctuation. However, so far the allocation of biomass to sporulation in the decline phase of Ulva populations has been almost overlooked. Practically, only the intrinsic traits involved in the increase phase, such as high growth rates or multiple reproduction modes, have been highlighted [3,26]. Recently, a few works examined the decline phase of the U. prolifera green tide in the Yellow Sea [10]. However, little coverage has been given to the allocation to sporulation.
2.4. Disappearance Phase
After the decline phase, Ulva thalli almost disappear in the autumn. In this disappearance phase, although evidence has not been provided from field research yet, small individual thalli of less than a few centimeters in size could release spores and disappear, whereby their settled spores grow up fast to small thalli and release spores again in the early developmental stage. This fast generation alternation may occur until the water temperature drops below 20 °C in temperate species. These predictions are derived from culture work in the laboratory as described next.
3. Individual Size Determined by Vegetative Growth and Sporulation Decay
Culture experiments using temperature-controlled incubators have confirmed that higher temperatures promote sporulation decay [27]. An asexual variant of U. prolifera originally isolated from the attached population in brackish water and its clonal offspring thalli were tested (Figure 3). According to this study, their growth rates increase as the temperature rises to 25 °C. However, sporulation at the apical part of the thallus occurs earlier as the temperature rises, and the amount of sporulation decay increases. At 30 °C after sporulation first occurs, the thalli repeatedly produce spores and as a whole continue to decrease, resulting in the disappearance in one and half months of culture. At 20 °C and 25 °C, the vegetative growth increment and the amount of decay due to sporulation are balanced, and the total length of the thalli cannot extend from about 10 cm. At 15 °C, because the vegetative growth exceeds the small amount of sporulation, the thalli continue to grow larger. At the low temperature of 10 °C, sporulation does not occur, and the thalli continue to grow slowly. From these findings, individual thallus mass (M) can be expressed by the two factors of vegetative growth (G) and the amount allocated to sporulation (S) as M = G − S. As the U. prolifera strain has the intrinsic trait of S ≥ G at ≥20 °C after the first sporulation, M becomes constant or decreases over 20 °C.
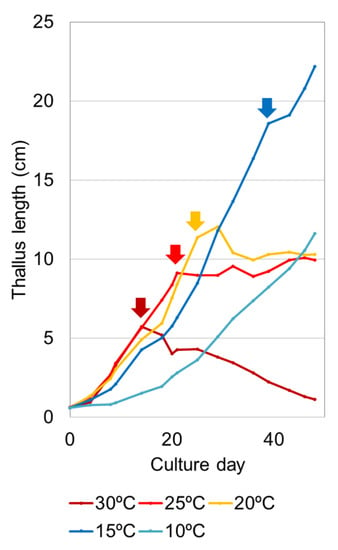
Figure 3.
Change of averaged thallus length of Ulva prolifera strain isolated from the attached population in the Yoshino River estuary, Japan, cultured at different temperatures. Each arrow indicates the day when sporulation first occurred. After that, sporulation occurred repeatedly. Only at 10 °C, no sporulation occurred. This figure was redrawn based on the data from [27].
4. Inhibition of Sporulation Leads to Green Tides
4.1. Ulva ohnoi Green Tide
Floating thalli which are free from the attached substrate can spread moderately and receive sufficient light. If the floating thalli acquires the property of inhibiting sporulation even at high temperatures, they would make a massive green tide. Here, the intrinsic trait to cause a large-scale green tide is expressed as S < G at ≥20 °C. Then, as S is nearly zero and G is usually the maximum growth rate that the Ulva species can attain, M increases exponentially. In addition to the high-temperature growth property, the structural feature of easily producing floating thalli and their fragments also promotes the magnification of the green tides. Ulva ohnoi is a typical species with these characteristics. When this species was reported as a new green tide-forming species, it was taxonomically described as having a large, thin, and fragile blade thallus easily torn into floating fragments in the diagnosis [28]. The field observation of the U. ohnoi green tide was first made in Tosa Bay, southwestern Japan, for two years [11]. It shows that the thallus fragments grew rapidly at over a five-fold growth rate per week as the summer water temperature rose to 28–30 °C, reaching an average length of about 50 cm, a maximum length of >1 m, and the largest biomass attained approximately 1 kg fresh mass m−2 in August. In the decline phase of the green tides, a small amount of sporulation occurred in June-August, but half and more parts of the well-developed blades frequently formed and released spores in October, resulting in the population decline (Figure 2A). The remarkable difference compared to the temperate attached population is that U. ohnoi continues to grow with no or little biomass allocation to sporulation during the period of the summer decline. The properties are summarized below.
