Abstract
In recent work, it was shown that the graminoid plants Cynodon dactylon (Poaceae), Cyperus exaltatus (Cyperaceae), and Panicum repens (Poaceae) have an ovipositional effect on the malaria vector Anopheles gambiae in olfactometric bioassays. In order to get a view of the diversity of semiochemicals present in the environment of the vector during olfactometric trials, in the present work, the volatile profiles of these graminoid plants were analyzed using headspace solid-phase microextraction (HS-SPME) together with gas chromatography–mass spectrometry (GC-MS). In addition, one-way ANOVA comparison of compounds detected in two or more headspace samples are presented in order to provide a basis for comparison of compounds that could constitute a starting point for novel blends of volatile organic compounds to be tested as oviposition attractants.
1. Introduction
The study of the influence of volatile compounds and their behavioral effects on insects is an important aspect of chemical ecology. The development of control strategies against disease vectors, based on the semiochemicals available from plants, has, for example, led to advances in new pest control concepts. One goal is to decrease the spread of diseases associated with certain species of insects. An example of such a severe problem is the spread of malaria in sub-Saharan Africa, which has declined [1] when compared to the goals set by the World Health Organization (WHO) in their Global Technical Strategy for Malaria (GTS) report from 2016 [2]. As of now, long-lasting insecticide nets (LLINs) and indoor residual spray (IRS) are the most commonly employed techniques to minimize the infection rate of malaria. However, these methods are limited to indoor use, and it has been shown that the effectiveness has subsided due to a decrease in usage [1]. Increased resistance in certain Anopheles mosquitoes towards the utilized insecticides has also been reported [3]. One of the main malaria vectors is the Anopheles gambiae mosquito, whose host-seeking [4,5] and oviposition behaviors [4,5,6] are impacted by the semiochemicals in its environment. In order to obtain new methods of vector control, the regulation of oviposition using semiochemicals has thus been suggested, focusing on outdoor settings, such as “lure-and-kill”, that can provide an alternative to the indoor methods [7,8]. The challenge in the development of these novel outdoor-focused control techniques is the identification of volatile organic compounds (VOCs) that could be used to influence the malaria vectors’ choice of egg-laying site.
To be able to determine the volatile compounds that elicit this behavior from the target disease vectors, the presence of the VOCs must be established. With the knowledge of what VOCs are present in the chemical environment of the vector as oviposition occurs, one can aim to relate the VOCs to the behavior of the vector. In addition to establishing VOCs in the chemical environment, the sources of these VOCs should be determined. In a study by Bokore et al., a correlation between graminoid plants and the occurrence of A. gambiae instars was shown. It was determined that the choice of egg laying sites is influenced by the presence of water and certain graminoid plants. From this study, it could also be seen that the plants associated with the occurrence of the instars of A. gambiae are Cyperus rotundus (Nut grass) and Cyperus exaltatus (Exaltatus Grass) from the Cyperaceae family, as well as Cynodon dactylon (Bermuda Grass) and Panicum repens (Torpedo Grass) from the Poaceae family [9]. The essential oil composition [10,11,12] of Cyperus rotundus has previously been studied, as well as the volatile headspace of its macerated rhizomes using headspace solid-phase microextraction (HS-SPME) [13]. As the volatile constituents of Nut grass has been mapped previously [14], and the fact that this grass has been studied for its ovipositional impact on the malaria vector A. gambiae [15], it was omitted in the present study.
Analysis of some graminoid plants has been performed previously, but this has been done on dried hay infusions of the Bermuda grass [16]. The geographical origin of a plant has been suggested to be an influencing factor in the composition of the volatiles and essential oil [17,18]. Furthermore, this was demonstrated in a comparison of the essential oil of rhizomes of Nut grass from two different locations in South Africa [19], adding to the incentive of determining the volatile profile of the graminoid plants from the specific location in Western Kenya suggested by Bokore et al. [9].
In addition, the olfactometric work presented by Bokore et al. shows that even uprooted plants still elicit some ovipositional effects on gravid A. gambiae [20]. In the study by Bokore et al., dynamic headspace analysis was reported for the three graminoid plants used in the present work. The composition of volatiles released from the uprooted grass shoots under the two-port olfactometric bioassay circumstances was studied. However, headspace analysis of the shoots and root parts of these plants separately has not been studied. Thus, there is a lack of insight into the auxiliary compounds that are present, and that potentially contribute to the ovipositional effect on the malaria vector in the conditions used in the previous study [20].
In the present study, headspace (HS) sampling was used in combination with solid-phase microextraction (SPME), as this offers a rapid, solvent-free, and qualitative analysis. The application of SPME in the study of gas phase composition is common practice today and is applied in the investigation of flora samples for the determination of chemical composition [21,22,23].
Here, HS-SPME GC-MS analysis was performed on shoots and root parts from the three graminoid plants: Cynodon dactylon, Cyperus exaltatus, and Panicum repens, to determine the composition of volatile compounds acquired from each plant in similar conditions to that of the two-port olfactometric setup [20]. In addition to this, the data collected were interpreted with one-way ANOVA and Tukey post-hoc test to determine if there was a significant difference in the relative composition between the three graminoid plants, and between the different parts of the plants under conditions emulating certain aspect of those described by Bokore et al. [20].
