Influence of Rootstock on the Leaf Volatile Organic Compounds of Citrus Scion Is More Pronounced after the Infestation with Diaphorina citri
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Asian Citrus Psyllids
Infestation with Diaphorina citri
2.3. DNA Extraction and qPCR
2.4. Sugar Belle Leaf VOC Extraction
2.5. Analysis of Leaf Volatiles
2.6. Statistical Analyses
3. Results
3.1. Volatile Organic Compounds Identified in Sugar Belle Leaves
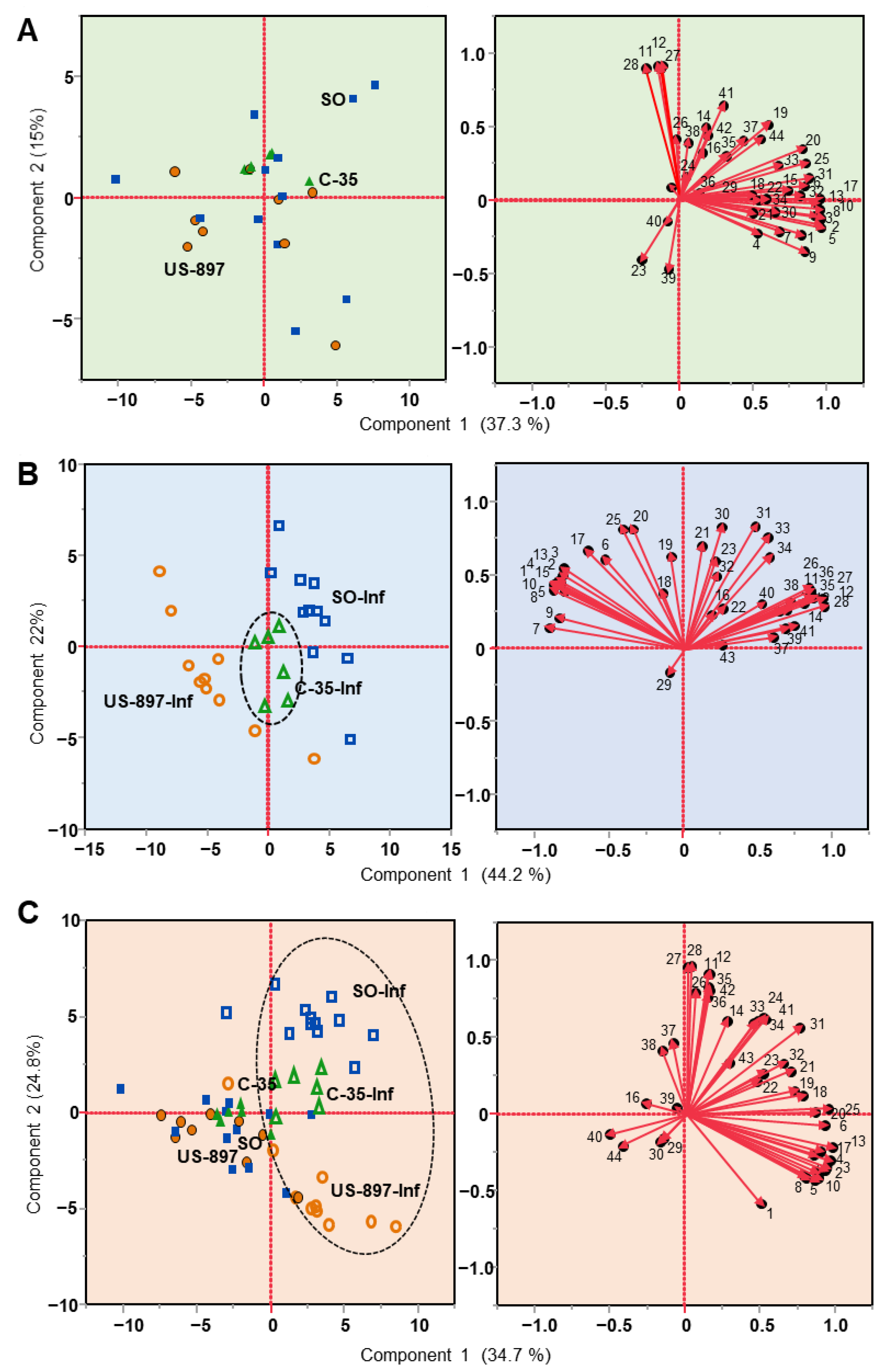
3.2. Principal Component Analysis
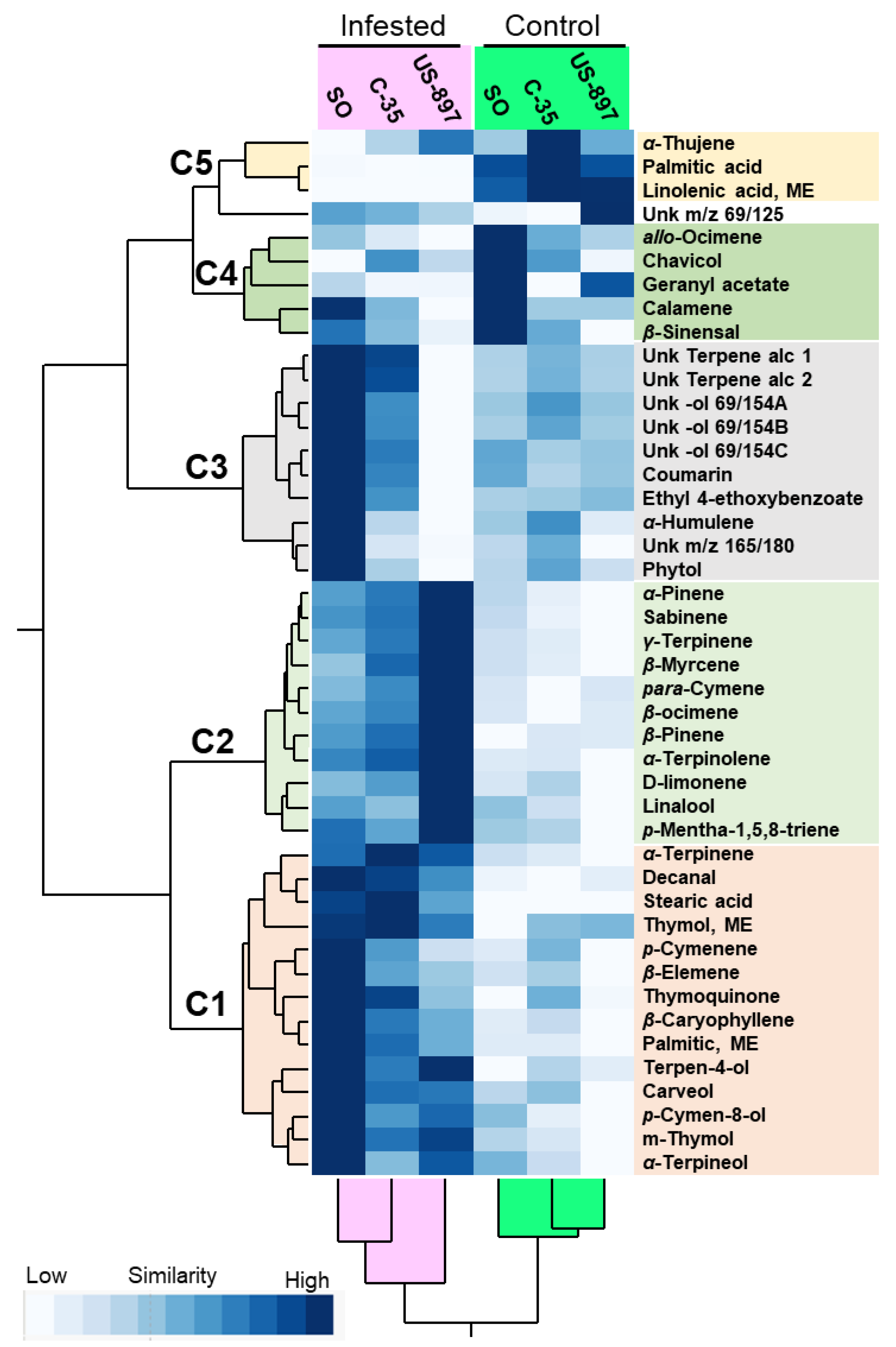
3.3. Two-Way Hierarchical Cluster Analysis and Heat Map
3.4. Volatile Profile Alteration after Infestation with Diaphorina citri
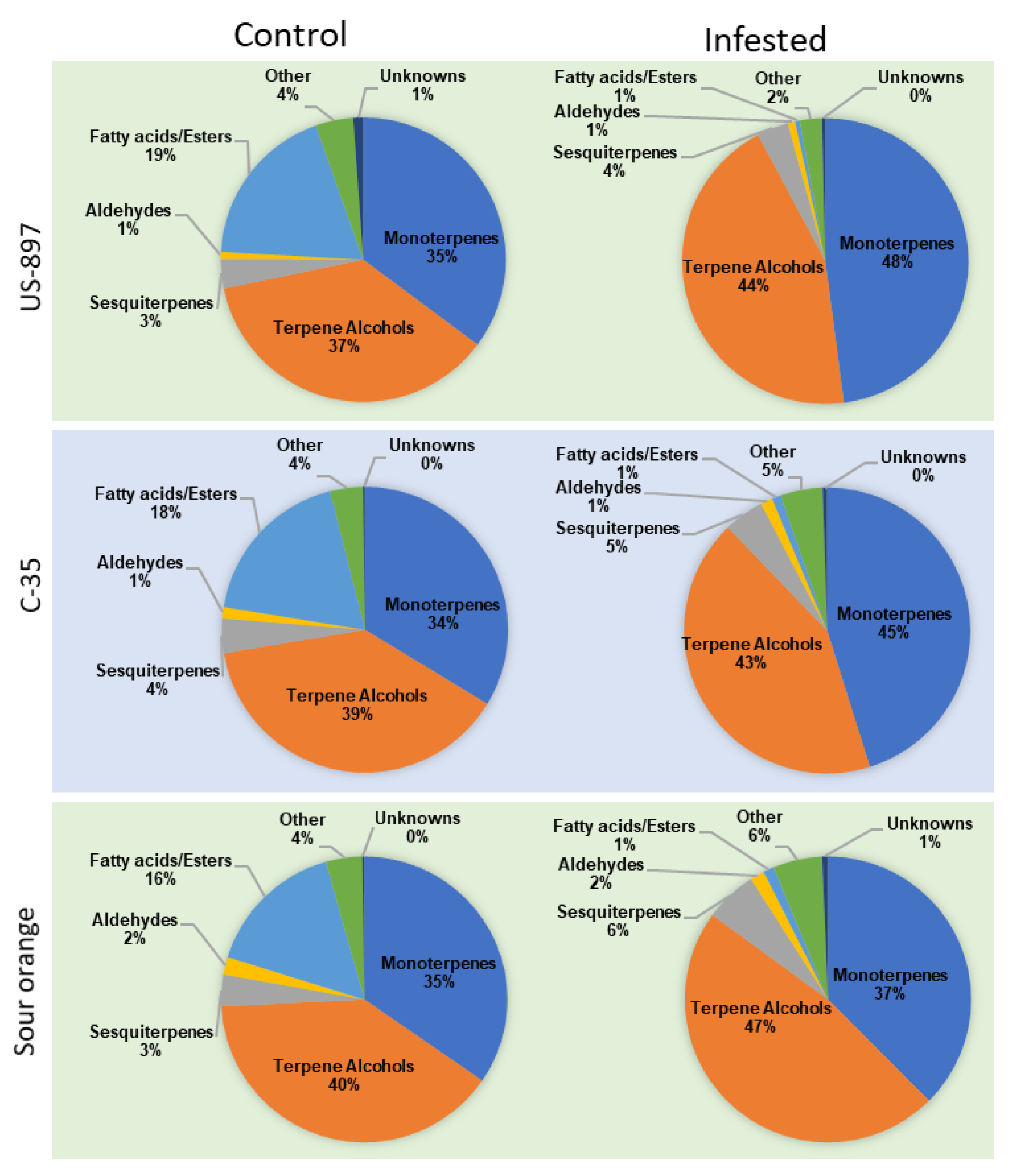
3.5. VOC Classes Percentage to Each Other (Pie Chart Analysis)
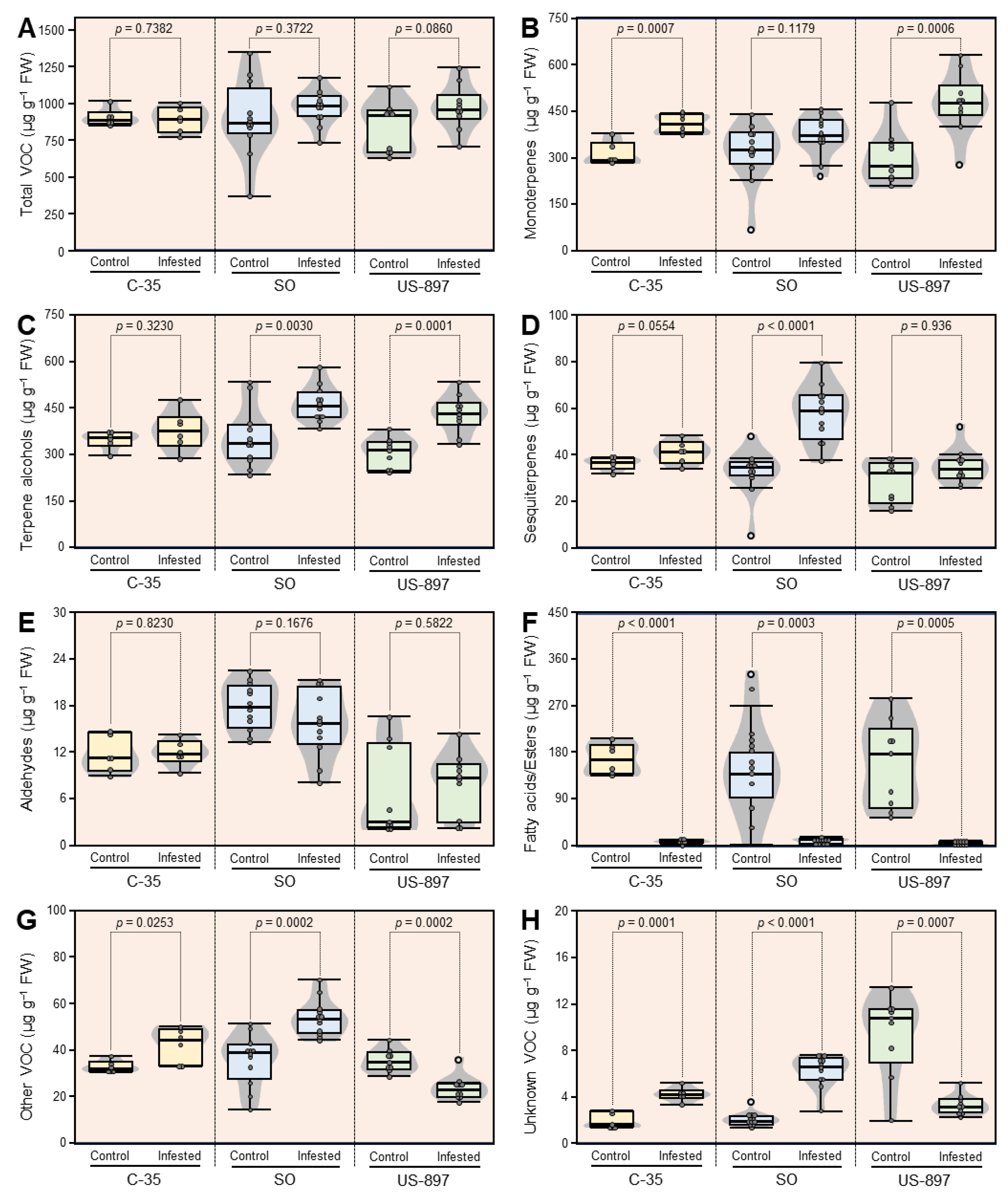
3.6. Concentrations of VOC Classes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Folimonova, S.Y.; Robertson, C.J.; Garnsey, S.M.; Gowda, S.; Dawson, W.O. Examination of the Responses of Different Genotypes of Citrus to Huanglongbing (Citrus Greening) Under Different Conditions. Phytopathology 2009, 99, 1346–1354. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, U.; Bowman, K.D. Tolerance of trifoliate citrus rootstock hybrids to Candidatus Liberibacter asiaticus. Sci. Hortic. 2012, 147, 71–80. [Google Scholar] [CrossRef]
- Ramadugu, C.; Keremane, M.L.; Halbert, S.E.; Duan, Y.P.; Roose, M.L.; Stover, E.; Lee, R.F. Long-Term Field Evaluation Reveals Huanglongbing Resistance in Citrus Relatives. Plant Dis. 2016, 100, 1858–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, K.D.; McCollum, G.; Albrecht, U. Performance of ‘Valencia’ orange (Citrus sinensis [L.] Osbeck) on 17 rootstocks in a trial severely affected by huanglongbing. Sci. Hortic. 2016, 201, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Miles, G.P.; Stover, E.; Ramadugu, C.; Keremane, M.L.; Lee, R.F. Apparent Tolerance to Huanglongbing in Citrus and Citrus-related Germplasm. HortScience 2017, 52, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, U.; Bowman, K.D. Reciprocal influences of rootstock and scion citrus cultivars challenged with Ca. Liberibacter asiaticus. Sci. Hortic. 2019, 254, 133–142. [Google Scholar] [CrossRef]