- Sporulation which is generally promoted at high temperatures is suppressed.
- At high temperatures, relatively high growth ability is exhibited.
- Free-floating thallus fragments are easily formed.
Of these, 1 is particularly important for the occurrence of green tides. If the species does not produce spores and does not reduce its total mass, the green tide biomass gradually develops even if the growth is not so fast. A fragmentation culture method available for investigating the likelihood of sporulation in Ulva has been presented [29]. Applying the method, sporulation can be induced in 2 to 3 days on Ulva thallus tissue collected from the attached population [30]. By the same method, however, the thallus blades of U. ohnoi and the other Ulva spp. sampled from the massive green tides showed no or a very low frequency of induced sporulation or took a longer time to sporulation [31]. Before U. ohnoi was taxonomically differentiated as a new species, this species blooming in Ohmura Bay, southwestern Japan, had been identified as a sterile mutant of U. pertusa (now U. australis) [32] and it is still believed to be so [33]. Migita [32] showed that 1 cm2 thallus fragments of his U. ohnoi strain displayed the maximum growth rate of two-fold growth rate per 2 days in laboratory experiments at 20 °C, and then transplanted into an outdoor tank and grew up to larger than 1 m2 in 2 months without any sporulation, while all the fragments of more than 10 wild U. australis thalli formed spores in the same culture conditions, resulting in the disappearance of the thallus. These results indicate that bloom-forming species have a physiological property of being less prone to sporulate, or they do not sporulate.
Ulva ohnoi distribute mainly in the subtropical region and are adapted to high temperatures. Therefore, it has spread to the temperate area and outbreaks in the summer. The spread of U. ohnoi has been increasingly reported from various regions [34,35,36]. Due to global warming, U. ohnoi may spread further into higher latitudes and cause green tides. However, in Tosa Bay, where the U. ohnoi green tide was first reported, this species has recently decreased sharply and instead, U. reticulata, which has a distribution centered in more tropical waters, has begun to increase [37]. This example suggests that each Ulva species has a temperature range that balances the vegetative growth and sporulation, and that individual thallus growth, or population growth, may not be possible if the temperature limit is exceeded even by a few degrees.
4.2. Ulva prolifera Green Tide
The U. prolifera green tides in the Yellow Sea regularly reach their biomass peak during June and July in summer (Figure 2B) [4,15]. The earliest free-floating Ulva patches are found in the coastal areas of the southern Yellow Sea from mid-April to early May [3,18]. These patches originally appear off the nearby rafts for purple laver (Neopyropia yezoensis) aquaculture, for which the coverage area is approximately 4.1 × 104 ha [3]. Annually, approximately 6500 t of the Ulva mass has been estimated to be released as macroalgal waste from mid-April to late-May after cleaning the Neopyropia aquaculture facilities [38]. In this early stage, the patches include multiple Ulva spp. such as U. linza, U. compressa, and U. aragoënsis (=U. flexuosa in [18,19]). However, the free-floating Ulva complexes move northward, associated with the seasonal monsoons and ocean currents, rapidly develop into long large bands ranging from hundreds of meters to tens of kilometers in the open sea area in late May [3], and then massive green tides are dominated by a single species, U. prolifera [18,19,39]. Only this species explosively grows, while the other species disappear over 20 °C in early summer. This suggests that the bloom-forming U. prolifera can continuously grow inhibiting allocation to sporulation in high temperatures, thereby differing from the other Ulva spp. in this region. Supporting this finding, the culture work showed that a few centimeters of the bloom-forming U. prolifera fragment can grow to more than 50 cm in length without sporulation at 20 °C (cf. Figure 12 in [40]). This growth characteristic is obviously different from that of the U. prolifera strain from the attached population, which cannot grow over 10 cm at ≥20 °C (Figure 3). However, the bloom-forming U. prolifera seems to allocate its biomass to sporulation around 25 °C, because it was observed that the green tide population began to decline at 25 °C from July to August [10].