2. Results and Discussion
2.1. Chemical Composition of Headspace Samples from C. dactylon, C. exaltatus, and P. repens
In Table 1, the 46 compounds that were identified in the roots and/or the shoots of the three different graminoid plants are listed. The compounds were detected and tentatively identified using MS as well as experimental retention index (RI). External analytical standards were used to confirm the identity of the compounds when possible. The compounds found were categorized into one of the following classes: terpenoids (TR), aliphatics (AL), benzenoids and phenylpropanoids (BP), C5-branched compounds (C5), nitrogen- or sulfur-containing compounds (NS), and cyclic miscellaneous compounds (Cyc) [24]. These chemical classes represent the continuation ofthe initial work by Knudsen et al. [25]. The categorization of compounds was based on the results from HS sampling of plants and covers over 1700 compounds [24]. Thus, it is appropriate to use these classes as many compounds have already been categorized accordingly. It should be noted that in the work by Knudsen et al. [24], 7 classes were used to classify the compounds. In this work, nitrogen- and sulfur-containing compounds were grouped under the same term, giving a total of 6 classes. In addition, a second classification method was used to classify the compounds based on their functional group, which can be seen in Table 1.

Table 1.
Volatile compounds detected in the shoots and root parts of the graminoid plants C. dactylon, C. exaltatus, and P. repens.
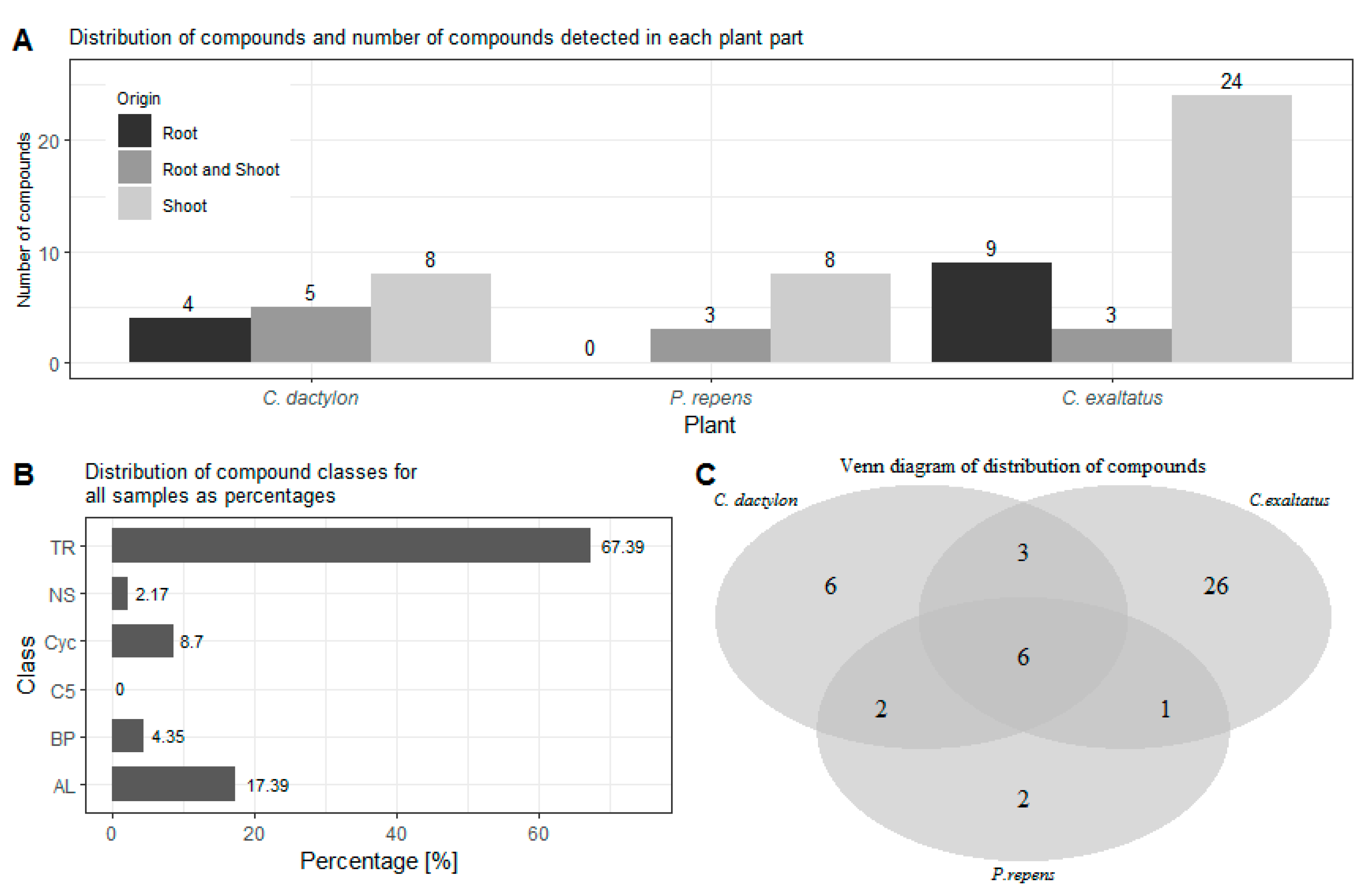
In order to compare the results of the analysis of the headspace of the graminoid plants, the first task was to identify the source tissue of the different compounds. In Figure 1A, the number of compounds detected in the root samples, shoot samples as well as in both types of samples are shown. Here, the largest number of compounds detected in both the root and shoot samples were obtained for C. dactylon. On the other hand, C exaltatus showed the largest total number of unique compounds detected in the root and shoot samples, respectively. Regarding P. repens, all the compounds found in the root samples were also found in the samples of the shoots. Finally, even though VOCs can be emitted by almost any plant tissue [26], it can be noted that the largest number of volatiles were identified in the samples from the shoots in all three species. After grouping the compounds under one of the 6 classes mentioned previously, it could be established that out of the 46 compounds found, 31 were terpenoids, 8 were aliphatic, 4 were cyclic compounds, 2 were a benzenoid/phenylpropanoid, and 1 was a nitrogen- or sulfur-containing compound. No C5-branched compounds were detected in the headspace samples. The distribution of the number of compounds in each of the classes, derived from the analysis of the three graminoid plants, follows the expected trends and is shown in Figure 1B. It is expected that the number of terpenoids, i.e., mono-and sesquiterpenes, should constitute the largest percentile of the identified compounds [27]. It is also more common that the number of fatty acid derivatives, such as the larger aliphatic compounds, appear in higher numbers than compounds containing a benzene ring [27]. Figure 1C shows how many of the compounds were detected for each of the graminoid plants as well as the number of compounds overlapping in two or more plant samples.
