Spermidine Suppressed the Inhibitory Effects of Polyamines Inhibitors Combination in Maize (Zea mays L.) Seedlings under Chilling Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Condition
- (1)
- 10 µM Co. (10 µM D-Arg, 10 µM DFMO, 10 µM MGBG and 100 µM Ag);
- (2)
- 100 µM Co. (100 µM D-Arg, 100 µM DFMO, 100 µM MGBG and 1000 µM Ag);
- (3)
- 500 µM Co. (500 µM D-Arg, 200 µM DFMO, 500 µM MGBG and 5 mM Ag);
- (4)
- 1000 µM Co. (1000 µM D-Arg, 200 µM DFMO, 1000 µM MGBG and 10 mM Ag);
- (5)
- Spd + 1000 µM Co. (1 mM Spd + 1000 µM D-Arg, 200 µM DFMO, 1000 µM MGBG, 10 mM Ag) and the control of seedlings were without any treatment of polyamine inhibitors.
2.2. Polyamine Assay
2.3. Polyamine Biosynthesis Enzymes under Chilling Stress
2.4. Polyamines’ Degradative Enzymes under Chilling Stress
2.5. Transcript Levels of PA Biosynthesis Genes
2.6. Statistical Analysis
3. Results
3.1. Polyamine Concentration in Roots of Maize Seedlings
3.2. Polyamine Concentration in Mesocotyl of Maize Seedlings
3.3. Polyamine Concentrations in Coleoptile of Maize Seedlings
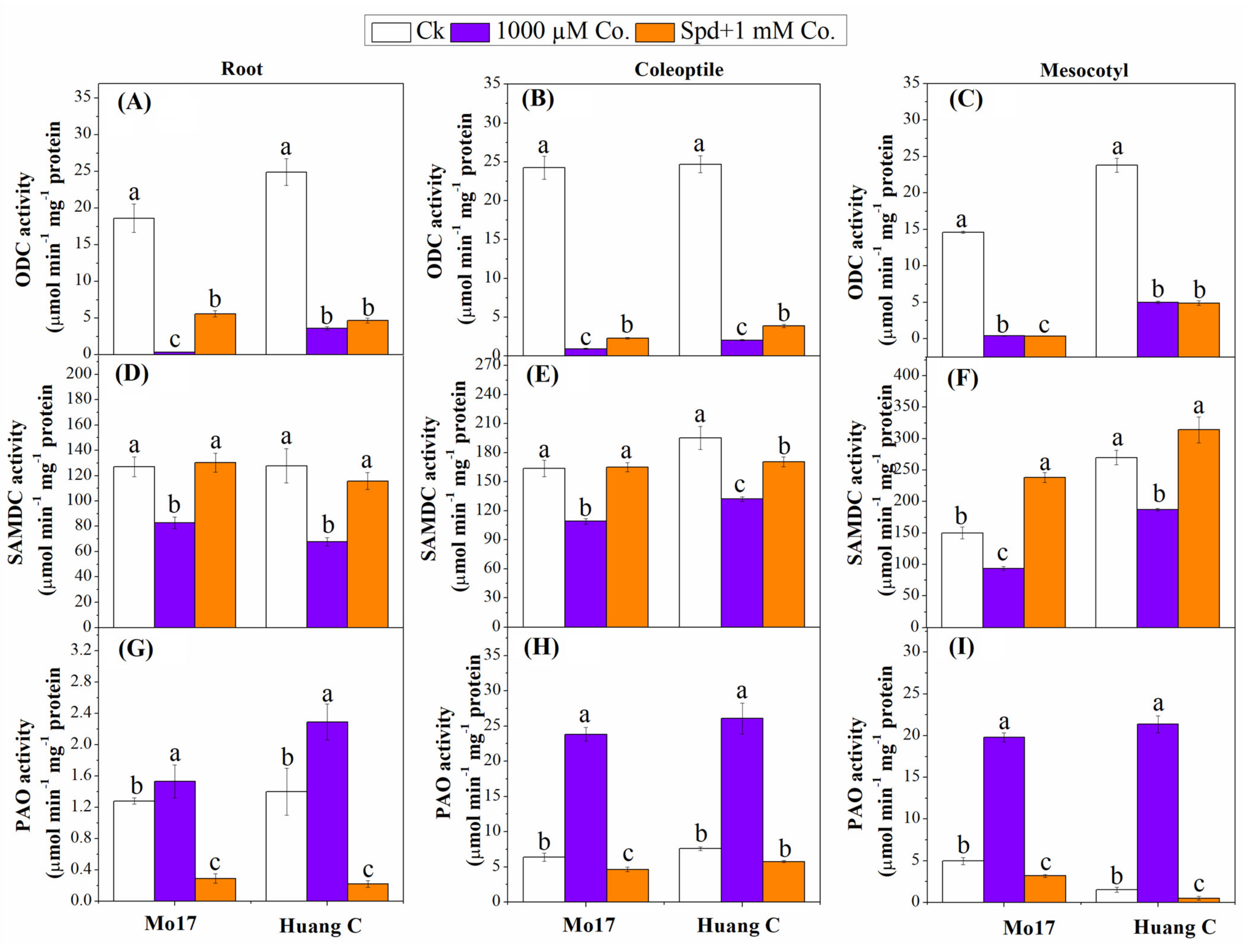
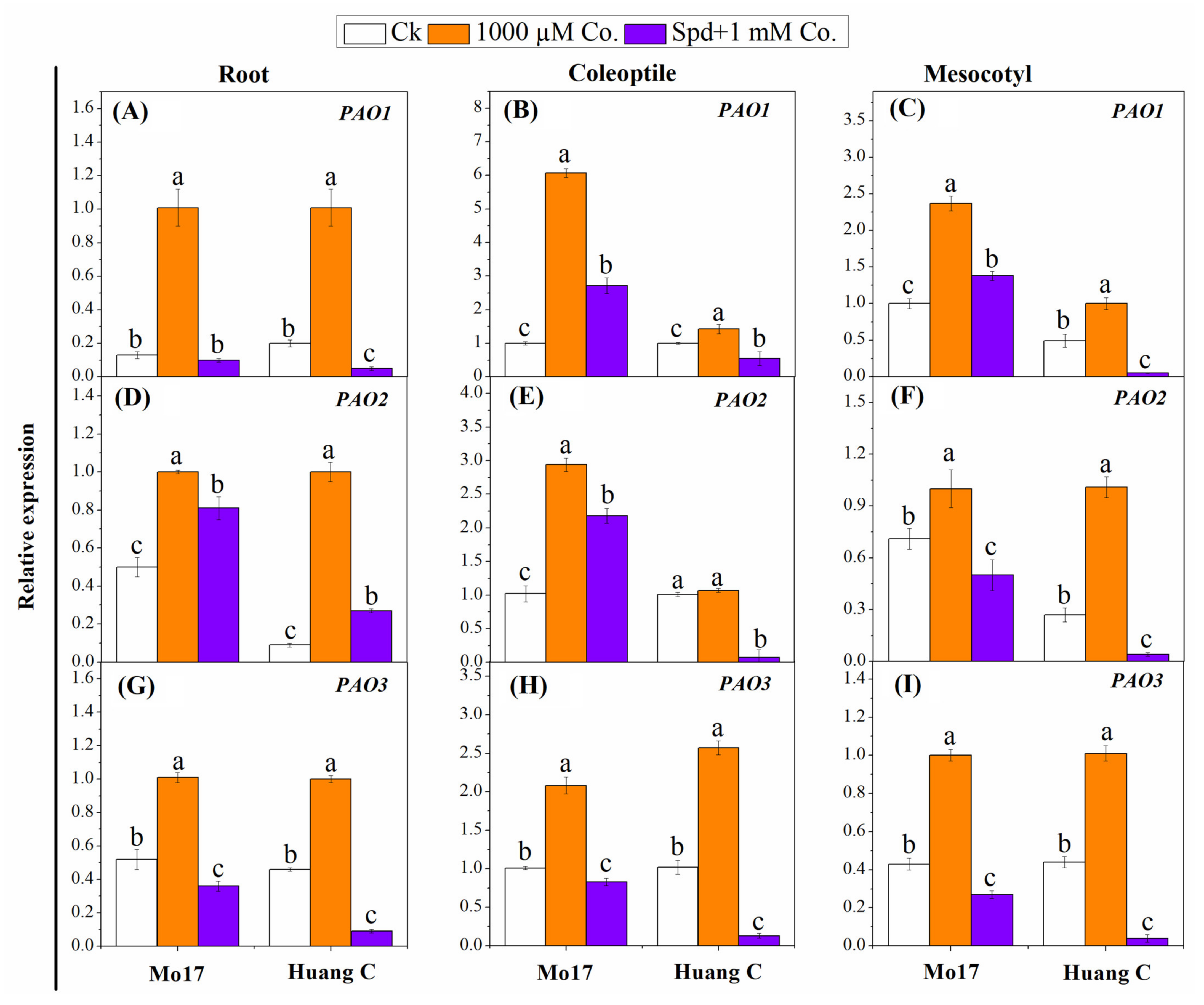
3.4. Polyamine Biosynthetic and Degradative Enzymes under Chilling Stress
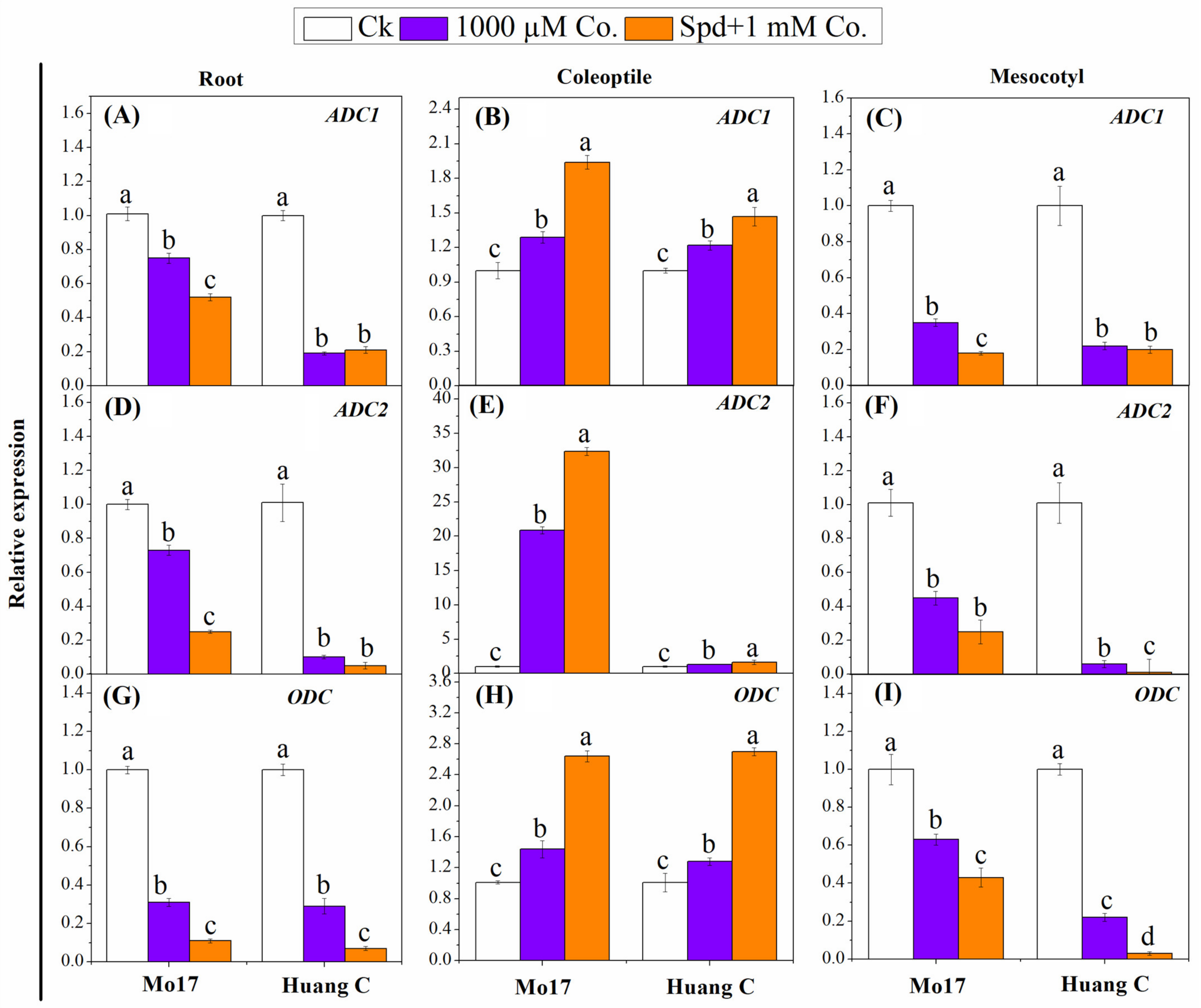
3.5. Polyamines Biosynthesis Genes in Response to PA Inhibitors under Chilling Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sheteiwy, M.S.; AbdElgawad, H.; Xiong, Y.C.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, H.; Hamoud, Y.A.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol. Plant. 2021, 172, 2153–2169. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Daly, P.; Sharma, A.; Shaghaleh, H.; Hamoud, Y.A.; El-Esawi, M.A.; Pan, R.; Wan, Q.; et al. Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine max L.) seedlings. J. Sci. Food Agri. 2021, 101, 2027–2041. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Ali, B.; El-Keblawy, A.; Ksiksi, T.; El-Esawi, M.A.; Jośko, I.; Ulhassan, Z.; Sheteiwy, M.S. Effect of Source–Sink Ratio Manipulation on Growth, Flowering, and Yield Potential of Soybean. Agriculture 2021, 11, 926. [Google Scholar] [CrossRef]
- Yang, S.; Ulhassan, Z.; Shah, A.M.; Khan, A.R.; Azhar, W.; Hamid, Y.; Hussain, S.; Sheteiwy, M.S.; Salam, A.; Zhou, W. Salicylic acid underpins silicon in ameliorating chromium toxicity in rice by modulating antioxidant defense, ion homeostasis and cellular ultrastructure. Plant Physiol. Biochem. 2021, 166, 1001–1013. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Gill, R.A.; Huang, H.; Ali, S.; Mwamba, T.M.; Ali, B.; Huang, Q.; Hamid, Y.; Khan, A.R.; Wang, J.; et al. Selenium mitigates the chromium toxicity in Brassicca napus L. by ameliorating nutrients uptake, amino acids metabolism and antioxidant defense system. Plant Physiol. Biochem. 2019, 145, 142–152. [Google Scholar] [CrossRef]
- Allen, D.J.; Ort, D.R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef]
- Hola, D.; Kocova, M.; Rothova, O.; Wilhelmova, N.; Benesova, M. Recovery of maize (Zea mays L.) inbreds and hybrids for chilling stress of various duration: Photosynthesis and antioxidant enzymes. J. Plant Physiol. 2007, 164, 868–877. [Google Scholar] [CrossRef]