Different from the bloom-forming type, the common attached type of U. prolifera form abundant populations on the substrate in brackish waters such as river estuaries [41,42]. Seasonal fluctuations of the largest attached U. prolifera population in Japan have been described in detail (Figure 2B) [13,14]. The biomass and thallus length of the attached population regularly reach their maximum from January to March and then disappear by July. Although natural U. prolifera mats develop in the Chinese coast located in the south of the Yellow Sea and are harvested as edible biomass, the peak harvest is also from January to March [43], which is consistent with the fluctuation pattern of the populations in Japan. Although the attached U. prolifera has been used as an expensive macroalgal ingredient for Japanese dishes for a long time, its harvest has become a ‘winter’ tradition [13]. However, in line with recent ocean warming, the annual yield is declining [44]. It can be explained that the biomass decrease of the attached population is due to the shortening of the period when the water temperature falls below 20 °C. The most significant difference of the ecophysiological property between the bloom-forming type and the attached type in U. prolifera is whether they can vegetatively grow inhibiting allocation to sporulation around 20 °C or not. The seasonal difference is described in Figure 4.
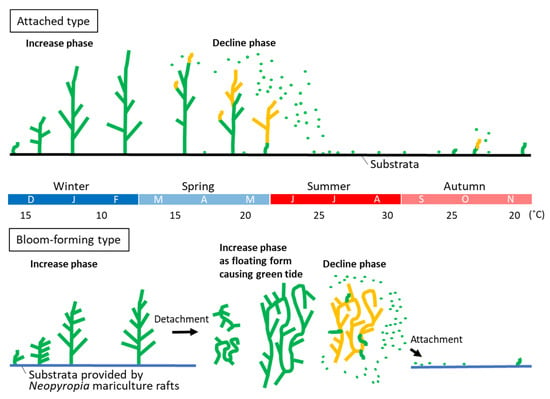
Figure 4.
Comparison of seasonal change of thallus state between the attached type, Ulva prolifera subsp. prolifera, and the bloom-forming type, U. prolifera subsp. qingdaoensis. Green and orange lines of thallus image show vegetative state and sporulating state, respectively. Green dots indicate microscopic propagules or spores released from the sporulated thalli. The two types have significantly different timings of the decline phase.
Although the issue of the massive green tide in China received a great deal of attention in 2008 [45], immediately after that in some research papers the bloom-forming type and the attached type were not distinguished, and both were confused because they formed a monophyletic clade together with the most closely related species, U. linza, by molecular analysis using the nuclear-encoded rDNA internal transcribed spacer (ITS) region, which is commonly used for Ulva species identification. However, studies of culture, hybridization, and phylogenetic analysis using a higher resolution DNA marker (5S rDNA spacer region) revealed that the bloom-forming type can cross with the attached type without any reproductive boundary, which was confirmed to be conspecific, but there are several genetic and ecophysiological differentiations (Table 1). Consistently, the other genetic analyses using inter-simple sequence repeat markers and a sequence-characterized amplified region marker indicated that the bloom-forming type is a unique ecotype of U. prolifera, genetically distinct from the attached types along the Chinese coast [46].

Table 1.