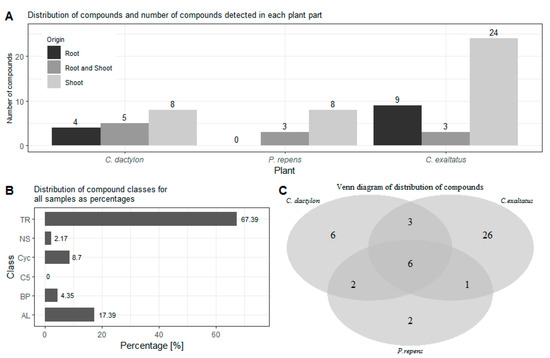
Figure 1.
(A)—Shows the distribution of compounds detected in the roots, shoots, and both plant parts for each of the graminoid plants. (B)—Shows the percentage distribution of the classes for the identified compounds for all samples analyzed. (C)—Shows a Venn diagram of the number of unique compounds detected for each of the three plants as well as the number of compounds found in two or all three plant species. TR—Terpenoid, AL—Aliphatics, BP—Benzenoid/Phenylpropanoids, C5—C5-branched compounds, NS—Nitrogen- or sulfur-containing compounds, Cyc—Cyclic miscellaneous compounds.
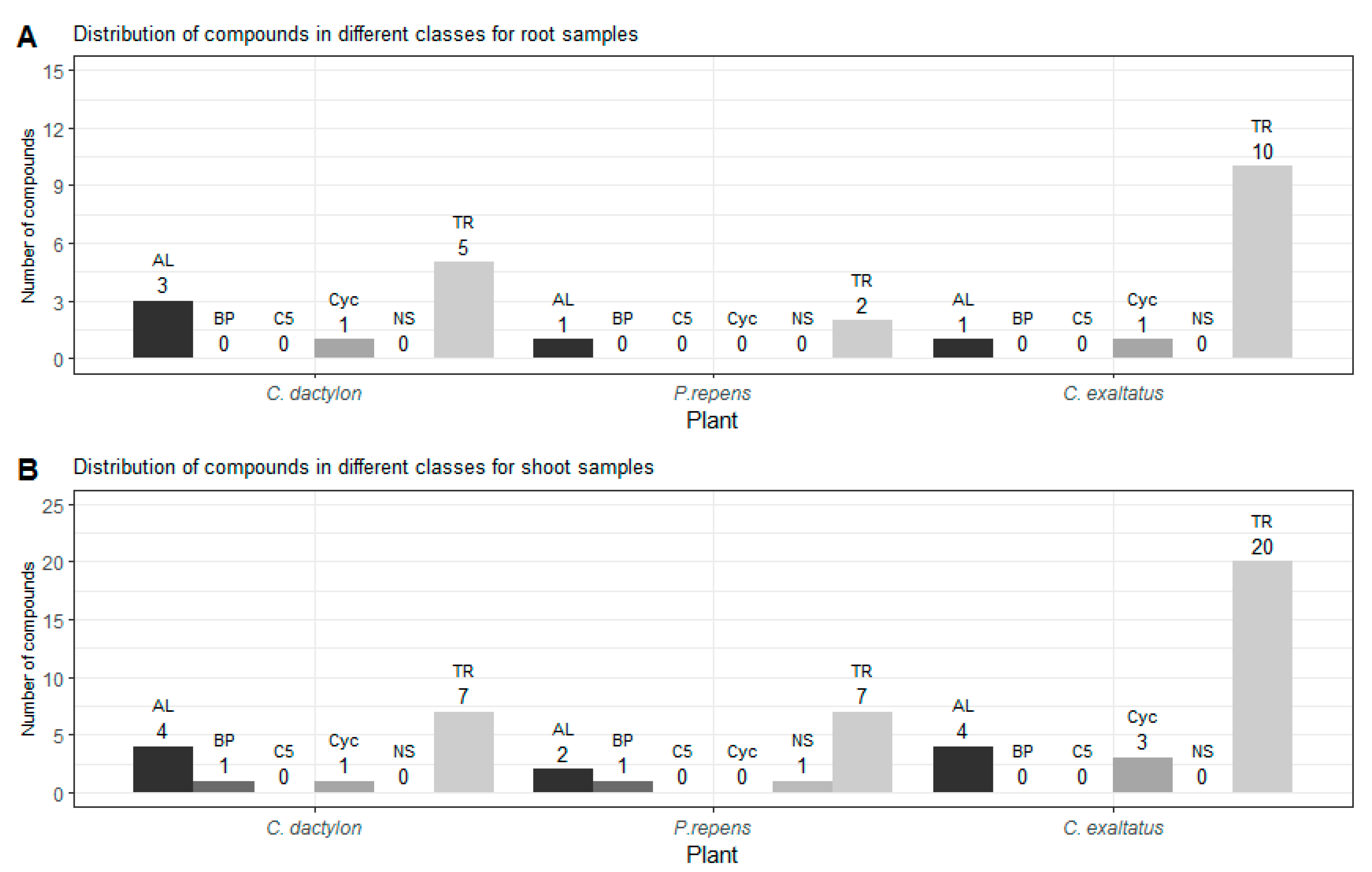
In Figure 2, the distribution of grouped compounds found in each of the plant species is shown separately. In Figure 2A, it can be seen that the highest number of the dominating group of terpenoids could be found in the root samples of C. exaltatus, when compared to the root samples of the other two plant species. The lowest total numbers of detected peaks were obtained from the root samples from P. repens. Nonetheless, in Table 1, it can be seen that the largest relative peak area was detected in P. repens across all samples. It appears that fewer VOCs originated from the torpedo grass roots compared to the other plant samples but that the roots emitted an amount represented by a higher relative area percentage of the said compounds. In Figure 2B, the distribution between substance classes detected in the shoot samples is shown. Here, it is visible that C. exaltatus shoot samples contained the largest number of terpenoids of all the samples analyzed, contributing to the trend of the terpenoid class constituting the largest percentile in the overall class distribution. In Figure 1C, the Venn diagram shows the overlap of the detected compounds in the different plants. From this figure, it can be observed that the number of common compounds found between the three plants are quite similar. Six compounds were found in all three plants, while one to three compounds were shared between pairs of plants. What is apparent in Figure 1A,C is the large number of compounds detected only in the headspace of the C. exaltatus samples. The larger number of compounds detected for this plant could be the basis for distinguishing the plants by family, as the more fragrant C. exaltatus belongs to the Cyperaceae family. Both C. dactylon and P. repens belong to the Poaceae family of plants, and when compared to that of C. exaltatus, they have a similarly low number of uniquely detected compounds in their headspace. Although grouping of plant families based on VOCs emission has been performed [28], it has not been done for the specific plants analyzed in this work. Therefore, differences and similarities based on belonging to a family can be suggested but not determined.