- He, F.; Shen, H.; Lin, C.; Fu, H.; Sheteiwy, M.S.; Guan, Y.; Huang, Y.; Hu, J. Transcriptome analysis of chilling-imbibed embryo revealed membrane recovery related genes in maize. Front. Plant Sci. 2017, 7, 1978. [Google Scholar] [CrossRef]
- Farooq, M.; Aziz, T.; Basra, S.M.A.; Wahid, A.; Khaliq, A.; Cheema, M.A. Exploring the role of calcium to improve chilling tolerance in hybrid maize. J. Agron. Crop Sci. 2008, 194, 350–359. [Google Scholar] [CrossRef]
- Guan, Y.J.; Hu, J.; Wang, X.J.; Shao, C.X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef]
- Imran, S.; Afzal, I.; Basra, S.M.A.; Saqib, M. Integrated seed priming with growth promoting substances enhances germination and seedling vigour of spring maize at low temperature. Int. J. Agric. Biol. 2013, 15, 1251–1257. [Google Scholar]
- Ruelland, E.; Vaultier, M.N.; Zachowski, A.; Hurry, V. Cold signallong and cold acclimation in plants. Adv. Bot. Res. 2009, 49, 35–150. [Google Scholar]
- Bano, S.; Aslam, M.; Saleem, M.; Basra, S.M.A.; Aziz, K. Evaluation of maize accessions under low temperature stress at early growth stages. J. Anim. Plant Sci. 2015, 25, 392–400. [Google Scholar]
- Sowinski, P.; Rudzinska-Langwald, A.; Adamczyk, J.; Kubica, I.; Fronk, J. Recovery of maize seedlings growth, development and photosynthetic efficiency after initial growth at low temperature. J. Plant Physiol. 2005, 162, 67–80. [Google Scholar] [CrossRef]
- Aroca, R.; Irigoyen, J.J.; Sanchez-Diaz, M. Drought enhances maize chilling tolerance. II. Photosynthetic traits and protective mechanisms against oxidative stress. Physiol. Plant. 2003, 117, 540–549. [Google Scholar] [CrossRef]
- Haldimann, P. How do changes in temperature during growth affect leaf pigment composition and photosynthesis in Zea mays genotypes differing in sensitivity to low temperature? J. Exp. Bot. 1999, 50, 543–550. [Google Scholar] [CrossRef]
- Pinhero, R.G.; Rao, M.V.; Paliyath, G.; Murr, D.P.; Fletcher, R.A. Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings. Plant Physiol. 1997, 114, 695–704. [Google Scholar] [CrossRef]
- Foyer, C.H.; Vanacker, H.; Gornez, L.D.; Harbinson, J. Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: Review. Plant Physiol. Biochem. 2002, 40, 659–668. [Google Scholar] [CrossRef]
- Skrudlik, G.; Baczek-Kwinta, R.; Koscielniak, J. The effect of short warm breaks during chilling on photosynthesis and the activity of antioxidant enzymes in plants sensitive to chilling. J. Agron. Crop. Sci. 2000, 184, 233–240. [Google Scholar] [CrossRef]
- Nayyar, H.; Chander, S. Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J. Agron. Crop. Sci. 2004, 190, 355–365. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Cuevas, J.C.; Chiesa, M.A.; Ruiz, O.A. Free spermidine and spermine content in Lotus glaber under long-term salt stress. Plant Sci. 2005, 168, 541–546. [Google Scholar] [CrossRef]