Comparison between the bloom-forming type and the attached type in Ulva prolifera.
From the phylogenetic analyses of U. linza and U. prolifera using the 5S sequence, it has been suggested that U. prolifera had adapted to brackish water and recently evolutionarily separated from marine U. linza [42]. Furthermore, crossing tests suggested that the bloom-forming type of U. prolifera completed the speciation from U. linza via intermediate brackish U. prolifera (=the attached type) because there is still a partial compatibility between the common brackish U. prolifera and U. linza, but a complete reproductive barrier exists between the bloom-forming U. prolifera and U. linza [48]. The brackish U. prolifera contains many regional populations that have genetically differentiated [42]. One of them may have acquired the ability to suppress sporulation and continue vegetative growth even at 20 °C or higher, resulting in creating the U. prolifera subsp. qingdaoensis that cause green tides. Interestingly, the U. prolifera subsp. qingdaoensis is characterized by a densely branching morphology (Table 1). This feature may facilitate the production and dispersal of large numbers of floating thallus fragments.
In contrast to U. ohnoi, widely reported from various regions, the occurrence of U. prolifera green tides has been limited to the Yellow Sea only. The special U. prolifera population unique to this region is fostered in the vast Neopyropia farm as its nursery bed, and is supplied annually as a large amount of floating mass [3,38]. The world’s largest green tide appears to be caused by such a very special production cycle supported by the aquaculture activities.
5. Mechanism of Sporulation
The multicellular body of Ulva is composed of mostly uniform blade cells (or somatic cells) except for a small number of rhizoid cells forming a small holdfast (Figure 1). Therefore, the allocation of the individual thallus tissue to vegetative growth and sporulation is almost attributed to the blade cell fate between the alternative courses, somatic cell division, and sporic cell division. Nordby [29] hypothesized that the cell fate is controlled by changes in the concentration of sporulation inhibitors. This inhibitor hypothesis inferred that the double-layered structure of the Ulva thallus (Figure 1B) could be responsible for maintaining a sufficient concentration of the sporulation inhibitor during vegetative growth. Supporting that, Stratmann et al. [49] revealed that the Ulva thallus produces at least two kinds of the sporulation inhibitor, one of which is a glycoprotein ‘Sporulation inhibitor-1′ (SI-1), and the other is a nonprotein of very low molecular mass (SI-2). The SI-1 is present in the cell wall of Ulva cells and appears to be secreted extracellularly. The SI-2 is in the inner space between the two blade cell layers. The excretion of the SI-1 decreases with maturation of the thallus, whereas the overall concentration of SI-2 in the thallus stays constant throughout the life cycle. The SI-2 affects different Ulva species whereas the SI-1 is species-specific. Although such characteristics of the inhibitors have been shown, their molecular structures have not yet been identified.
The fragmentation culture method can synchronously induce sporulation in Ulva thallus tissue within 48 h, when fragmented single-layered thalli are transferred to fresh medium at a low density of fragments and cultured in optimal conditions [29]. It is explained by the inhibitor hypothesis that the inhibitors leak out from the circumference of fragmented thalli and the somatic cells that sense the decrease in the concentration of the inhibitors go to sporulation. As already mentioned, the bloom-forming Ulva spp. hardly, or do not, allocate the somatic cells to sporulation, even in high temperatures. Considering the inhibitor hypothesis, it is possibly thought that the bloom-forming species produce a large amount of the inhibitory substances or have an inhibitor-sensing system in which it is hardly relieved from the inhibition. The entire genome of Ulva has already been announced [50]. Therefore, if the inhibitors are structurally identified, it is expected that the elucidation of the switching mechanism between the somatic cell division and the sporic cell division will be achieved.
6. Conclusions and Perspective
By comparing attached populations and green tide populations, it became clear that the bloom-forming species continue to grow vegetatively with almost no spore formation even at high temperatures. However, few field surveys have been conducted on the process of the decline of green tide populations from the viewpoint of the allocation to sporulation, and future investigations are required.