- Castle, W.S. Rootstock as a fruit quality factor in citrus and deciduous tree crops. N. Z. J. Crop. Hortic. Sci. 1995, 23, 383–394. [Google Scholar] [CrossRef]
- Florida Citrus Rootstock Selection Guide, 3rd ed.; EDIS Publication SP-248; Available online: https://crec.ifas.ufl.edu/extension/citrus_rootstock/ (accessed on 15 January 2018).
- dos Santos, I.C.; de Almeida, A.-A.F.; Pirovani, C.P.; Costa, M.G.C.; Silva, M.F.D.G.F.D.; Bellete, B.S.; Freschi, L.; Filho, W.S.; Filho, M.A.C.; Gesteira, A.D.S. Differential accumulation of flavonoids and phytohormones resulting from the canopy/rootstock interaction of citrus plants subjected to dehydration/rehydration. Plant Physiol. Biochem. 2017, 119, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Johnson, E.G.; Gottwald, T.R.; Irey, M.S. Presymptomatic Fibrous Root Decline in Citrus Trees Caused by Huanglongbing and Potential Interaction with Phytophthora spp. Plant Dis. 2013, 97, 1195–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosser, J.W.; Chandler, J.; Duncan, L.W. Production of mandarin+pummelo somatic hybrid citrus rootstocks with potential for improved tolerance/resistance to sting nematode. Sci. Hortic. 2007, 113, 33–36. [Google Scholar] [CrossRef]
- Javed, N.; Javed, M.; Ilyas, M.B.; Khan, M.M.; Inam-Ul-Haq, M. Reactions of various citrus root stocks (germplasm) against citrus root nematode (Tylenchulus semipenetrans Cobb.). Pak. J. Bot. 2008, 40, 2693–2696. [Google Scholar]
- Gonçalves, L.P.; Camargo, R.L.B.; Takita, M.A.; Machado, M.A.; Filho, W.S.D.S.; Costa, M.G.C. Rootstock-induced molecular responses associated with drought tolerance in sweet orange as revealed by RNA-Seq. BMC Genom. 2019, 20, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedroso, F.K.; Prudente, D.A.; Bueno, A.C.R.; Machado, E.C.; Ribeiro, R.V. Drought tolerance in citrus trees is enhanced by rootstock-dependent changes in root growth and carbohydrate availability. Environ. Exp. Bot. 2014, 101, 26–35. [Google Scholar] [CrossRef]
