1. Introduction
Salinity has become one of the major abiotic stresses that negatively affect plant growth and agricultural production worldwide [
1]. According to the Food and Agriculture Organization (FAO), approximately 19.5% of the irrigated land and 2.1% of dry land is saline affected [
2]. In addition, in arid and semi-arid regions, the salinization process occurs because of high evaporation and inadequate amounts of precipitation, leading to considerable leaching [
3]. Sorghum (
Sorghum bicolor (L.) Moench), a C
4 plant, is the fifth most important cereal crop in the world, well adapted to various abiotic stresses with a wide range of nutritional values [
4]. It is highly biomass productive, water-efficient, and widely cultivated in arid and semiarid tropics [
5]. In recent years, it has transformed from being consumed as feed to raw material for the production of biofuels [
6,
7].
It is well known that climate change and environmental extremes induce and enhance the impact of abiotic stresses on plant fitness and performance [
8,
9]. Salinity has a significant impact on plant metabolism, generating ion toxicity, osmotic stress, mineral inadequacies, and physiological, biochemical, and metabolic alterations, eventually influencing plant growth, development, and productivity [
10,
11]. Accumulation of higher concentrations of inorganic cations such as sodium, magnesium, and calcium, and anions such as chloride and sulfate, disturb the membrane potential [
12]. The high concentration of salts in the soil has a destructive effect on the cell membrane’s integrity, photosynthetic reactions, activation of several proteases, and absorption of nutrients [
13]. Excess salts compromise the physiological and biochemical functions of plants, causing osmotic stress that results in disturbances of water conductance [
2]. Tolerant plants use ions as an alternative to organic compounds for osmotic modification, which requires the synthesis of more energy (ATP) [
14]. Under salinity stress, plants adapt by initiating multiple molecular and physiochemical changes, which results in modifications to metabolic pathways to reach a new homeostatic equilibrium [
15]. Furthermore, defense through protective enzymes superoxide dismutase, catalase and peroxidase against salt-induced ROS over-generation and membrane lipid peroxidation is attributed to the protection of cellular membranes, which leads to salt tolerance [
16].
One of the most significant processes in plants’ adaptation to stress conditions is the accumulation of reserve compounds (carbohydrates, proteins, and lipids) in seeds [
17]. The function of these reserves is to provide energy for the formation of carbon skeletons within seedling tissues [
18]. Salinity alters plant metabolism by impeding the mobilization of reserves and changing the embryonic axis membranes, but it is crucial to understand the reason for this process [
19].
Salinity is also considered to activate alternative gene expression patterns that synthesize, degrade, or embellish metabolites from related pathways [
20]. Monitoring metabolite patterns is critical for understanding the physiological and molecular responses of plants to salinity [
21], as well as highlighting the functions of genes as crucial tools in functional genomics and systems biology for developing new breeding and screening strategies to enhance salt tolerance in crops [
22].
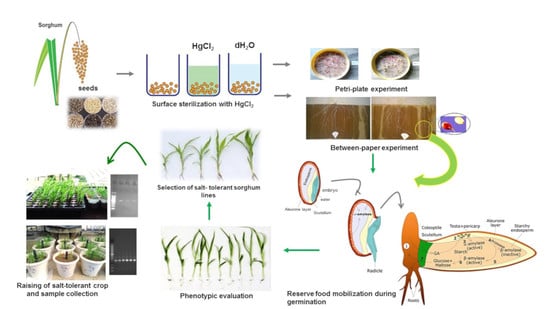
The development of salt-tolerant crops has been a major objective of plant breeding programs to maintain crop productivity in semi-arid and saline areas has been a major objective of plant breeding programs [
23]. This lack of information makes it imperative to study the damage caused by salinity during the mobilization of reserves in sorghum tissues since the germination and seedling establishment phases are vital for the success of the production. Understanding such mechanisms may contribute to the domestication as well as the genetic improvement of this species, making it profitable and competitive in semi-arid regions [
24]. Screening of salt-tolerant sorghum genotypes is a practical approach for successful identification and plantation in saline-affected areas by the implementation of agronomic practices for high-yielding genotypes that includes soil preparation, variety selection, and plant management system. Thus, the objective of the present study was to examine the effect of salinity on the metabolism of reserve mobilization and partition of macromolecules in sorghum seedlings, through the quantification of antioxidants during the germination period for salt tolerance.