Though the sporulation inhibitors were partially characterized in 1996, their molecular structures have not yet been determined. These substances are the key to determining the fate of somatic cell division or sporic cell division. It is highly possible that the causative species of the green tide have different reaction systems involving the sporulation inhibitors. Such research studies are more likely to detect minor differences when comparing taxa containing blooming strains and non-blooming strains within the same species. In that sense, U. prolifera would be excellent experimental material.
Ulva is a promising organism for carbon dioxide fixation and bioproduct production due to its high productivity [51,52]. Understanding the allocation system of vegetative growth and sporulation decay will enable greater control of biomass production and will contribute to the development of the bioeconomy.
Funding
This research was supported by the Kochi University research project of the Biomass Refinery of Marine Algae, the JST-OPERA Program (Grant Number JPMJOP1832), and the Wood and Cabinet Office grant in aid, the Advanced Next-Generation Greenhouse Horticulture by IoP (Internet of Plants), Japan.
Conflicts of Interest
The author declares no conflict of interest.
References
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication; National University of Ireland: Galway, Ireland; Available online: http://www.algaebase.org (accessed on 4 September 2021).
- Joniver, C.F.H.; Photiades, A.; Moore, P.J.; Winters, A.L.; Woolmer, A.; Adams, J.M.M. The global problem of nuisance macroalgal blooms and pathways to its use in the circular economy. Algal Res. 2021, 58, 102407. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Li, H.; Li, G.; Liu, J.; Jiao, F.; Zhang, J.; Huo, Y.; Shi, X.; Su, R. Ulva prolifera green-tide outbreaks and their environmental impact in the Yellow Sea, China. Nat. Sci. Rev. 2019, 6, 825–838. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Xing, Q.; Shang, S. Long-term trend of Ulva prolifera blooms in the western Yellow Sea. Harmful Algae 2016, 58, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Rivers, J.S.; Peckol, P. Summer decline of Ulva lactuca (Chlorophyta) in a eutrophic embayment: Interactive effects of temperature and nitrogen availability? J. Phycol. 1995, 31, 223–228. [Google Scholar] [CrossRef]
- Choi, T.S.; Kim, J.H.; Kim, K.Y. Seasonal changes in the abundance of Ulva mats on a rocky intertidal zone of the southern coast of Korea. Algae 2001, 16, 337–341. [Google Scholar]
- Kim, K.Y.; Choi, T.S.; Kim, J.H.; Han, T.; Shin, H.W.; Garbary, D.J. Physiological ecology and seasonality of Ulva pertusa on a temperate rocky shore. Phycologia 2004, 43, 483–492. [Google Scholar] [CrossRef]
- Hiraoka, M.; Yoshida, G. Temporal variation in isomorphic phase and sex ratios of a natural population of Ulva pertusa (Chlorophyta). J. Phycol. 2010, 46, 882–888. [Google Scholar] [CrossRef]
- Hao, Y.; Guan, C.; Zhao, X.; Qu, T.; Tang, X.; Wang, Y. Investigation of the decline of Ulva prolifera in the Subei Shoal and Qingdao based on physiological changes. J. Ocean. Limnol. 2021, 39, 918–927. [Google Scholar] [CrossRef]