- Takahashi, T.; Kakehi, J.I. Polyamines: Ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 2010, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tabor, C.W.; Tabor, H. Polyamines. Annu. Rev. Biochem. 1984, 53, 749–790. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Polyamines: Mysterious modulators of cellular functions. Bioch. Biophys. Res. Commun. 2000, 271, 559–564. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Jing, W.; Liu, Y.; Zhang, W. Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 2008, 59, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Schuber, F. Influence of polyamines on membrane functions. Biochem. J. 1989, 260, 1–10. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Basu, S.; Sengupta, D.N. Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J. Plant Physiol. 2011, 168, 317–328. [Google Scholar] [CrossRef]
- Amini, S.; Maali-Amiri, R.; Kazemi-Shahandashti, S.; Miguel, L.; Sadeghzadeh, B.; Sobhani-Najafabadi, A.; Kariman, K. Effect of cold stress on polyamine metabolism and antioxidant responses in chickpea. J. Plant Physiol. 2021, 258–259, 153387. [Google Scholar] [CrossRef]
- Bouchereau, A.; Aziz, A.; Larher, F.; Martin-Tanguy, J. Polyamines and environmental challenges: Recent development. Plant Sci. 1999, 140, 103–125. [Google Scholar] [CrossRef]
- Besford, R.T.; Richardson, C.M.; Campos, J.L.; Tiburcio, A.F. Effect of polyamines on stabilization of molecular complexes in thylakoid membranes of osmotically stressed oat leaves. Planta 1993, 189, 201–206. [Google Scholar] [CrossRef]
- Kalia, V.C.; Gong, C.; Patel, S.K.S.; Lee, J.-K. Regulation of Plant Mineral Nutrition by Signal Molecules. Microorganisms 2021, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Guo, S.R.; Sun, J.; Yuan, L.Y. Effects of salt stress on the structure and function of the photosynthetic apparatus in Cucumis sativus and its protection by exogenous putrescine. Physiol. Plant. 2012, 146, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Yoshida, N.; Fujita, M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glycoxalase system. Plant Growth Regul. 2014, 73, 31–44. [Google Scholar] [CrossRef]
- Yamamoto, A.; Shim, I.S.; Fujihara, S. Chilling-stress responses by rice seedlings grown with different ammonium concentration and its relationship to leaf spermidine content. J. Plant Biol. 2012, 55, 191–197. [Google Scholar] [CrossRef]
- Kasukabe, Y.; He, L.; Nada, K.; Misawa, S.; Iharu, I.; Tachibana, S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stress and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 712–722. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, C.; He, F.; Li, Z.; Guan, Y.; Hu, Q.; Hu, J. Exogenous spermidine improves seed germination of sweet corn via involvement in phytohormone interactions, H2O2 and relevant gene expression. BMC Plant Biol. 2017, 17, 1. [Google Scholar] [CrossRef]
- Du, H.Y.; Wang, J.; Liu, H.P.; Yang, Q.H. Effects of seed soaking with spermidine on the germination of maize seeds. J. Anhui Agric. Sci. 2007, 35, 11009–11010. [Google Scholar]
- Xin, S.Q.; Gao, Y.; Zhao, J.M.; Liu, X.M. Effect of seed soaking in spermidine (Spd) under salt stress on rice seed germination. North Rice 2010, 40, 23–25. [Google Scholar]