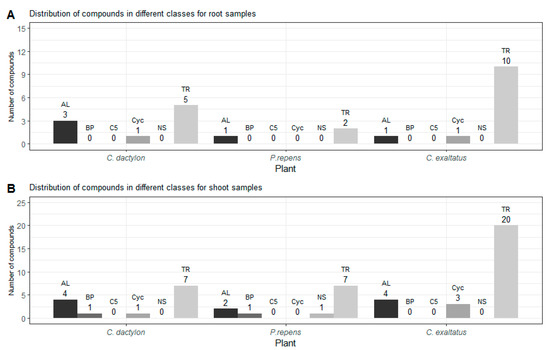
Figure 2.
(A)—Shows the distribution of compounds for each class detected in the root samples for the three plants. (B)—Shows the distribution of compounds for each class detected in the shoot samples for the three plants. TR—Terpenoid, AL—Aliphatics, BP—Benzenoid/Phenylpropanoids, C5—C5-branched compounds, NS—Nitrogen- or sulfur-containing compounds, Cyc—Cyclic miscellaneous compounds.
In total, 26 compounds were detected for C. exaltatus alone, where β-elemene and cyperene showed the highest relative area. In the headspace of C. dactylon, six compounds, the highest relative areas are reported for germacrene D and mesitylene, were uniquely detected. Regarding P. repens, only two compounds, myrtanylamine and phenylethyl alcohol, were uniquely detected. In the case of myrtanylamine, the sparse information available in the literature regarding its presence in the headspace of plants should be noted. This shows an excerpt of VOCs that separates the chemical profile of the plants from each other, and that could be a factor in why these plant species show different ovipositional strength [20]. Understanding the diversity of the compounds originating from all the graminoid plants is important for the selection of compounds to be tested as oviposition attractants. However, it is also important to investigate compounds that are shared between the headspace samples of multiple plants, and to evaluate the differences in the abundance between different plants. Compounds, such as α-pinene, β-pinene, and limonene, have been shown to elicit antennal responses from A. arabiensis [29]. Moreover, it has been shown that a synthetic blend of compounds, with a naturally observed ratio, could trigger a short-range response from A. arabiensis at a certain dosage [29]. Nonetheless, the same synthetic blend showed no response at higher dosages, or even provoked an avoiding response from the mosquitoes [29]. It has been suggested that compound blends are significant in the development of ovipositional attractive odors, and that not only must the individual compounds be determined, but also the ratio at which they occur in relation to each other [30,31]. This would suggest that a different response could be elicited for a blend of the same compounds with different ratios. Thus, compounds that appear in several plant samples could induce a positive ovipositional response, if the ratio of the compounds is suitable for the specific target. On the other hand, the response could vary between plant species due to the different abundances of the same compound. Therefore, it is valuable to determine if there is a significant difference in the relative peak area of the detected compounds between the different plants.