- Ohno, M. Seasonal changes of the growth of green algae, Ulva sp. in Tosa Bay, southern Japan. Mar. Fouling 1988, 7, 13–17. [Google Scholar] [CrossRef]
- Uchimura, M.; Yoshida, G.; Hiraoka, M.; Komatsu, T.; Arai, S.; Terawaki, T. Ecological studies of green tide, Ulva spp. (Chlorophyta) in Hiroshima Bay, the Seto Inland Sea. Jpn. J. Phycol. 2004, 52, 17–22. [Google Scholar]
- Ohno, M.; Mizutani, S.; Taino, S.; Takahashi, I. Ecology of the edible green alga Enteromorpha prolifera in Shimanto River, southern Japan. Bull. Mar. Sci. Fish. Kochi Univ. 1999, 19, 27–35. [Google Scholar]
- Hiraoka, M.; Higa, M. Novel distribution pattern between coexisting sexual and obligate asexual variants of the true estuarine macroalga Ulva prolifera. Ecol. Evol. 2016, 6, 3658–3671. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zeng, K.; Hu, C.; He, M.X. On the remote estimation of Ulva prolifera areal coverage and biomass. Remote Sens. Environ. 2019, 223, 194–207. [Google Scholar] [CrossRef]
- Teichberg, M.; Fox, S.E.; Olsen, Y.S.; Valiela, I.; Martinetto, P.; Iribarne, O.; Muto, E.; Petti, M.A.V.; Corbisier, T.N.; Soto-Jiménez, M.; et al. Eutrophication and macroalgal blooms in temperate and tropical coastal waters: Nutrient enrichment experiments with Ulva spp. Glob. Chang. Biol. 2010, 16, 2624–2637. [Google Scholar] [CrossRef]
- Yabe, T.; Ishii, Y.; Amano, Y.; Koga, T.; Hayashi, S.; Nohara, S.; Tatsumoto, H. Green tide formed by free-floating Ulva spp. at Yatsu tidal flat, Japan. Limnology 2009, 10, 239–245. [Google Scholar] [CrossRef]
- Han, W.; Chen, L.P.; Zhang, J.H.; Tian, X.L.; Hua, L.; He, Q.; Huo, Y.Z.; Yu, K.F.; Shi, D.J.; Ma, J.H.; et al. Seasonal variation of dominant free-floating and attached Ulva species in Rudong coastal area, China. Harmful Algae 2013, 28, 46–54. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Yu, R.C.; Chen, Z.F.; Qiu, L.M.; Wang, Y.F.; Kong, F.Z.; Geng, H.X.; Zhao, Y.; Jiang, P.; Yan, T.; et al. Genetic evidence in tracking the origin of Ulva prolifera blooms in the Yellow Sea, China. Harmful Algae 2018, 78, 86–94. [Google Scholar] [CrossRef]
- Niesenbaum, R.A. The ecology of sporulation by the macroalga Ulva lactuca L. (Chlorophyceae). Aquat. Bot. 1988, 32, 155–166. [Google Scholar] [CrossRef]
- Fillit, M. Seasonal changes in the photosynthetic capacities and pigment content of Ulva rigida in a Mediterranean coastal lagoon. Bot. Mar. 1995, 38, 271–280. [Google Scholar] [CrossRef]
- Munda, I.M.; Markham, J.W. Seasonal variations of vegetation patterns and biomass constituents in the rocky eulittoral of Helgoland. Helgoländer Meeresunters 1982, 35, 131–151. [Google Scholar] [CrossRef][Green Version]
- Garbary, D.; Galway, M.E. Macroalgae as underexploited model systems for stem cell research. In Deferring Development; Bishop, C.D., Hall, B.K., Eds.; CRC Press: New York, NY, USA, 2020; pp. 87–105. [Google Scholar]
- Wichard, T.; Charrier, B.; Mineur, F.; Bothwell, J.H.; De Clerck, O.; Coates, J.C. The green seaweed Ulva: A model system to study morphogenesis. Front. Plant Sci. 2015, 6, 72. [Google Scholar] [CrossRef]
- Hiraoka, M.; Kinoshita, Y.; Higa, M.; Tsubaki, S.; Monotilla, A.P.; Onda, A.; Dan, A. Fourfold daily growth rate in multicellular marine alga Ulva meridionalis. Sci. Rep. 2020, 10, 12606. [Google Scholar] [CrossRef] [PubMed]