- Santos, J.Z.; Almeida, L.A.; Filho, W.S.S.; Bizzo, H.R.; Santos, M.C.D.S.; Mattos, J.K.; Silva, J.P.; Vieira, R.F. Chemical characterization of the essential oils from leaves of mandarins Sunki, Cleopatra and their hybrids. J. Essent. Oil Res. 2014, 27, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hijaz, F.; Nehela, Y.; Killiny, N. Possible role of plant volatiles in tolerance against huanglongbing in citrus. Plant Signal. Behav. 2016, 11, e1138193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Coletta-Filho, H.D.; Targon, M.L.P.N.; Takita, M.; De Negri, J.D.; Pompeu, J.; Machado, M.A.; Amaral, A.M.D.; Muller, G.W. First Report of the Causal Agent of Huanglongbing (“Candidatus Liberibacter asiaticus”) in Brazil. Plant Dis. 2004, 88, 1382. [Google Scholar] [CrossRef]
- Halbert, S.E.; Manjunath, K.L. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Fla. Entomol. 2004, 87, 330. [Google Scholar] [CrossRef]
- Court, C.D.; Hodges, A.W.; Stair, C.; Rahmani, M. Economic Contributions of the Florida Citrus Industry in 2016–2017; University of Florida: Gainesville, FL, USA, 2018. [Google Scholar]
- Jagoueix, S.; Bove, J.-M.; Garnier, M. The Phloem-Limited Bacterium of Greening Disease of Citrus Is a Member of the Subdivision of the Proteobacteria. Int. J. Syst. Bacteriol. 1994, 44, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Nehela, Y.; Killiny, N. Revisiting the Complex Pathosystem of Huanglongbing: Deciphering the Role of citrus Metabolites in Symptom Development. Metabolites 2020, 10, 409. [Google Scholar] [CrossRef]
- Gottwald, T.R. Current Epidemiological Understanding of Citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDACS. Florida Citrus Statistics 2012–2013; Florida Department of Agriculture and Consumer Services: Tallahassee, FL, USA, 2014.