From the Venn diagram in Figure 1C, it can be seen that there are six compounds common to the headspace samples of all three species when the results from the roots and shoots are combined. These compounds are α-pinene, β-pinene, 3-octanone, β-myrcene, limonene, and citronellol. Three compounds were shared between C. dactylon and C. exaltatus: 2-pentyl-furan, nonanal, and 2,2,6-trimethyl-cyclohexanone, while C. dactylon and P. repens only shared two compounds, namely decane and β-ionone. The only compound shared by C. exaltatus and P. repens was 3-carene. Since the results are also split into roots and shoot samples, a similar comparison can be made for compounds common in both parts of the plants, to investigate if there are significant differences in the abundance of VOCs in the different plant parts. It has been suggested that compounds emitted into the rhizosphere play a role in plant–plant interaction [32], as well as plant–herbivore interactions [33]. However, the interaction of rhizosphere compounds and the malaria vector has not been fully explored, with regards to whether they contribute to long-range cues or short-range cues. Knowledge of abundance variation could offer additional insight into which compounds are important for oviposition. For C. dactylon, the compounds α-pinene, β-pinene, 3-octanone, decane, and limonene were present in both root and shoot samples. In the same sense, the compounds β-pinene, cyperene, and β-selinene were present in the samples of C. exaltatus. For P. repens, the compounds β-pinene and 3-octanone were present.
2.2. Statistical Evaluation of Results
The present study does not provide a concentration-based chemical profile, but rather, the relative peak areas were utilized. Nonetheless, determination of the significant differences in the detected VOCs is important to obtain an understanding of the sources that influence the chemical environment of the vector, which could affect the behavioral choices, as mentioned previously. Thus, one-way ANOVA was applied to identify differences in the composition and their significance. When applying one-way ANOVA, homoscedasticity, which is equal variance, and normality in the dataset are assumed for the robustness of the analysis. In order to obtain this, there has to be a repetitive detection of the compounds that can be analyzed with ANOVA.
Evaluation of the differences in the abundance of the detected compounds, and the significance of these differences, between plants is important to understand the role of a VOC as a potential attractant. Out of the compounds mentioned previously, the analysis could not be applied to the compounds α-pinene, limonene, decane, β-Ionone, 2-pentyl-furan, nonanal, and 2,2,6-trimethyl-cyclohexanone as these compounds were not detected in a repetitive fashion, to assume homoscedasticity and normality of the data. Therefore, one-way ANOVA was applied to all three plant species for the compounds β-pinene and 3-octanone, for α-pinene and citronellol when comparing C. dactylon with P. repens, and β-myrcene when comparing C. exaltatus with P. repens. After the one-way ANOVA analysis was performed, the post-hoc Tukey test was also performed in order to identify if any significant difference in the mean relative peak area could be found between any of the plants (Table 2).

Table 2.
Post-hoc Tukey analysis of five compounds found in the headspace of at least two of the three graminoid plants.
Similarly, the evaluation of differences was done for the previously mentioned compounds comparing the roots and shoots of the plants (Table 3).

Table 3.
Post-hoc Tukey analysis of six compounds found in the headspace samples of both the root and shoot samples of each of the three graminoid plants.
Here, it can be seen that there were no significant differences in the detected relative peak areas except for the peak areas of β-pinene in the P. repens sample when compared to the other two plants. Furthermore, in Table 1, it can be seen that the relative peak area for β-pinene is very large when compared to the other compounds detected in the headspace of the torpedo grass. A tendency towards this could also be discerned when comparing the percentages in the other plants. The results shown in this study suggest that there are very small differences in the normalized amount detected in the analysis of the overlapping compounds, which were compared based on the criteria described earlier. With the exception of β-pinene, the relative peak areas were not significantly different in the 95% confidence interval for any of the compounds. Furthermore, it can be seen in Table 3 that when comparing the compounds found in both the shoots and the roots of the different plants, no significant difference could be seen for the normalized peak area for any of the compounds listed for C. dactylon. Similarly, no significant difference could be shown for the normalized peak area of β-selinene in C. exaltatus or for 3-octanone in P. repens. A significant difference could be observed for β-pinene in both C. exaltatus and P. repens, where the normalized peak area of β-pinene was significantly larger in the root tissue samples than in the shoot samples. The same result was obtained for cyperene in the comparison of the roots and shoots for C. exaltatus.