4. Discussion
Plants can tolerate salinity, but the extent to which they can counteract this menace depends on the nature of a species or even a cultivar [
45]. Sorghum displays a significant intraspecific difference in salinity tolerance [
7]. Seedling growth is a critical stage for the establishment of plant population under saline conditions. Salinity primarily reduces the osmotic ability of soil solutions to impede water intake by seed, which affects the seed germination rate. It inhibits seed germination by reducing the ease with which the water can be absorbed by the seeds or the Na
+ and Cl
− ions [
46] and even in the case of salt-tolerant plants. The germination index, dry leaf weight, and root length are the most significant factors and are recommended as the main indexes to identify alkaline tolerance of sorghum at germination [
47]. Salinity stress drastically decreased the seedling vigor of screened sorghum genotypes due to an increase in salt concentration, which may cause delayed emergence of plumule and radical [
9]. The growth rate of shoots controls the varietal difference in that lower shoot growth is associated with a higher level of NaCl in the leaves. Reduced shoot growth could also be due to reduced leaf initiation and progression and accelerated leaf abscission and internode growth [
48]. The percent of root biomass showed a downward trend because the root was the first contacting organ to the treatment solution. This was demonstrated by the phenomenon that the root accumulated more Na
+ and suffered more severe iron toxicity. This mechanism operates in salt-sensitive and salt-tolerant genotypes but with different efficiency levels [
49]. The analysis revealed that germination rate, leaf weight, and root length, which were the most significant loads in the germination factors, leaf, and root, respectively, will be used as the main indexes to screen for sorghum salt tolerance. Progress has been made in identifying traits that are good indicators of salt tolerance in sorghum. Sun et al. [
50] studied the responses of 42 sorghum genotypes to salt stress to identify genotypes with tolerance to salinity during germination.
Stress affects several physiological processes throughout the plasma membrane by impairing photosynthesis (especially PSII damage), resulting from inadequate water supply and its translocation together with ions and organic solutes. Stress also reduces the capacity of leaf osmosis [
51]. Saline stress generally results in a reduction in chlorophyll and photosynthetic thresholds [
7]. Total chlorophyll content was significantly affected by an increment of salinity levels, which showed that higher salt concentrations could ramp up chlorophyll decomposition in the body and reduce the photosynthetic efficiency of sorghum plants. In high salinity, the chlorophyll content initially increased and decreased afterward, suggesting that the optimal salt concentration may contribute to more chlorophyll synthesis, absorbing more light for photosynthesis [
52].
The protective antioxidant system is crucial for a plant’s survival under stress conditions. Salt stress significantly impacts the lipid peroxidation of the plasma membrane, suggesting disruption and leakage of membranes under stressful situations [
53]. MDA content increased due to the accumulation of reactive oxygen species (ROS) synthesized under stress conditions [
54,
55]. The increase in membrane lipid peroxidation during oxidative stress results from increased production of reactive oxygen species [
56]. However, biological membrane stability has been considered an effective tool to evaluate the negative impacts of salinity stress. Similar to our results, low MDA content has been shown to be essential in salt tolerance, as other researchers represent. Salt-tolerant barley cultivar and the salt-resistant canola plant [
57] also had lower lipid peroxidation levels, which is an essential hint of higher oxidative damage limiting growth capacity under salinity. However, salt-sensitive rice and maize varieties had higher MDA content and electrolyte leakage in response to salt stress [
19]. It has been suggested that a decrease in membrane stability reflects the extent of lipid peroxidation caused by ROS [
58].
Ascorbic acid is a common constituent of a plant cell synthesized in the cytosol and then translocated to the apoplast [
59]. The primary function of ascorbate is to protect plant cells against oxidative damage by scavenging oxygen-free radicals directly. A higher level of ascorbic acid content in tolerant genotypes in the present study is in accordance with the observations of Almeselmani et al. [
60], who reported higher ascorbic acid content in tolerant wheat genotypes. Compatible solutes, antioxidants, and soluble sugars constitute the biochemical basis of varietal differences associated with salt tolerance [
2]. Total soluble carbohydrates act as an osmolyte inside the plant cell, and their accumulation increases the resistance against stress conditions [
15]. Generally, the plant’s osmotic regulators include organic compounds and inorganic ions. In this study, with the increasing salt stress, proline and soluble carbohydrate content in leaves of sorghum seedlings also increased to mitigate oxidative stress. Proline and soluble sugar are the most common compounds that accumulate as compatible osmolytes [
16]. Plants survive in saline conditions due to osmotic adjusting, including intracellular division and separation of toxic ions from the cytosol via energy-related transport into the vacuole survives [
61,
62]. The lower cytosolic Na
+ ion and K
+/Na
+ homeostasis generally has been reported to be an essential for salinity tolerance, and higher concentrations of K
+/Na
+ have been suggested in the salt-tolerant lines [
1]. A large proportion of Na
+ translocates into the plant’s body under saline stress, affecting K
+ absorption and the disruption of the equilibrium between Na
+ and K
+ [
63]. With the increasing saline stress, the Mg
2+ ion and Ca
2+ ion in leaves of sorghum seedlings were decreased significantly. This showed that salt stress affected the Ca
2+ and Mg
2+ processes [
64]. The Cl
- and SO
42- anions showed a similarly increasing trend of increasing salinity, whereas the accumulation of Cl
- ions was significantly higher than the SO
42- ion. With the reduced concentration of Ca
2+ ions, the function of the cell membrane is disrupted, which maintains the integrity and regulates the selective transport of Na
+ and K
+ ions [
24].