- Garcia-Munoz, M.C.; Henao-Rojas, J.C.; Moreno-Rodriguez, J.M.; Botina-Azain, B.L.; Romero-Barrera, Y. Effect of rootstock and environmental factors on fruit quality of Persian lime (Citrus latifolia Tanaka) grown in tropical regions. J. Food Compos. Anal. 2021, 103. [Google Scholar] [CrossRef]
- Morales, J.; Bermejo, A.; Navarro, P.; Forner-Giner, M.Á.; Salvador, A. Rootstock effect on fruit quality, anthocyanins, sugars, hydroxycinnamic acids and flavanones content during the harvest of blood oranges ‘Moro’ and ‘Tarocco Rosso’ grown in Spain. Food Chem. 2021, 342, 128305. [Google Scholar] [CrossRef]
- Şekerli, L.; Tuzcu, O. Fruit quality of ‘Valencia’ orange trees grafted on Volkameriana and sour orange rootstocks grown in two different regions in northern Cyprus. Pak. J. Bot. 2020, 52, 1803–1808. [Google Scholar] [CrossRef]
- Caruso, M.; Continella, A.; Modica, G.; Pannitteri, C.; Russo, R.; Salonia, F.; Arlotta, C.; Gentile, A.; Russo, G. Rootstocks Influence Yield Precocity, Productivity, and Pre-Harvest Fruit Drop of Mandared Pigmented Mandarin. Agronomy 2020, 10, 1305. [Google Scholar] [CrossRef]
- Brito, M.E.B.; Fernandes, P.D.; Gheyi, H.R.; Soares, L.A.D.A.; Filho, W.D.S.S.; Suassuna, J.F. Screening of citrus scion-rootstock combinations for tolerance to water salinity during seedling formation. Acta Sci. Agron. 2020, 43, e48163. [Google Scholar] [CrossRef]
- Aparicio-Durán, L.; Hervalejo-García, A.; Calero-Velázquez, R.; Arjona-López, J.M.; Arenas-Arenas, F.J. Salinity Effect on Plant Physiological and Nutritional Parameters of New Huanglongbing Disease-Tolerant Citrus Rootstocks. Agronomy 2021, 11, 653. [Google Scholar] [CrossRef]
- Kunwar, S.; Grosser, J.; Gmitter, F.G.; Castle, W.S.; Albrecht, U. Field Performance of ‘Hamlin’ Orange Trees Grown on Various Rootstocks in Huanglongbing-endemic Conditions. HortScience 2021, 56, 244–253. [Google Scholar] [CrossRef]
- Bowman, K.D.; Albrecht, U. Rootstock Influences on Health and Growth Following Candidatus Liberibacter asiaticus Infection in Young Sweet Orange Trees. Agronomy 2020, 10, 1907. [Google Scholar] [CrossRef]
- Qureshi, J.A.; Rogers, M.E.; Hall, D.G.; Stansly, P.A. Incidence of invasive Diaphorina citri (Hemiptera: Psyllidae) and its introduced parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) in Florida citrus. J. Econ. Entomol. 2009, 102, 247–256. [Google Scholar] [CrossRef]
- Morgan, K.T.; Rouse, R.E.; Ebel, R.C. Foliar Applications of Essential Nutrients on Growth and Yield of ‘Valencia’ Sweet Orange Infected with Huanglongbing. HortScience 2016, 51, 1482–1493. [Google Scholar] [CrossRef]
- Killiny, N.; Kishk, A. Delivery of dsRNA through topical feeding for RNA interference in the citrus sap piercing-sucking hemipteran, Diaphorina citri. Arch. Insect Biochem. Physiol. 2017, 95, 21394. [Google Scholar] [CrossRef]
- Santos-Ortega, Y.; Killiny, N. Silencing of sucrose hydrolase causes nymph mortality and disturbs adult osmotic homeostasis in Diaphorina citri (Hemiptera: Liviidae). Insect Biochem. Mol. Biol. 2018, 101, 131–143. [Google Scholar] [CrossRef]
- Stover, E.; Inch, S.; Richardson, M.L.; Hall, D.G. Conventional Citrus of Some Scion/Rootstock Combinations Show Field Tolerance under High Huanglongbing Disease Pressure. HortScience 2016, 51, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Killiny, N.; Valim, M.F.; Jones, S.E.; Omar, A.; Hijaz, F.; Gmitter, F.G.; Grosser, J.W. Metabolically speaking: Possible reasons behind the tolerance of ‘Sugar Belle’ mandarin hybrid to huanglongbing. Plant Physiol. Biochem. 2017, 116, 36–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killiny, N.; Jones, S.E.; Nehela, Y.; Hijaz, F.; Dutt, M.; Gmitter, F.G.; Grosser, J.W. All roads lead to Rome: Towards understanding different avenues of tolerance to huanglongbing in citrus cultivars. Plant Physiol. Biochem. 2018, 129, 1–10. [Google Scholar] [CrossRef]
- Killiny, N.; Valim, M.F.; Jones, S.E.; Hijaz, F. Effect of different rootstocks on the leaf metabolite profile of ‘Sugar Belle’ mandarin hybrid. Plant Signal. Behav. 2018, 13, e1445934. [Google Scholar] [CrossRef]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.; Tumlinson, J.H. Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef] [Green Version]
- Hijaz, F.; El-Shesheny, I.; Killiny, N. Herbivory by the insect Diaphorina citri induces greater change in citrus plant volatile profile than does infection by the bacterium, Candidatus Liberibacter asiaticus. Plant Signal. Behav. 2013, 8, e25677. [Google Scholar] [CrossRef] [Green Version]
- Dardouri, T.; Gomez, L.; Ameline, A.; Costagliola, G.; Schoeny, A.; Gautier, H. Non-host volatiles disturb the feeding behavior and reduce the fecundity of the green peach aphid, Myzus persicae. Pest Manag. Sci. 2021, 77, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Scutareanu, P.; Drukker, B.; Bruin, J.; Posthumus, M.A.; Sabelis, M.W. Volatiles from Psylla-Infested Pear Trees and Their Possible Involvement in Attraction of Anthocorid Predators. J. Chem. Ecol. 1997, 23, 2241–2260. [Google Scholar] [CrossRef] [Green Version]