2.3. Relating Findings to the Olfactometric Results
Since β-pinene stands out in the obtained results, it is of interest to discuss this component’s possible response effects. It was reported earlier [29] that β-pinene elicits an antennal response from A. arabiensis, which would suggest that a similar response from A. gambiae is plausible as these mosquitos belong to the same family. From the work presented by Bokore et al. [20], it was suggested that in the short-range olfactometry tests, all three graminoid plants generated a similar preference from the malaria vector when compared to lake water. However, during long-range attraction trials, P. repens showed the weakest attraction when compared to lake water. Relating these olfactory measurements to the results reported here, regarding the occurrence of β-pinene is not straight-forward. It could be argued that the reduced attraction of P. repens at long range could be a result of β-pinene showing a significantly higher abundance in the roots, which were more exposed in short-range experiments, than in the shoots of the plant. This could suggest the role of β-pinene as a short-range cue, while not acting as a long-range cue. C. exaltatus showed similar attraction effects as C. dactylon, despite the fact that a significantly larger normalized peak was detected for β-pinene in the root sample of C. exaltatus. A “blend” effect from the combined compounds present in the headspace of the plants is possible as discussed in Section 2.1. In order to move forward with identifying new attractants for the mosquitoes, using the blends of compounds reported for C. dactylon and/or C. exaltatus in Table 3 could be a starting point. This choice is motivated by the fact that it was reported that P. repens induced a lower attraction at long range, while the other two graminoid plants were reported to show both short- and long-range attraction. Still, no significant difference in the abundance of the compounds common to these two plants could be elucidated. This suggests that investigating different ratios of the compounds that are common for the roots and shoots of the plants should be considered for the testing of new blends. These compounds could be involved in both the short- and long-range attraction of the vector, suggesting their potential as “blend-effect” chemicals. After these blends are evaluated, the addition of individual compounds to the blend could be investigated for potential increased effect on the malaria vector. Examples of such compounds could be β-elemene, which was detected in C. exaltatus shoot samples, or germacrene D, which was only detected in the root samples of C. dactylon. However, the addition of single compounds will be more of a “hit-and-miss” approach, although the tentative selection of compounds has been narrowed down with the results reported in this study.
3. Materials and Methods
Grass samples were collected in the summer of 2020 and shipped to KTH Royal Institute of Technology, Stockholm, Sweden. C. exaltatus was collected from Rusinga Island (0°23′47.3″ S 34°12′13.1″ E) while C. dactylon and P. repens were collected in Mbita (0°26′06.19″ S 34°12′53.13″ E). The plants were divided into roots and shoots prior to import due to permits from the Swedish Board of Agriculture. Roots include any plant tissue that is present below the ground, while shoots refer to any part of the plant tissue that is above the ground. Botanical identification was performed at the International Centre of Insect Physiology and Ecology Thomas Odhiambo Mbita Campus (ICIPE) in Kenya. Upon arrival, both parts (shoots and roots) of the grass were washed in order to remove any residual soil left on the plant material. The plant material was washed using doubly distilled water (Milli-Q) from a Synergy 185 water purification system (Merck, Kenilworth, NJ, USA) with a resistivity of 18.2 MΩ*cm at 25 °C. Excess water from the washing of the plant material was then allowed to evaporate at room temperature from the samples. The samples were then stored in a −80 °C freezer until further sample preparation and analysis.
3.1. Solid-Phase Micro Extraction
In total, 10 g of sample were placed in a 250 mL round-bottom flask, covered with 100 mL of Milli-Q water, and sealed. The water-grass mixture was allowed to sit at room temperature for 30 min before the SPME fiber was introduced to the round-bottom flask neck. A picture showing a sample collection setup is provided in the Supplementary Materials Figure S1. The fiber was exposed to the headspace for 4 h. The SPME fibers used were 1 cm Polydimethylsiloxane/Divinylbenzene (PDMS/DVB) fibers with a 24 gauge needle (57310-U, Supelco, Bellefonte, PA, USA). Each shoot and root headspace sample collection was performed with two different samples on two different days (day 1 and 2), due to the long extraction time. Two different fibers were used for sample extraction on day 1 and 2, so that the total number of analyses for each root and shoot sample was 4. The fibers were conditioned prior to sample and blank analyses by being placed in the injector port of the GC instrument for 3 min, while the temperature in the injector was 40 °C for 6 s, then ramped to 260 °C at a rate of 12 °C/s, and then held for 3 min. The resulting chromatograms were studied for any contaminations and undesired compounds to determine if the fiber was ready to be utilized for sample extraction. After extraction, the fiber was withdrawn, injected into the GC injector port, and the GC method was started. A desorption time of 30 s in the port was allowed before removing the fiber. The sample collection method was based on the work by Svenberg et al. [34].
3.2. Gas Chromatography–Mass Spectrometry Parameters
Analysis was performed using an Agilent 7890A GC (Agilent Technologies, Santa Clara, CA, USA) coupled to a 5975C Triple axis MS (Agilent Technologies). The temperature program started at 40 °C and was held for 1 min, after which the temperature was ramped to 260 °C at 10 °C/min. After reaching 260 °C, the temperature was held for 5 min. A 30 m × 0.25 mm × 0.25 µm DB-5 column (Agilent Technologies) was used for all analyses. The temperature program for the inlet was the same as the program described in the previous section. The carrier gas used was 6.0 LAB LINE helium (Strandmöllen AB, Ljungby, Sweden) and the mass range of the 5975C MS was set to 35–400 m/z. All data handling was performed and exported using the Data analysis software in Chemstation (Agilent Technologies), and identification with mass spectrometry was done with the National Institute of Standards and Technology (NIST) MS Search 2.0 program for the NIST/Environmental Protection Agency (EPA)/National Institute of Health (NIH) Mass Spectral Library version 2.0 g, build 2009.