Starch is the most abundant storage carbohydrate and the primary energy supplier [
6,
16]. Wang et al. [
65] found that starch content in sorghum genotypes ranged between 64 and 74% of dry grain weight. SSG 59-3 synthesized maximum proteins after exposure to salt treatment, whereas it was slower in PC-5. Thus, germinating seeds of SSG 59-3 showed higher starch mobilization and enhanced de novo protein synthesis at higher salinity levels [
18]. The starch granules are surrounded by a protein matrix that can limit the access of enzymes [
66]. Thus, its utilization depends upon the genetic make-up of cultivars for its effective mobilization [
67]. As starch comprises about 71.2% of reserve food in sorghum seed, it is the primary energy source for germinating seeds [
14].
In the present study, attempts were made to establish a correlation between antioxidative defense mechanisms and salinity-induced changes in sorghum seedlings. During oxidative stress, the excess production of ROS is scavenged by a complex enzymatic antioxidative system, which controls ROS production and ultimately protects the plant against oxidative damage [
15]. This salinity-induced defense mechanism is differential and primarily dependent on differential antioxidant enzymes, salinity extent, and exposure time [
68]. SOD is the most effective intracellular enzymatic antioxidant, ubiquitous in all aerobic organisms and all subcellular compartments prone to ROS-mediated oxidative stress [
2,
10]. Lee et al. [
69] reported that transgenic tobacco plants overexpressing Cu/Zn-SOD showed tolerance to salt and water stresses. The induction of catalase activity was reported on the accumulation of H
2O
2 and is seemingly consistent with this enzyme’s role in scavenging enhanced H
2O
2 levels [
24]. Salinity-induced stimulation in POD activity in tolerant genotypes, suggesting their possible role in efficiently removing H
2O
2 in tolerant genotypes [
70]. Enhanced peroxidase activity under various stresses was linked to protection from oxidative damage, lignification, and cross-linking of the cell wall. APX and GPX are specific enzymes that scavenge chloroplastic H
2O
2 using ascorbate as an electron donor in the first step of the ascorbate–glutathione cycle and are considered the essential plant peroxidases in H
2O
2 detoxification [
71]. The enhanced activity of APX and GPX concomitant with an enhanced ascorbic acid content and glutathione may help satisfy ROS [
72]. GR is essential to recycle GSH in the ascorbate–glutathione cycle in an NADPH-dependent reaction. Similarly, a higher induction in GR activity in tolerant varieties than susceptible varieties was reported in
Macrotyloma uniflorum and chickpea [
73]. Similar results were also reported in maize nine and wheat differing in salt tolerance [
74].
Several genes have been described to play an essential role in numerous physiological processes of embryo development, germination, and maturation [
75]. During the germination process, the genes involved are catabolism or α-amylase synthesis and degradation. Under high salinity, there is rapid mobilization of reserved food stored [
18]. The scutellum is the initial site of
α-amy1 expression and subsequent α-amylase synthesis. GBSS plays a minor role in amylose synthesis in non-storage tissue [
76]. The differential expression of ion transporter genes under different salinity levels indicated their adaptive behavior for Na
+ ion exclusion. Salt stress elicits a cytosolic calcium signal [
77]. The increased intracellular concentration of Ca
2+ ions is sensed by
SOS3, which interacts and activates
SOS2, a serine/threonine-protein kinase [
78]. Both
SOS2 and
SOS3 regulate the expression level of
SOS1, a salt tolerance gene that encodes a sodium proton antiporter (
NHX) [
79]. Co-expression of
H+-PPase together with
NHX dramatically enhances the salt tolerance capacity in plants [
80]. One of the largest families of plant-specific transcription factors includes
NAC regulating several other genes’ transcription in response to abiotic stress [
2]. Overexpression of
SbVPPase in transgenic finger millet enhances the plant’s performance under salt stress [
81]. Sun et al. [
82] elucidated that the complex regulatory network involving non-coding RNAs underpin the sorghum tolerance to salt stress. Varoquaux et al. [
83] assessed the molecular underpinnings of drought responses in sorghum and reported that, under stressed conditions, massive changes occurred in the transcriptome, impacting more than 40% of all expressed genes encompassing a wide variety of molecular mechanisms.
The principal component analysis is a multivariate technique for examining the relationships among several quantitative variables [
84]. Principal component analysis (PCA) provides mechanisms to describe relationships between the germination potential and adaptive means of sorghum seedlings under high salinity [
85]. This tool had led us to identify factors that will be the focus on future efforts to perform targeted changes to affect sorghum adaptability and tolerance behavior positively. Analysis of variability among traits and knowledge of associations among traits contributing to yield would be of great importance in planning a successful breeding program [
72]. Abraha et al. [
86] reported four principal components with eigenvalues greater than one, which explained > 75% of the total variation for agronomic traits.