- Fort, A.; Mannion, C.; Fariñas-Franco, J.M.; Sulpice, R. Green tides select for fast expanding Ulva strains. Sci. Total Environ. 2020, 698, 134337. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, M.; Dan, A.; Hagihira, M.; Ohno, M. Growth and maturity of clonal thalli in Enteromorpha prolifera under different temperature conditions. Nippon Suisan Gakkaishi 1999, 65, 302–303. [Google Scholar] [CrossRef][Green Version]
- Hiraoka, M.; Shimada, S.; Uenosono, M.; Masuda, M. A new green-tide-forming alga, Ulva ohnoi Hiraoka et Shimada sp. nov. (Ulvales, Ulvophyceae) from Japan. Phycol. Res. 2004, 52, 17–29. [Google Scholar] [CrossRef]
- Nordby, Ø. Optimal conditions for meiotic spore formation in Ulva mutabilis Føyn. Bot. Mar. 1977, 20, 19–28. [Google Scholar] [CrossRef]
- Hiraoka, M.; Enomoto, S. The induction of reproductive cell formation of Ulva pertusa Kjellman (Ulvales, Ulvophyceae). Phycol. Res. 1998, 46, 199–203. [Google Scholar] [CrossRef]
- Hiraoka, M.; Ohno, M.; Kawaguchi, S.; Yoshida, G. Crossing test among floating Ulva thalli forming ‘green tide’ in Japan. Hydrobiologia 2004, 512, 239–245. [Google Scholar] [CrossRef]
- Migita, S. The sterile mutant of Ulva pertusa Kjellman from Oumra Bay. Bull. Fac. Fish. Nagasaki Univ. 1985, 57, 33–37. [Google Scholar]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwella, G.S. Reproductive sterility increases the capacity to exploit the green seaweed Ulva rigida for commercial applications. Algal Res. 2017, 24, 64–71. [Google Scholar] [CrossRef]
- Melton, J.T., III; Collado-Vides, L.; Lopez-Bautista, J.M. Molecular identification and nutrient analysis of the green tide species Ulva ohnoi M. Hiraoka & S. Shimada, 2004 (Ulvophyceae, Chlorophyta), a new report and likely nonnative species in the Gulf of Mexico and Atlantic Florida, USA. Aquat. Invasions 2016, 11, 225–237. [Google Scholar]
- Krupnik, N.; Rinkevich, B.; Paz, G.; Douek, J.; Lewinsohn, E.; Israel, A.; Carmel, N.; Mineur, F.; Maggs, C.A. Native, invasive and cryptogenic Ulva species from the Israeli Mediterranean Sea: Risk and potential. Mediterr. Mar. Sci. 2018, 19, 132–146. [Google Scholar] [CrossRef]
- Chávez-Sánchez, T.; Piñón-Gimate, A.; Melton, J.T., III; López-Bautista, J.M.; Casas-Valdez, M. First report, along with nomenclature adjustments, of Ulva ohnoi, U. tepida and U. torta (Ulvaceae, Ulvales, Chlorophyta) from northwestern Mexico. Bot. Mar. 2019, 62, 113–123. [Google Scholar] [CrossRef]
- Hiraoka, M.; Tanaka, K.; Yamasaki, T.; Miura, O. Replacement of Ulva ohnoi in the type locality under rapid ocean warming in southwestern Japan. J. Appl. Phycol. 2020, 32, 2489–2494. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, J.; Fan, S.; Li, Y.; Liu, X.; Liu, D. Who made the world’s largest green tide in China?—An integrated study on the initiation and early development of the green tide in Yellow Sea. Limnol. Oceanogr. 2015, 60, 1105–1117. [Google Scholar] [CrossRef]