- Sheteiwy, M.S.; Shen, H.; Xu, J.; Guan, Y.; Song, W.; Hu, J. Seed polyamines metabolism induced by seed priming with Spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ. Exp. Bot. 2017, 137, 58–72. [Google Scholar] [CrossRef]
- Galston, A.W.; Sawhney, R.K. Polyamines in plant physiology. Plant Physiol. 1990, 94, 406–410. [Google Scholar] [CrossRef]
- Bezold, T.N.; Loy, J.B.; Minocha, S.C. Changes in the cellular content of polyamines in different tissues of seed and fruit of a normal and a hull-less seed variety of pumpkin during development. Plant Sci. 2003, 164, 743–752. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Belle, N.A.; Dalmolin, G.D.; Fonini, G.; Rubin, M.A.; Rocha, J.B. Polyamines reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res. 2004, 2, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Wu, J.; Cona, A.; Tavladoraki, P.; Angelini, R.; Roubelakis, K.A. The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J. Exp. Bot. 2012, 63, 5003–5015. [Google Scholar] [CrossRef]
- Arun, M.; Chinnathambi, A.; Subramanyam, K.; Karthik, S.; Sivanandhan, G.; Theboral, J.; Alharbi, S.A.; Kim, C.K.; Ganapathi, A. Involvement of exogenous polyamines enhances regeneration and Agrobacterium-mediated genetic transformation in half-seeds of soybean. 3 Biotech 2016, 6, 148. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Gupta, S.; Sengupta, D.N.; Ghosh, B. Expression of arginine decarboxylase in seedlings of indica rice (Oryza sativa L.) cultivars as affected by salinity stress. Plant Mol. Biol. 1997, 34, 477–493. [Google Scholar] [CrossRef]
- Mo, H.; Pua, E.C. Up-regulation of arginine decarboxylase gene expression and accumulation of polyamines in mustard (Brassica juncea) in response to stress. Physiol. Plant. 2002, 114, 439–449. [Google Scholar] [CrossRef]
- Hao, Y.J.; Kitashiba, H.; Honda, C.; Nada, K.; Moriguchi, T. Expression of arginine decarboxylase and ornithine decarboxylase genes in apple cells and stressed shoots. J. Exp. Bot. 2005, 56, 1105–1115. [Google Scholar] [CrossRef]
- Legocka, J.; Kluk, A. Effect of salt and osmotic stress on changes in polyamine content and arginine decarboxylase activity in Lupinus luteus seedlings. J. Plant Physiol. 2005, 162, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Nada, K.; Honda, C.; Kitashiba, H.; Wen, X.P.; Pang, X.M.; Moriguchi, T. Polyamine biosynthesis of apple callus under salt stress: Importance of the arginine decarboxylase pathway in stress response. J. Exp. Bot. 2006, 57, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Chen, S.Y. Differential accumulation of the S-adenosylmethionine decarboxylase transcript in rice seedlings in response to salt and drought stresses. Theor. Appl. Genet. 2000, 100, 782–788. [Google Scholar] [CrossRef]
- Tian, A.G.; Zhao, J.Y.; Zhang, J.S.; Gai, J.Y.; Chen, S.Y. Genomic characterization of the S-adenosylmethionine decarboxylase genes from soybean. Theor. Appl. Genet. 2004, 108, 842–850. [Google Scholar] [CrossRef]
- Li, Z.Y.; Chen, S.Y. Isolation and characterization of a salt and drought-inducible gene for S-adenosylmethionine decarboxylase from wheat (Triticum aestivum L.). J. Plant Physiol. 2000, 156, 386–393. [Google Scholar] [CrossRef]