- Cabedo-López, M.; Cruz-Miralles, J.; Vacas, S.; Navarro-Llopis, V.; Pérez-Hedo, M.; Flors, V.; Jaques, J.A. The olfactive responses of Tetranychus urticae natural enemies in citrus depend on plant genotype, prey presence, and their diet specialization. J. Pest Sci. 2019, 92, 1165–1177. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Q.; Erb, M.; Turlings, T.C.J.; Ge, L.; Hu, L.; Li, J.; Han, X.; Zhang, T.; Lu, J.; et al. Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol. Lett. 2012, 15, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Zimba, K.; Hill, M.; Moore, S.D.; Heshula, U. Agathis bishopi (Hymenoptera: Braconidae) as a Potential Tool for Detecting Oranges Infested with Thaumatotibia leucotreta (Lepidoptera: Tortricidae). J. Insect Behav. 2015, 28, 618–633. [Google Scholar] [CrossRef]
- Ananthakrishnan, G.; Choudhary, N.; Roy, A.; Sengoda, V.G.; Postnikova, E.; Hartung, J.S.; Stone, A.L.; Damsteegt, V.D.; Schneider, W.L.; Munyaneza, J.E.; et al. Development of Primers and Probes for Genus and Species Specific Detection of ‘Candidatus Liberibacter Species’ by Real-Time PCR. Plant Dis. 2013, 97, 1235–1243. [Google Scholar] [CrossRef] [Green Version]
- Harper, S.J.; Cowell, S.J. The past and present status of Citrus tristeza virus in Florida. J. Citrus Pathol. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Albrecht, U.; McCollum, G.; Bowman, K.D. Influence of rootstock variety on Huanglongbing disease development in field-grown sweet orange (Citrus sinensis [L.] Osbeck) trees. Sci. Hortic. 2012, 138, 210–220. [Google Scholar] [CrossRef]
- Benjamin, G.; Tietel, Z.; Porat, R. Effects of Rootstock/Scion Combinations on the Flavor of Citrus Fruit. J. Agric. Food Chem. 2013, 61, 11286–11294. [Google Scholar] [CrossRef]
- Raddatz-Mota, D.; Franco-Mora, O.; Mendoza-Espinoza, J.A.; Rodríguez-Verástegui, L.L.; de León-Sánchez, F.D.; Rivera-Cabrera, F. Effect of different rootstocks on Persian lime (Citrus latifolia T.) postharvest quality. Sci. Hortic. 2019, 257, 108716. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Tolerance of the Trifoliate Citrus Hybrid US-897 (Citrus reticulata Blanco × Poncirus trifoliata L. Raf.) to Huanglongbing. HortScience 2011, 46, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Mann, R.S.; Ali, J.G.; Hermann, S.L.; Tiwari, S.; Pelz-Stelinski, K.S.; Alborn, H.T.; Stelinski, L.L. Induced Release of a Plant-Defense Volatile ‘Deceptively’ Attracts Insect Vectors to Plants Infected with a Bacterial Pathogen. PLoS Pathog. 2012, 8, e1002610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, R.S.; Tiwari, S.; Smoot, J.M.; Rouseff, R.L.; Stelinski, L.L. Repellency and toxicity of plant-based essential oils and their constituents against Diaphorina citri Kuwayama (Hemiptera: Psyllidae). J. Appl. Entomol. 2010, 136, 87–96. [Google Scholar] [CrossRef]
- Onagbola, E.O.; Rouseff, R.L.; Smoot, J.M.; Stelinski, L.L. Guava leaf volatiles and dimethyl disulphide inhibit response of Diaphorina citri Kuwayama to host plant volatiles. J. Appl. Entomol. 2010, 135, 404–414. [Google Scholar] [CrossRef]
- Martini, X.; Kuhns, E.H.; Hoyte, A.; Stelinski, L.L. Plant volatiles and density-dependent conspecific female odors are used by Asian citrus psyllid to evaluate host suitability on a spatial scale. Arthropod-Plant Interact. 2014, 8, 453–460. [Google Scholar] [CrossRef]
- Alquézar, B.; Rodríguez, A.; De La Peña, M.; Peña, L. Genomic Analysis of Terpene Synthase Family and Functional Characterization of Seven Sesquiterpene Synthases from Citrus sinensis. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Dudareva, N.; Klempien, A.; Muhlemann, J.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Simkin, A.J.; Guirimand, G.; Papon, N.; Courdavault, V.; Thabet, I.; Ginis, O.; Bouzid, S.; Giglioli-Guivarc’H, N.; Clastre, M. Peroxisomal localisation of the final steps of the mevalonic acid pathway in planta. Planta 2011, 234, 903–914. [Google Scholar] [CrossRef]
- Mohammed, K.; Agarwal, M.; Du, X.B.; Newman, J.; Ren, Y. Behavioural responses of the parasitoid Aphytis melinus to volatiles organic compounds (VOCs) from Aonidiella aurantii on its host fruit Tahitian lime fruit Citrus latifolia. Biol. Control. 2019, 133, 103–109. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Ozek, G.; Ozek, T.; Aytac, Z.; Bernier, U.R.; Agramonte, N.M.; Baser, K.H.C.; Khan, I.A. Essential Oils of Echinophora lamondiana (Apiales: Umbelliferae): A Relationship Between Chemical Profile and Biting Deterrence and Larvicidal Activity Against Mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2015, 52, 93–100. [Google Scholar] [CrossRef]