Peak detection was performed by using the integration function in Chemstation data analysis software. In the method for peak detection, the parameters were set to initial area reject of 1,500,000, initial peak width of 0.02, shoulder detection off, and initial threshold of 16.0. For determination of the total area in the chromatogram, the parameters of the integration method were set to initial area reject of 1, initial peak width of 0.02, shoulder detection off, and initial threshold of 15.0. Retention indices were calculated by comparison of the retention times to that of the 49452-U C7-C40 alkane standard (Supelco, Bellefonte, PA, USA) (10 µg of each in hexane), and external standard identification was performed by comparing the sample analyte peak retention times to that of the retention times of compounds present in the CRM40755 Cannabis Terpene Mix A (Sigma Aldrich) (10 µg of each in hexane). The mix contained the following 20 terpenes: α-pinene, β-pinene, camphene, 3-carene, α-terpenine, R-(+)-limonene, γ-terpinene, L-(−)-fenchone, fenchol, (1R)-(+)-camphor, isoborneol, menthol, citronellol, (+)-pulegone, geranyl acetate, α-cedrene, α-humulene, nerolidol, (+)-cedrol, and (−)-α-bisabolol. The standards of β-caryophyllene standard 22075 (Sigma-Aldrich) and the (−)-caryophyllene oxide 91034 (Sigma-Aldrich) were also used to confirm the identity of the detected compounds. Analysis parameters, peak detection parameters and peak selection were based on the work by Svenberg et al. [34].
All statistical analysis and graphical work were performed in the Rstudios R software. Source code, libraries required, and data tables are provided in the Supplementary Materials.
4. Conclusions
In the present work, 46 volatiles were detected and identified in the root and/or shoot parts of the three graminoid plant species: C. dactylon, C. exaltatus, and P. repens. It was shown that out of the 46 detected compounds, 26 were unique to C. exaltatus, 6 were unique to C. dactylon, and 2 were unique to P. repens, with 12 compounds overlapping in combinations between the studied plants. Furthermore, it was shown, with one-way ANOVA analysis and Tukey post-hoc test, that there was no significant difference in the total relative peak areas detected in the shoot and root samples for any of the overlapping compounds except for β-pinene. The relative peak area for this compound was significantly higher in P. repens compared to the other two plants, while no significant difference was shown for this substance between C. dactylon and C. exaltatus. The comparison of C. dactylon and C. exaltatus showed that for the overlapping compounds, there is no significant difference in the relative area of the compounds detected in this study. In addition to this, it was shown that when comparing the relative area of compounds detected in both the root and shoots of the different plants, there are significant difference in the areas detected in this work. The knowledge about these compounds appearing both in the roots and shoots, in combination with the previously shown attraction strength of the different grasses, offers new starting points for blends of compounds. Blends of compounds including, e.g., β-pinene, cyperene, and β-selinene could be possible starting points, based on data reported for C. exaltatus in this work. These blends have to be investigated further to determine the ratio that should be used to elicit a response from the vector. Once this has been determined, the individual unique compounds found in the present study, such as β-elemene, can be added to the proposed plant-specific blends.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants10112423/s1. Figure S1: experimental setup for headspace collection. Source code s1: R_code_figures—R code with libraries to generate Figure 1 and Figure 2 shown in this work. Source code s2: R_code_ANOVA_tbl2—R code with libraries to perform the one-way ANOVA analysis shown in Table 2 of this work. Source code s3: R_code_ANOVA_tbl3—R code with libraries to perform the one-way ANOVA analysis shown in Table 3 of this work. CSV file s1: Figure_data_1—CSV file containing data required to run source code 1. CSV file s2: Figure_data_2—CSV file containing data required to run source code 1. CSV file s3: Figure_data_3—CSV file containing data required to run source code 1. CSV file s4: ANOVA_tbl2 CSV file containing data required to run source code 2. CSV file s5: ANOVA_tbl3 CSV file containing data required to run source code 3.
Author Contributions
Conceptualization, L.S. and Å.E.; methodology, L.S.; software, L.S.; validation, L.S. and Å.E.; formal analysis, L.S.; investigation, L.S.; resources, Å.E.; data curation, L.S.; writing—original draft preparation, L.S.; writing—review and editing, L.S. and Å.E.; visualization, L.S.; supervision, Å.E.; project administration, Å.E.; funding acquisition, Å.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Swedish Research Council (2015-03159) and the Carl Trygger foundation (CTS18:97 and CTS20:119).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Ulrike Fillinger and Getachew Eticha Bokore from the International Centre of Insect Physiology and Ecology Thomas Odhiambo Mbita Campus for their help in supplying the graminoid plants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges; WHO: Geneva, Switzerland, 2020; pp. 22, 60, 62. [Google Scholar]
- World Health Organization. Global Technical Strategy for Malaria 2016–2030; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Ranson, H.; Lissenden, N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef]
- Bentley, M.D.; Day, J.F. Chemical ecology and behavioral aspects of mosquito oviposition. Annu. Rev. Entomol. 1989, 34, 401–421. [Google Scholar] [CrossRef]
- Takken, W.; Knols, B.G.J. Odor-mediated behaviour of afrotropical malaria mosquitoes. Annu. Rev. Entomol. 1999, 44, 131–157. [Google Scholar] [CrossRef]