- Urano, K.; Yoshiba, Y.; Nanjo, T.; Igarashi, Y.; Seki, M.; Sekiguchi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Characterization of Arabidopsis genes involved in biosynthesis of polyamines in abiotic stress responses and developmental stages. Plant Cell Environ. 2003, 26, 1917–1926. [Google Scholar] [CrossRef]
- Hao, Y.J.; Zhang, Z.; Kitashiba, H.; Honda, C.; Ubi, B.; Kita, M.; Moriguchi, T. Molecular cloning and functional characterization of two apple S-adenosylmethionine decarboxylase genes and their different expression in fruit development, cell growth and stress responses. Gene 2005, 350, 41–50. [Google Scholar] [CrossRef]
- Rodríguez-Kessler, M.; Alpuche-Solísm, A.; Ruiz, O.; Jiménez-Bremont, J. Effect of salt stress on the regulation of maize (Zea mays L.) genes involved in polyamine biosynthesis. Plant Growth Regul. 2006, 48, 175–185. [Google Scholar] [CrossRef]
- Seiler, N. Catabolism of polyamines: Review Article. Amino Acids 2004, 26, 217–233. [Google Scholar] [CrossRef]
- Marocco, A.; Lorenzoni, C.; Fracheboud, Y. Chilling stress in maize. Maydica 2004, 50, 571–580. [Google Scholar]
- Wallace, H.M.; Fraser, A.V. Inhibitors of polyamine metabolism: Review article. Amino Acids 2004, 26, 353–365. [Google Scholar] [CrossRef]
- Gao, C.H.; Hu, J.; Zhang, S.; Zheng, Y.Y.; Knapp, A. Association of polyamines in governing the chilling sensitivity of maize genotypes. Plant Growth Regul. 2009, 57, 31–38. [Google Scholar] [CrossRef]
- Joseph, M.; Tomaso, D.; Jonathan, J.H.; Leon, V.K. Transport kinetics and metabolism of exogenously applied putrescine in roots of intact maize seedlings. Plant Physiol. 1992, 98, 611–620. [Google Scholar]
- Lee, T.M.; Lur, H.S.; Chu, C. Role of abscisic acid in chilling tolerance of rice (Oryza sativa L.) seedlings. Modulation of free polyamine levels. Plant Sci. 1997, 126, 1–10. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 3, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Hamoud, Y.A.; Shaghaleh, H.; Khan, N.U.; Yang, R.; Tang, B. GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int. J. Mol. Sci. 2019, 20, 5709. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.H.; Du, W.K.; Song, H.N.; Shao, H.B.; Qi, W.C.; Sheteiwy, M.S.A.; Yu, D.Y. Identification of responsive miRNAs involved in combination stresses of phosphate starvation and salt stress in soybean root. Environ. Exp. Bot. 2019, 167, 103823. [Google Scholar] [CrossRef]
- Gao, C.; Sheteiwy, M.S.; Han, J.; Dong, Z.; Pan, R.; Guan, Y.; Alhaj Hamoud, Y.; Hu, J. Polyamine biosynthetic pathways and their relation with the cold tolerance of maize (Zea mays L.) seedlings. Plant Signal. Behav. 2020, 15, 1807722. [Google Scholar] [CrossRef]
- Pang, X.; Zang, Z.; Wen, X.; Ban, Y.; Moriguchi, T. Polyamines all-purpose players in response to environment stress in plants. Plant Stress 2007, 1, 173–188. [Google Scholar]
- Hummel, I.; Couée, I.; El Amrani, A.; Martin-Tanguy, J.; Hennion, F. Involvement of polyamines in root development at low temperature in the subantarctic cruciferous speciecs Pringlea antiscorbutica. J. Exp. Bot. 2002, 53, 1463–1473. [Google Scholar] [CrossRef][Green Version]