- Tabari, M.A.; Youssefi, M.R.; Maggi, F.; Benelli, G. Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae). Vet. Parasitol. 2017, 245, 86–91. [Google Scholar] [CrossRef]
- Hall, D.G.; Borovsky, D.; Chauhan, K.R.; Shatters, R.G. An evaluation of mosquito repellents and essential plant oils as deterrents of Asian citrus psyllid. Crop. Prot. 2018, 108, 87–94. [Google Scholar] [CrossRef]
- Verdi, M.Z.; Abbasipour, H.; Chegini, S.G. Phytochemical and Insecticidal Study of the Avishan-e-denaii (Thymus daenensis Celak.) Essential Oil against the Melon Aphid (Aphis gossypii Glover). J. Essent. Oil Bear. Plants 2019, 22, 545–553. [Google Scholar] [CrossRef]
- Patt, J.M.; Robbins, P.S.; Niedz, R.; Mccollum, G.; Alessandro, R. Exogenous application of the plant signalers methyl jasmonate and salicylic acid induces changes in volatile emissions from citrus foliage and influences the aggregation behavior of Asian citrus psyllid (Diaphorina citri), vector of Huanglongbing. PLoS ONE 2018, 13, e0193724. [Google Scholar] [CrossRef] [Green Version]
- Waliwitiya, R.; Kennedy, C.J.; Lowenberger, C.A. Larvicidal and oviposition-altering activity of monoterpenoids, trans-anithole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Pest Manag. Sci. 2009, 65, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, F.; Al-Rimawi, F.; Manthey, J.A.; Killiny, N. Phenolics, flavonoids and antioxidant capacities in Citrus species with different degree of tolerance to Huanglongbing. Plant Signal. Behav. 2020, 15, 1752447. [Google Scholar] [CrossRef] [PubMed]
- Glasser, F.; Doreau, M.; Maxin, G.; Baumont, R. Fat and fatty acid content and composition of forages: A meta-analysis. Anim. Feed. Sci. Technol. 2013, 185, 19–34. [Google Scholar] [CrossRef]
- Gueta-Dahan, Y.; Yaniv, Z.; Zilinskas, B.A.; Ben-Hayyim, G. Salt and oxidative stress: Similar and specific responses and their relation to salt tolerance in Citrus. Planta 1997, 203, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Stover, E.; Hall, D.G.; Grosser, J.; Gruber, B.; Moore, G.A. Huanglongbing-related Responses of ‘Valencia’ Sweet Orange on Eight Citrus Rootstocks during Greenhouse Trials. HortTechnology 2018, 28, 776–782. [Google Scholar] [CrossRef]
- Killiny, N.; Nehela, Y. Metabolomic Response to Huanglongbing: Role of Carboxylic Compounds in Citrus sinensis Response to ‘Candidatus Liberibacter asiaticus’ and Its Vector, Diaphorina citri. Mol. Plant-Microbe Interact. 2017, 30, 666–678. [Google Scholar] [CrossRef] [Green Version]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Chippendale, G.M.; Beck, S.D.; Strong, F.M. Methyl Linolenate as an Essential Nutrient for the Cabbage Looper, Trichoplusia ni (Hübner). Nature 1964, 204, 710–711. [Google Scholar] [CrossRef]
- Sivapalan, P.; Gnanapragasam, N. The influence of linoleic acid and linolenic acid on adult moth emergence of Homona coffearia from meridic diets in vitro. J. Insect Physiol. 1979, 25, 393–398. [Google Scholar] [CrossRef]
- Fougeron, A.-S.; Farine, J.-P.; Flaven-Pouchon, J.; Everaerts, C.; Ferveur, J.-F. Fatty-Acid Preference Changes during Development in Drosophila melanogaster. PLoS ONE 2011, 6, e26899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfaress, S.; Hijaz, F.; Killiny, N. Chemical composition of cornicle secretion of the brown citrus aphid Toxoptera citricida. Physiol. Entomol. 2015, 41, 38–47. [Google Scholar] [CrossRef]
- Baker, E.A.; Procopiou, J.; Hunt, G.M. The cuticles of Citrus species. Composition of leaf and fruit waxes. J. Sci. Food Agric. 1975, 26, 1093–1101. [Google Scholar] [CrossRef]
- Bowman, K.D.; Faulkner, L.; Kesinger, M. New Citrus Rootstocks Released by USDA 2001–2010: Field Performance and Nursery Characteristics. HortScience 2016, 51, 1208–1214. [Google Scholar] [CrossRef] [Green Version]
- Gonzáles, W.; Ramirez, C.; Olea, N.; Niemeyer, H. Host plant changes produced by the aphid Sipha flava: Consequences for aphid feeding behaviour and growth. Entomol. Exp. Appl. 2002, 103, 107–113. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.M.; Wang, H.Y.; Khajuria, C.; Reese, J.C.; Whitworth, R.J.; Welti, R.; Chen, M.S. Rapid Mobilization of Membrane Lipids in Wheat Leaf Sheaths during Incompatible Interactions with Hessian Fly. Mol. Plant Microbe Interact. 2012, 25, 920–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Horst, D.J.; van Doorn, J.M.; Passier, P.C.C.M.; Vork, M.M.; Glatz, J.F.C. Role of fatty acid-binding protein in lipid metabolism of insect flight muscle. Mol. Cell. Biochem. 1993, 123, 145–152. [Google Scholar] [CrossRef]
| Compound No. | Leaf VOC | VOC Class | Compound No. | Leaf VOC | VOC Class |
|---|---|---|---|---|---|
| 1 | α-Thujene | Monoterpene | 23 | Thymol, ME | Terpene alcohol |
| 2 | α-Pinene | Monoterpene | 24 | Thymoquinone | Other |
| 3 | Sabinene | Monoterpene | 25 | m-Thymol | Terpene alcohol |