- Wang, S.; Huo, Y.; Zhang, J.; Cui, J.; Wang, Y.; Yang, L.; Zhou, Q.; Lu, Y.; Yu, K.; He, P. Variations of dominant free-floating Ulva species in the source area for the world’s largest macroalgal blooms, China: Differences of ecological tolerance. Harmful Algae 2018, 74, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Monotilla, A.P.; Zhu, W.; Takano, Y.; Shimada, S.; Ichihara, K.; Matsui, T.; He, P.; Hiraoka, M. Taxonomic reassessment of Ulva prolifera (Ulvophyceae, Chlorophyta) based on specimens from the type locality and Yellow Sea green tides. Phycologia 2018, 57, 692–704. [Google Scholar] [CrossRef]
- Hiraoka, M.; Dan, A.; Shimada, S.; Hagihira, M.; Migita, M.; Ohno, M. Different life histories of Enteromorpha prolifera (Ulvales, Chlorophyta) from four rivers on Shikoku Island Japan. Phycologia 2003, 42, 275–284. [Google Scholar] [CrossRef]
- Shimada, S.; Yokoyama, N.; Arai, S.; Hiraoka, M. Phylogeography of the genus Ulva (Ulvophyceae, Chlorophyta), with special reference to the Japanese freshwater and brackish taxa. J. Appl. Phycol. 2008, 20, 979–989. [Google Scholar] [CrossRef]
- Liu, F.; Pang, S.; Zhao, X. Molecular phylogenetic analyses of Ulva (Chlorophyta, Ulvophyceae) mats in the Xiangshan Bay of China using high-resolution DNA markers. J. Appl. Phycol. 2013, 25, 1287–1295. [Google Scholar] [CrossRef]
- Hiraoka, M. Seaweed bed shifts under enhanced ocean warming and measures to cover fishery losses. Aquabiology 2012, 34, 314–318. [Google Scholar]
- Leliaert, F.; Zhang, X.; Ye, N.; Malta, E.; Engelen, A.H.; Mineur, F.; Verbruggen, H.; De Clerck, O. Identity of the Qingdao algal bloom. Phycol. Res. 2009, 57, 147–151. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, P.; Qin, S.; Liu, X.; Liu, Z.; Lin, H.; Li, F.; Chen, H.; Wu, C. Genetic analyses of floating Ulva prolifera in the Yellow Sea suggest a unique ecotype. Estuar. Coast. Shelf Sci. 2015, 163, 96–102. [Google Scholar] [CrossRef]
- Duan, W.; Guo, L.; Sun, D.; Zhu, S.; Chen, X.; Zhu, W.; Xu, T.; Chen, C. Morphological and molecular characterization of free-floating and attached green macroalgae Ulva spp. in the Yellow Sea of China. J. Appl. Phycol. 2012, 24, 97–108. [Google Scholar] [CrossRef]
- Hiraoka, M.; Ichihara, K.; Zhu, W.; Ma, J.; Shimada, S. Culture and hybridization experiments on an Ulva clade including the Qingdao strain blooming in the Yellow Sea. PLoS ONE 2011, 6, e19371. [Google Scholar] [CrossRef]
- Stratmann, J.; Paputsoglu, G.; Oertel, W. Differentiation of Ulva mutabilis (Chlorophyta) gametangia and gamete release are controlled by extracellular inhibitors. J. Phycol. 1996, 32, 1009–1021. [Google Scholar] [CrossRef]
- De Clerck, O.; Kao, S.M.; Bogaert, K.A.; Blomme, J.; Foflonker, F.; Kwantes, M.; Vancaester, E.; Vanderstraeten, L.; Aydogdu, E.; Boesger, J.; et al. Insights into the evolution of multicellularity from the sea lettuce genome. Curr. Biol. 2018, 28, 2921–2933.e5. [Google Scholar] [CrossRef] [PubMed]
- Lawton, R.J.; Sutherland, J.E.; Glasson, C.R.; Magnusson, M.E. Selection of temperate Ulva species and cultivars for land-based cultivation and biomass applications. Algal Res. 2021, 56, 102320. [Google Scholar] [CrossRef]
- Moreira, A.; Cruz, S.; Marques, R.; Cartaxana, P. The underexplored potential of green macroalgae in aquaculture. Rev. Aquac. 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).