- Alcazar, R.; Marco, F.; Cuevas, J.C.; Patrón, M.; Ferrando, A.; Carrasco, P.; Tiburcio, A.F.; Altabella, T. Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Liu, K.; Wang, Z.; Liu, L. Involvement of polyamines in the drought resistance of rice. J. Exp. Bot. 2007, 58, 1545–1555. [Google Scholar] [CrossRef]
- Torrigiani, P.; Bressanin, D.; Ruiz, K.B.; Tadiello, A.; Trainotti, L.; Bonghi, C.; Ziosi, V.; Costa, G. Spermidine application to young developing peach fruits leads to a slowing down of ripening by impairing ripening-related ethylene and auxin metabolism and signaling. Physiol. Plant. 2012, 146, 86–98. [Google Scholar] [CrossRef]
- Kakkar, R.K.; Sawhney, V.K. Polyamine research in plants—A changing perspective. Physiol. Plant. 2002, 116, 281–292. [Google Scholar] [CrossRef]
- Alcazar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef]
- Krishnamurthy, R.; Bhagwat, K.A. Polyamines as modulators of salt tolerance in rice cultivars. Plant Physiol. 1989, 91, 500–504. [Google Scholar] [CrossRef]
- Santa-Cruz, A.; Perez-Alfocea, F.; Caro, M.; Acosta, M. Polyamines as short-term salt tolerance traits in tomato. Plant Sci. 1998, 138, 9–16. [Google Scholar] [CrossRef]
- Liu, H.P.; Dong, B.H.; Zhang, Y.Y.; Liu, Z.P.; Liu, Y.L. Relationship between osmotic stress and the levels of free, conjugated, and bound polyamines in leaves of wheat seedlings. Plant Sci. 2004, 166, 1261–1267. [Google Scholar] [CrossRef]
- Moschou, P.N.; Paschalidis, K.A.; Delis, I.D.; Andriopoulou, A.H.; Lagiotis, G.D.; Yakoumakis, D.I.; Kalliopi, A.R. Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 2008, 20, 1708–1724. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Zhang, X.Q.; Ma, X.; Huang, L.; Yan, Y. Exogenous spermidine improves seed germination of white clover under water stress via involvement in starch metabolism, antioxidant defenses and relevant gene expression. Molecules 2014, 19, 18003–18024. [Google Scholar] [CrossRef]
- Xu, L.X.; Han, L.B.; Huang, B.R. Antioxidant enzyme activities and gene expression patterns in leaves of Kentucky bluegrass in response to drought and post-drought recovery. J. Am. Soc. Hortic. Sci. 2011, 136, 247–255. [Google Scholar] [CrossRef]
- Duan, J.; Li, J.; Guo, S.; Kang, Y. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J. Plant Physiol. 2008, 165, 1620–1635. [Google Scholar] [CrossRef]
- Tassoni, A.; Buuren, M.V.; Franceschetti, M.; Fornale, S.; Bagni, N. Polyamine content and metabolism in Arabidopsis thaliana and effect of spermidine on plant development. Plant Physiol. Biochem. 2000, 38, 383–393. [Google Scholar] [CrossRef]
- De-Agazio, M.; Zacchini, M.; Federico, R.; Grego, S. Putrescine accumulation in maize roots treated with spermidine: Evidence for spermidine to putrescine conversion. Plant Sci. 1995, 111, 181–185. [Google Scholar] [CrossRef]
- De-Agazio, M.; Grego, S.; Zacchini, M.; De Cesare, F.; Cellai, L.; Rizea-Savu, S.; Silvestro, L. 1-N-Acetylspermidine in roots of maize seedlings. Plant Sci. 1996, 121, 143–149. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Altabella, T.; Borrell, A.; Masgrau, C. Polyamines metabolism and its regulation. Physiol. Plant. 1997, 100, 664–674. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef]