| 4 | β-Pinene | Monoterpene | 26 | Unk -ol m/z 69/154A | Terpene alcohol |
| 5 | β-Myrcene | Monoterpene | 27 | Unk -ol m/z 69/154B | Terpene alcohol |
| 6 | α-Terpinene | Monoterpene | 28 | Unk -ol m/z 69/154C | Terpene alcohol |
| 7 | p-Cymene | Monoterpene | 29 | Chavicol | Terpene alcohol |
| 8 | D-limonene | Monoterpene | 30 | Geranyl acetate | Monoterpene ester |
| 9 | β-ocimene | Monoterpene | 31 | β-Elemene | Sesquiterpene |
| 10 | γ-Terpinene | Monoterpene | 32 | β-Caryophyllene | Sesquiterpene |
| 11 | Unk Terpene alc 1 | Terpene alcohol | 33 | α-Humulene | Sesquiterpene |
| 12 | Unk Terpene alc 2 | Terpene alcohol | 34 | Unk m/z 165/180 | Unknown |
| 13 | α-Terpinolene | Monoterpene | 35 | Coumarin | Other |
| 14 | p-Cymenene | Monoterpene | 36 | Ethyl 4-ethoxybenzoate | Other |
| 15 | Linalool | Terpene alcohol | 37 | Calamene | Sesquiterpene |
| 16 | allo-Ocimene | Monoterpene | 38 | β-Sinensal | Aldehyde |
| 17 | p-Mentha-1,5,8-triene | Monoterpene | 39 | Unk m/z 69/125 | Unknown |
| 18 | Terpen-4-ol | Terpene alcohol | 40 | Palmitic acid | Fatty acid |
| 19 | p-Cymen-8-ol | Terpene alcohol | 41 | Palmitic, ME | Fatty acid ester |
| 20 | α-Terpineol | Terpene alcohol | 42 | Phytol | Terpene alcohol |
| 21 | Decanal | Aldehyde | 43 | Stearic acid | Fatty acid |
| 22 | Carveol | Terpene alcohol | 44 | Linolenic acid, ME | Fatty acid ester |
| Cmpd No. | LRI | Leaf VOC | SB/US-897 | SB/C-35 | SB/Sour Orange | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | ACP-Infested | p Value | Fold Change | Control | ACP-Infested | p Value | Fold Change | Control | ACP-Infested | p Value | Fold Change | |||
| 1 | 900 | α-Thujene b | 22.03 ± 7.06 | 23.80 ± 6.08 | 0.566 | 1.08 | 25.98 ± 2.84 | 20.62 ± 1.62 | 0.002 | −1.26 | 21.03 ± 5.44 | 18.40 ± 3.52 | 0.173 | −1.14 |
| 2 | 909 | α-Pinene a | 29.46 ± 9.43 | 49.77 ± 12.71 | 0.001 | 1.69 | 31.01 ± 4.01 | 43.06 ± 3.29 | 0.000 | 1.39 | 34.30 ± 12.22 | 39.44 ± 7.25 | 0.224 | 1.15 |
| 3 | 962 | Sabinene a | 2.88 ± 0.87 | 4.58 ± 1.13 | 0.002 | 1.59 | 2.97 ± 0.45 | 4.06 ± 0.32 | 0.001 | 1.37 | 3.25 ± 1.09 | 3.82 ± 0.67 | 0.139 | 1.17 |
| 4 | 966 | β-Pinene a | 26.25 ± 8.25 | 45.44 ± 11.42 | 0.001 | 1.73 | 26.56 ± 3.84 | 39.37 ± 2.77 | 0.000 | 1.48 | 23.85 ± 14.36 | 34.92 ± 6.86 | 0.025 | 1.46 |
| 5 | 985 | β-Myrcene a | 8.91 ± 2.90 | 12.84 ± 3.12 | 0.011 | 1.44 | 9.25 ± 1.25 | 11.82 ± 0.86 | 0.002 | 1.28 | 9.56 ± 3.09 | 10.11 ± 2.10 | 0.620 | 1.06 |
| 6 | 1018 | α-Terpinene a | 1.87 ± 1.12 | 4.64 ± 2.24 | 0.004 | 2.49 | 2.30 ± 0.55 | 5.16 ± 0.75 | 0.000 | 2.24 | 2.59 ± 1.64 | 4.37 ± 1.18 | 0.006 | 1.69 |
| 7 | 1026 | p-Cymene a | 38.37 ± 8.88 | 66.19 ± 10.64 | 0.000 | 1.72 | 34.81 ± 4.73 | 51.83 ± 6.33 | 0.000 | 1.49 | 38.48 ± 10.83 | 44.50 ± 9.73 | 0.166 | 1.16 |
| 8 | 1034 | D-limonene a | 14.41 ± 4.47 | 21.56 ± 5.33 | 0.006 | 1.50 | 16.04 ± 3.33 | 17.61 ± 1.34 | 0.311 | 1.10 | 15.24 ± 6.12 | 16.61 ± 3.02 | 0.494 | 1.09 |
| 9 | 1055 | β-ocimene a | 41.10 ± 17.25 | 69.27 ± 14.30 | 0.001 | 1.69 | 38.01 ± 9.09 | 56.24 ± 7.14 | 0.003 | 1.48 | 41.76 ± 13.97 | 50.99 ± 10.09 | 0.077 | 1.22 |
| 10 | 1071 | γ-Terpinene a | 93.61 ± 24.92 | 145.50 ± 32.00 | 0.001 | 1.55 | 98.47 ± 12.75 | 127.78 ± 9.42 | 0.001 | 1.30 | 102.45 ± 33.20 | 115.82 ± 19.63 | 0.243 | 1.13 |
| 11 | 1094 | Terpene alc 1 c | 3.60 ± 0.47 | 2.56 ± 0.38 | 0.000 | −1.41 | 4.01 ± 0.21 | 5.32 ± 0.18 | 0.000 | 1.32 | 3.56 ± 0.88 | 5.56 ± 0.31 | 0.000 | 1.56 |
| 12 | 1099 | Terpene alc 2 c | 2.21 ± 0.28 | 1.58 ± 0.22 | 0.000 | −1.40 | 2.49 ± 0.11 | 3.25 ± 0.11 | 0.000 | 1.30 | 2.19 ± 0.55 | 3.45 ± 0.17 | 0.000 | 1.58 |
| 13 | 1101 | α-Terpinolene a | 7.67 ± 3.02 | 15.96 ± 4.58 | 0.000 | 2.08 | 8.84 ± 1.37 | 14.36 ± 1.10 | 0.000 | 1.62 | 8.68 ± 3.61 | 12.94 ± 2.42 | 0.003 | 1.49 |
| 14 | 1110 | p-Cymenene b | 9.58 ± 1.22 | 10.19 ± 2.71 | 0.543 | 1.06 | 10.90 ± 1.04 | 11.46 ± 2.10 | 0.569 | 1.05 | 9.95 ± 3.32 | 13.83 ± 2.27 | 0.003 | 1.39 |
| 15 | 1114 | Linalool a | 184.35 ± 35.17 | 296.81 ± 68.44 | 0.000 | 1.61 | 202.80 ± 22.99 | 219.40 ± 47.27 | 0.457 | 1.08 | 218.88 ± 76.07 | 235.18 ± 40.42 | 0.519 | 1.07 |
| 16 | 1160 | allo-Ocimene b | 0.74 ± 0.21 | 0.61 ± 0.16 | 0.168 | −1.20 | 0.81 ± 0.18 | 0.67 ± 0.15 | 0.179 | −1.21 | 1.22 ± 0.48 | 0.76 ± 0.23 | 0.006 | −1.61 |
| 17 | 1172 | p-Mentha-1,5,8-triene b | 2.29 ± 0.68 | 3.27 ± 0.74 | 0.008 | 1.43 | 2.60 ± 0.49 | 2.82 ± 0.31 | 0.384 | 1.08 | 2.66 ± 0.92 | 3.03 ± 0.54 | 0.237 | 1.14 |
| 18 | 1225 | Terpen-4-ol a | 0.44 ± 0.52 | 1.16 ± 0.18 | 0.001 | 2.66 | 0.61 ± 0.48 | 0.93 ± 0.24 | 0.165 | 1.54 | 0.34 ± 0.26 | 1.16 ± 0.25 | 0.000 | 3.39 |
| 19 | 1224 | p-Cymen-8-ol a | 0.29 ± 0.21 | 0.70 ± 0.20 | 0.000 | 2.41 | 0.34 ± 0.17 | 0.59 ± 0.21 | 0.047 | 1.75 | 0.50 ± 0.46 | 0.81 ± 0.23 | 0.050 | 1.62 |
| 20 | 1236 | α-Terpineol a | 2.50 ± 0.59 | 3.69 ± 0.97 | 0.006 | 1.47 | 2.84 ± 0.58 | 3.12 ± 0.58 | 0.426 | 1.10 | 3.16 ± 0.78 | 3.90 ± 0.78 | 0.030 | 1.23 |