- Zwiebel, L.J.; Takken, W. Olfactory regulation of mosquito–host interactions. Insect Biochem. Mol. Biol. 2004, 34, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Govella, N.J.; Ferguson, H.M. Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front. Physiol. 2012, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, H.; Dornhaus, A.; Beeche, A.; Borgemeister, C.; Gottlieb, M.; Mulla, M.S.; Gimnig, J.E.; Fish, D.; Killeen, G.F. Ecology: A prerequisite for malaria elimination and eradication. PLoS Med. 2010, 7, e1000303. [Google Scholar] [CrossRef] [Green Version]
- Bokore, G.E.; Ouma, P.; Onyango, P.O.; Bukhari, T.; Fillinger, U. A cross-sectional observational study investigating the association between sedges (swamp grasses, Cyperaceae) and the prevalence of immature malaria vectors in aquatic habitats along the shore of Lake Victoria, western Kenya. F1000Research 2020, 9, 1032. [Google Scholar] [CrossRef]
- Abo-Altmene, R.A.; Al-Shammari, A.M.; Shawkat, M.S. GC-MS analysis and chemical composition identification of Cyperus rotundus L. from Iraq. Energy Procedia 2019, 157, 1462–1474. [Google Scholar] [CrossRef]
- Janaki, S.; Zandi-Sohani, N.; Ramezani, L.; Szumny, A. Chemical composition and insecticidal efficacy of Cyperus rotundus essential oil against three stored product pests. Int. Biodeterior. Biodegrad. 2018, 133, 93–98. [Google Scholar] [CrossRef]
- Yagi, S.; Babiker, R.; Tzanova, T.; Schohn, H. Chemical composition, antiproliferative, antioxidant and antibacterial activities of essential oils from aromatic plants growing in Sudan. Asian Pac. J. Trop. Med. 2016, 9, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Poyraz, I.E.; Demirci, B.; Kucuk, S. Volatiles of Turkish Cyperus rotundus L. Roots. Rec. Nat. Prod. 2017, 12, 222–228. [Google Scholar] [CrossRef]
- Kamala, A.; Middha, S.K.; Karigar, C.S. Plants in traditional medicine with special reference to Cyperus rotundus L.: A review. 3 Biotech 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Eneh, L.K.; Saijo, H.; Borg-Karlson, A.-K.; Lindh, J.M.; Rajarao, G.K. Cedrol, a malaria mosquito oviposition attractant is produced by fungi isolated from rhizomes of the grass cyperus rotundus. Malar. J. 2016, 15, 478–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eneh, L.K.; Okal, M.N.; Borg-Karlson, A.K.; Fillinger, U.; Lindh, J.M. Gravid Anopheles gambiae sensu stricto avoid ovipositing in Bermuda grass hay infusion and it’s volatiles in two choice egg-count bioassays. Malar. J. 2016, 15, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Knudsen, J.T. Variation in floral scent composition within and between populations of Geonoma macrostachys (Arecaceae) in the Western Amazon. Am. J. Bot. 2002, 89, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.A.; Oyedeji, A.O. Chemical Composition of the Essential Oils of Cyperus rotundus L. from South Africa. Molecules 2009, 14, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Bokore, G.E.; Svenberg, L.; Tamre, R.; Onyango, P.; Bukhari, T.; Emmer, Å.; Fillinger, U. Grass-like plants release general volatile cues attractive for gravid Anopheles gambiae sensu stricto mosquitoes. Parasit. Vectors 2021, 14, 552. [Google Scholar] [CrossRef]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.; Schnitzler, J.-P. Practical approaches to plant volatile analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.-S.; Ramya, M.; An, H.-R.; Park, P.-M.; Lee, S.-Y.; Baek, N.-I.; Park, P.-H. Volatiles Profile of the Floral Organs of a New Hybrid Cymbidium, ‘Sunny Bell’ Using Headspace Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry Analysis. Plants 2019, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-G.; Choi, W.-S.; Yang, S.-O.; Hwang-Bo, J.; Kim, H.-G.; Fang, M.; Yi, T.-H.; Kang, S.C.; Lee, Y.-H.; Baek, N.-I. Volatile Profiles of Five Variants of Abeliophyllum distichum Flowers Using Headspace Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry (HS-SPME-GC-MS) Analysis. Plants 2021, 10, 224. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and Distribution of Floral Scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollstein, L.; Bergström, L.G. Floral scents—A checklist of voaltile compounds isolated by headspace techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusiá, J. The complexity of factors driving volatile organic compound emissions by plants. Biol. Plant. 2001, 44, 481–487. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [Green Version]
- Vivaldo, G.; Masi, E.; Taiti, C.; Caldarelli, G.; Mancuso, S. The network of plants volatile organic compounds. Sci. Rep. 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Wondwosen, B.; Birgersson, G.; Seyoum, E.; Tekie, H.; Torto, B.; Fillinger, U.; Hill, S.R.; Ignell, R. Rice volatiles lure gravid malaria mosquitoes, Anopheles arabiensis. Sci. Rep. 2016, 6, 37930. [Google Scholar] [CrossRef] [Green Version]
- Wooding, M.; Naude, Y.; Rohwer, E.; Bouwer, M. Controlling mosquitoes with semiochemicals: A review. Parasit. Vectors 2020, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects—Finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef]
- Gfeller, V.; Huber, M.; Förster, C.; Huang, W.; Köllner, T.; Erb, M. Root volatiles in plant–plant interactions I: High root sesquiterpene release is associated with increased germination and growth of plant neighbours. Plant Cell Environ. 2019, 42, 1950–1963. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Gfeller, A.; Erb, M. Root volatiles in plant–plant interactions II: Root volatiles alter root chemistry and plant–herbivore interactions of neighbouring plants. Plant Cell Environ. 2019, 42, 1964–1973. [Google Scholar] [CrossRef] [Green Version]
- Svenberg, L.; Emmer, Å.; Lindh, J. Analysis of six secondary metabolites from Cyperus rotundus—Comparing different methods for determining volatile compounds in laboratory and field settings. 2021. Submited. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).