- Soyka, S.; Heyer, A.G. Arabidopsis knockout mutation of ADC2 gene reveals inducibility by osmotic stress. FEBS Lett. 1999, 458, 219–223. [Google Scholar] [CrossRef]
- Do, P.T.; Degenkolbe, T.; Erban, A.; Heyer, A.G.; Kopka, J.; Karine, I.K.; Hincha, D.K.; Ellen, Z. Dissecting rice polyamine metabolism under controlled long-term drought stress. PLoS ONE 2013, 8, e60325. [Google Scholar]
- Fincato, P.; Moschou, P.N.; Spedaletti, V.; Tavazza, R.; Angelini, R.; Federico, R.; Roubelakis-Angelakis, K.A.; Tavladoraki, P. Functional diversity inside the Arabidopsis polyamine oxidase gene family. J. Exp. Bot. 2011, 62, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Yoda, H.; Hiroi, Y.; Sano, H. Polyamine oxidase is one of the key elememts for oxidative burst to induce programmed cell death in tobacco cultured cell. Plant Physiol. 2006, 142, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Kim, D.W.; Watanable, K.; Sasaki, A.; Niitsu, M.; Berberich, T.; Tusano, T.; Takahashi, Y. Constitutively and highly expressed Oryza sativa polyamine oxidase localize in peroxisomes and catalyze polyamine back conversion. Amino Acids 2012, 42, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Cona, A.; Angelini, R.; Polticelli, F.; Federico, R.; Mariottini, P. Barley polyamine oxidase isoform with distinct structural features and subcellular localization. Eur. J. Biochem. 2001, 268, 3816–3830. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Tavladoraki, P.; Agostino, S.D.; Angelini, R.; Federico, R.; Mariottini, P. Isolation and characterization of three polyamine oxidase genes from Zea mays. Plant Physiol. Biochem. 2000, 38, 667–677. [Google Scholar] [CrossRef]

| Locus | Primer Name | Primer Orientation | Sequence (5′–3′) |
|---|---|---|---|
| NC_008332.1 | 18s rRNA | Forward | ACATGCGCCTAAGGAGAAATAG |
| 18s rRNA | Reverse | ACCTCCATGCTCACTGGTACTT | |
| NM_001323076 | ZmADC1 | Forward | GCTACGGCTCAAGGTACCAG |
| ZmADC1 | Reverse | CCGAACTCCACAATGTCCTC | |
| NM_001138726 | ZmADC2 | Forward | GGAGCCACTCATGACCAAAG |
| ZmADC2 | Reverse | CAGGGACCTTGTATTCGTTGA | |
| NM_001148682 | ZmODC | Forward | GCGCCTACTCCACAGGTTC |
| ZmODC | Reverse | CGTAGATCTTAATCTCCGACGTG | |
| NM_001155838 | ZmSPDS | Forward | TGTTCAATTCCATCCCCTAAA |
| ZmSPDS-R | Reverse | TCCACTGAACTGTGTCTGGAA | |
| NM_001112243 | ZmSAMDC2 | Forward | TGTGGCTACTCCATGAATGC |
| ZmSAMDC2 | Reverse | CGTAACTGGCGTAGCTGAAA | |
| NM_001111636 | ZmPAO1 | Forward | CCAGCAGCAGGAGAGGTTAC |
| ZmPAO1 | Reverse | GCGTCAGGGTACTGCTTCTC | |
| NM_001329439 | ZmPAO2 | Forward | CACACACACCATCCGCTATT |
| ZmPAO2 | Reverse | CATCAGCAGCAGCAAGACC | |
| XM_008652490 | ZmPAO3 | Forward | AAAGCCACACACACCATCTG |
| ZmPAO3 | Reverse | CAGCAGCAGCAAGACCTGTA |
| Treatment | Put | Spd | Spm | Total Polyamine | ||||
|---|---|---|---|---|---|---|---|---|
| Mo17 | Huang C | Mo17 | Huang C | Mo17 | Huang C | Mo17 | Huang C | |
| 0 (CK) | 639.5 b | 673.3 b | 178.7 a | 302.9 a | 23.6 a | 13.6 a | 841.8 b | 989.8 b |