| 21 | 1243 | Decanal a | 2.64 ± 1.07 | 3.23 ± 0.58 | 0.147 | 1.22 | 2.54 ± 0.24 | 3.62 ± 0.47 | 0.001 | 1.42 | 2.59 ± 0.87 | 3.71 ± 0.80 | 0.004 | 1.43 |
| 22 | 1259 | Carveol a | 0.29 ± 0.32 | 0.47 ± 0.19 | 0.150 | 1.62 | 0.40 ± 0.13 | 0.48 ± 0.26 | 0.537 | 1.19 | 0.36 ± 0.26 | 0.53 ± 0.25 | 0.127 | 1.45 |
| 23 | 1268 | Thymol, Me a | 0.64 ± 0.12 | 0.77 ± 0.09 | 0.012 | 1.21 | 0.61 ± 0.05 | 0.90 ± 0.10 | 0.000 | 1.49 | 0.23 ± 0.30 | 0.89 ± 0.11 | 0.000 | 3.91 |
| 24 | 1250 | Thymoquinone b | 0.28 ± 0.09 | 0.52 ± 0.11 | 0.000 | 1.86 | 0.57 ± 0.27 | 0.88 ± 0.15 | 0.033 | 1.55 | 0.26 ± 0.09 | 0.94 ± 0.17 | 0.000 | 3.57 |
| 25 | 1324 | m-Thymol a | 65.70 ± 12.55 | 95.31 ± 14.30 | 0.000 | 1.45 | 71.27 ± 7.29 | 89.72 ± 8.48 | 0.002 | 1.26 | 75.99 ± 18.96 | 97.42 ± 15.94 | 0.007 | 1.28 |
| 26 | 1336 | Unk -ol 69/154A c | 3.29 ± 2.63 | ND | NA | ∞ | 2.86 ± 3.16 | 5.84 ± 0.83 | 0.049 | 2.04 | 4.37 ± 1.75 | 8.22 ± 1.29 | 0.000 | 1.88 |
| 27 | 1342 | Unk -ol 69/154B c | 4.03 ± 0.77 | 2.39 ± 0.83 | 0.000 | −1.69 | 4.91 ± 0.32 | 5.05 ± 0.48 | 0.585 | 1.03 | 3.99 ± 1.16 | 6.51 ± 0.42 | 0.000 | 1.63 |
| 28 | 1347 | Unk -ol 69/154C c | 2.47 ± 0.50 | 1.51 ± 0.61 | 0.002 | −1.63 | 2.96 ± 0.05 | 3.27 ± 0.37 | 0.064 | 1.11 | 2.42 ± 0.74 | 4.33 ± 0.52 | 0.000 | 1.79 |
| 29 | 1378 | Chavicol a | 1.40 ± 0.61 | 1.53 ± 0.68 | 0.659 | 1.10 | 1.74 ± 0.34 | 1.78 ± 0.13 | 0.806 | 1.02 | 2.13 ± 0.80 | 1.38 ± 0.37 | 0.007 | −1.55 |
| 30 | 1385 | Geranyl acetate a | 1.35 ± 0.49 | 0.45 ± 0.11 | 0.000 | −3.01 | 0.42 ± 0.07 | 0.45 ± 0.13 | 0.640 | 1.07 | 1.55 ± 0.58 | 0.65 ± 0.14 | 0.000 | −2.40 |
| 31 | 1396 | β-Elemene a | 12.04 ± 3.95 | 16.50 ± 4.09 | 0.028 | 1.37 | 16.12 ± 1.25 | 18.91 ± 2.90 | 0.056 | 1.17 | 14.40 ± 4.95 | 29.46 ± 5.17 | 0.000 | 2.05 |
| 32 | 1461 | β-Caryophyllene a | 10.78 ± 3.79 | 13.90 ± 2.72 | 0.053 | 1.29 | 12.38 ± 1.01 | 16.10 ± 1.91 | 0.002 | 1.30 | 11.52 ± 4.05 | 18.90 ± 7.90 | 0.009 | 1.64 |
| 33 | 1512 | α-Humulene a | 4.20 ± 1.81 | 3.85 ± 0.89 | 0.586 | −1.09 | 6.01 ± 0.58 | 4.67 ± 0.68 | 0.004 | −1.29 | 4.93 ± 1.22 | 7.99 ± 1.63 | 0.000 | 1.62 |
| 34 | 1525 | Unk m/z 165/180 c | 1.59 ± 0.49 | 1.61 ± 0.37 | 0.954 | 1.01 | 1.96 ± 0.31 | 1.71 ± 0.35 | 0.232 | −1.14 | 1.79 ± 0.60 | 3.01 ± 0.75 | 0.000 | 1.69 |
| 35 | 1534 | Coumarin b | 17.47 ± 2.50 | 11.98 ± 2.63 | 0.000 | −1.46 | 16.27 ± 1.29 | 21.67 ± 3.79 | 0.008 | 1.33 | 19.26 ± 3.88 | 26.77 ± 4.11 | 0.000 | 1.39 |
| 36 | 1554 | Ethyl 4-ethoxybenzoate b | 17.18 ± 2.21 | 11.86 ± 2.78 | 0.000 | −1.45 | 16.42 ± 1.33 | 19.97 ± 3.43 | 0.039 | 1.22 | 15.96 ± 8.07 | 26.26 ± 3.51 | 0.001 | 1.65 |
| 37 | 1724 | Calamene b | 1.22 ± 0.26 | 0.91 ± 0.31 | 0.031 | −1.35 | 1.22 ± 0.11 | 1.29 ± 0.32 | 0.608 | 1.06 | 1.68 ± 0.37 | 1.67 ± 0.82 | 0.974 | −1.01 |
| 38 | 1734 | β-Sinensal a | 4.23 ± 6.40 | 4.94 ± 3.86 | 0.767 | 1.17 | 9.28 ± 2.38 | 8.47 ± 1.30 | 0.481 | −1.10 | 15.40 ± 2.96 | 12.14 ± 4.42 | 0.045 | −1.27 |
| 39 | 1746 | Unk m/z 69/125 c | 8.01 ± 3.41 | 1.89 ± 0.65 | 0.000 | −4.24 | 0.21 ± 0.33 | 2.69 ± 0.40 | 0.000 | 12.69 | 0.47 ± 0.12 | 3.39 ± 1.12 | 0.000 | 7.28 |
| 40 | 1967 | Palmitic acid a | 62.38 ± 32.71 | 0.19 ± 0.22 | 0.000 | −329.73 | 72.34 ± 7.33 | 0.18 ± 0.43 | 0.000 | −409.03 | 63.44 ± 46.08 | 0.95 ± 0.66 | 0.000 | −66.80 |
| 41 | 2000 | Palmitic, ME a | 0.71 ± 0.49 | 3.69 ± 1.30 | 0.000 | 5.15 | 1.45 ± 0.31 | 6.34 ± 2.39 | 0.001 | 4.37 | 1.46 ± 2.57 | 8.62 ± 2.91 | 0.000 | 5.90 |
| 42 | 2075 | Phytol a | 39.69 ± 16.43 | 29.36 ± 30.49 | 0.379 | −1.35 | 56.24 ± 12.13 | 44.53 ± 17.15 | 0.202 | −1.26 | 42.51 ± 19.75 | 98.62 ± 26.98 | 0.000 | 2.32 |
| 43 | 2088 | Stearic acid a | 0.41 ± 1.22 | 1.24 ± 1.98 | 0.292 | 3.06 | ND | 2.75 ± 3.07 | NA | ∞ | 1.34 ± 2.23 | 2.51 ± 3.89 | 0.374 | 1.88 |
| 44 | 2094 | Linolenic acid, ME a | 95.70 ± 53.18 | ND | NA | ∞ | 95.96 ± 23.47 | ND | NA | ∞ | 78.46 ± 54.65 | ND | NA | ∞ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, S.E.; Killiny, N. Influence of Rootstock on the Leaf Volatile Organic Compounds of Citrus Scion Is More Pronounced after the Infestation with Diaphorina citri. Plants 2021, 10, 2422. https://doi.org/10.3390/plants10112422
Jones SE, Killiny N. Influence of Rootstock on the Leaf Volatile Organic Compounds of Citrus Scion Is More Pronounced after the Infestation with Diaphorina citri. Plants. 2021; 10(11):2422. https://doi.org/10.3390/plants10112422
Chicago/Turabian StyleJones, Shelley E., and Nabil Killiny. 2021. "Influence of Rootstock on the Leaf Volatile Organic Compounds of Citrus Scion Is More Pronounced after the Infestation with Diaphorina citri" Plants 10, no. 11: 2422. https://doi.org/10.3390/plants10112422
APA StyleJones, S. E., & Killiny, N. (2021). Influence of Rootstock on the Leaf Volatile Organic Compounds of Citrus Scion Is More Pronounced after the Infestation with Diaphorina citri. Plants, 10(11), 2422. https://doi.org/10.3390/plants10112422