| 10 µM Co. | 551.7 c | 559.9 c | 100.9 c | 289.1 c | 17.2 b | 9.8 b | 669.8 c | 858.7 c |
| 100 µM Co. | 311.6 d | 424.8 d | 85.4 c | 282.0 c | 11.8 c | 1.4 d | 408.8 d | 708.1 d |
| 500 µM Co. | 239.6 e | 358.3 e | 92.5 c | 194.7 d | 4.4 d | 0.0 e | 336.4 e | 553.0 e |
| 1000 µM Co. | 243.0 e | 356.4 e | 94.0 c | 155.4 e | 0.0 e | 0.0 e | 337.0 e | 511.8 f |
| Spd + 1 mM Co. | 1269.7 a | 905.6 a | 129.8 b | 312.1 a | 13.5 c | 6.4 c | 1412.9 a | 1224.1 a |
| Treatment | Put | Spd | Spm | Total Polyamines | ||||
|---|---|---|---|---|---|---|---|---|
| Mo17 | Huang C | Mo17 | Huang C | Mo17 | Huang C | Mo17 | Huang C | |
| 0 (CK) | 1369.8 a | 738.4 a | 195.8 a | 245.0 a | 25.0 b | 31.4 a | 1589.6 a | 1018.6 a |
| 10 µM Co. | 953.7 c | 398.9 c | 107.0 d | 127.9 b | 24.5 b | 12.8 b | 1085.2 c | 539.7 c |
| 100 µM Co. | 805.0 d | 400.4 c | 99.5 e | 75.4 d | 13.3 c | 8.8 d | 917.8 d | 484.6 d |
| 500 µM Co. | 781.2 d | 335.0 d | 111.2 c d | 51.4 e | 8.5 d | 3.6 e | 900.9 d | 390.0 e |
| 1000 µM Co. | 790.9 d | 332.4 d | 115.1 c | 39.1 f | 3.2 e | 2.10 f | 906.0 d | 371.5 e |
| Spd + 1 mM Co. | 1166.0 b | 444.3 b | 161.5 b | 117.6 c | 41.4 a | 11.5 c | 1368.9 b | 573.3 b |
| Treatment | Put | Spd | Spm | Total Polyamines | ||||
|---|---|---|---|---|---|---|---|---|
| Mo17 | Huang C | Mo17 | Huang C | Mo17 | Huang C | Mo17 | Huang C | |
| 0 (CK) | 2044.7 a | 932.9 a | 535.3 a | 619.9 a | 80.5 a | 81.2 a | 2660.5 a | 1634.0 a |
| 10 µM Co. | 620.5 c | 319.7 c | 439.4 b | 464.4 b | 51.4 b | 16.7 c | 1111.3 b | 800.6 b |
| 100 µM Co. | 604.3 c | 326.4 c | 338.4 c d | 390.8 c | 43.4 c | 12.9 d | 986.1 c | 730.1 c |
| 500 µM Co. | 556.2 d | 244.6 d | 363.5 c | 235.2 f | 46.3 c | 13.6 d | 965.9 c | 493.3 d |
| 1000 µM Co. | 529.0 e | 247.0 d | 231.6 e | 258.0 e | 13.9 e | 9.3 e | 744.4 d | 514.3 d |
| Spd + 1 mM Co. | 778.4 b | 369.0 b | 315.0 d | 314.2 d | 31.7 d | 32.7 b | 1125.0 b | 715.8 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Sheteiwy, M.S.; Lin, C.; Guan, Y.; Ulhassan, Z.; Hu, J. Spermidine Suppressed the Inhibitory Effects of Polyamines Inhibitors Combination in Maize (Zea mays L.) Seedlings under Chilling Stress. Plants 2021, 10, 2421. https://doi.org/10.3390/plants10112421
Gao C, Sheteiwy MS, Lin C, Guan Y, Ulhassan Z, Hu J. Spermidine Suppressed the Inhibitory Effects of Polyamines Inhibitors Combination in Maize (Zea mays L.) Seedlings under Chilling Stress. Plants. 2021; 10(11):2421. https://doi.org/10.3390/plants10112421
Chicago/Turabian StyleGao, Canhong, Mohamed S. Sheteiwy, Chen Lin, Yajing Guan, Zaid Ulhassan, and Jin Hu. 2021. "Spermidine Suppressed the Inhibitory Effects of Polyamines Inhibitors Combination in Maize (Zea mays L.) Seedlings under Chilling Stress" Plants 10, no. 11: 2421. https://doi.org/10.3390/plants10112421
APA StyleGao, C., Sheteiwy, M. S., Lin, C., Guan, Y., Ulhassan, Z., & Hu, J. (2021). Spermidine Suppressed the Inhibitory Effects of Polyamines Inhibitors Combination in Maize (Zea mays L.) Seedlings under Chilling Stress. Plants, 10(11), 2421. https://doi.org/10.3390/plants10112421







