Plant Parasitic Nematodes: A Review on Their Behaviour, Host Interaction, Management Approaches and Their Occurrence in Two Sites in the Republic of Ireland
Abstract
1. Introduction
2. Plant Parasitic Nematode Species and Their Distribution
3. Irish Case Studies
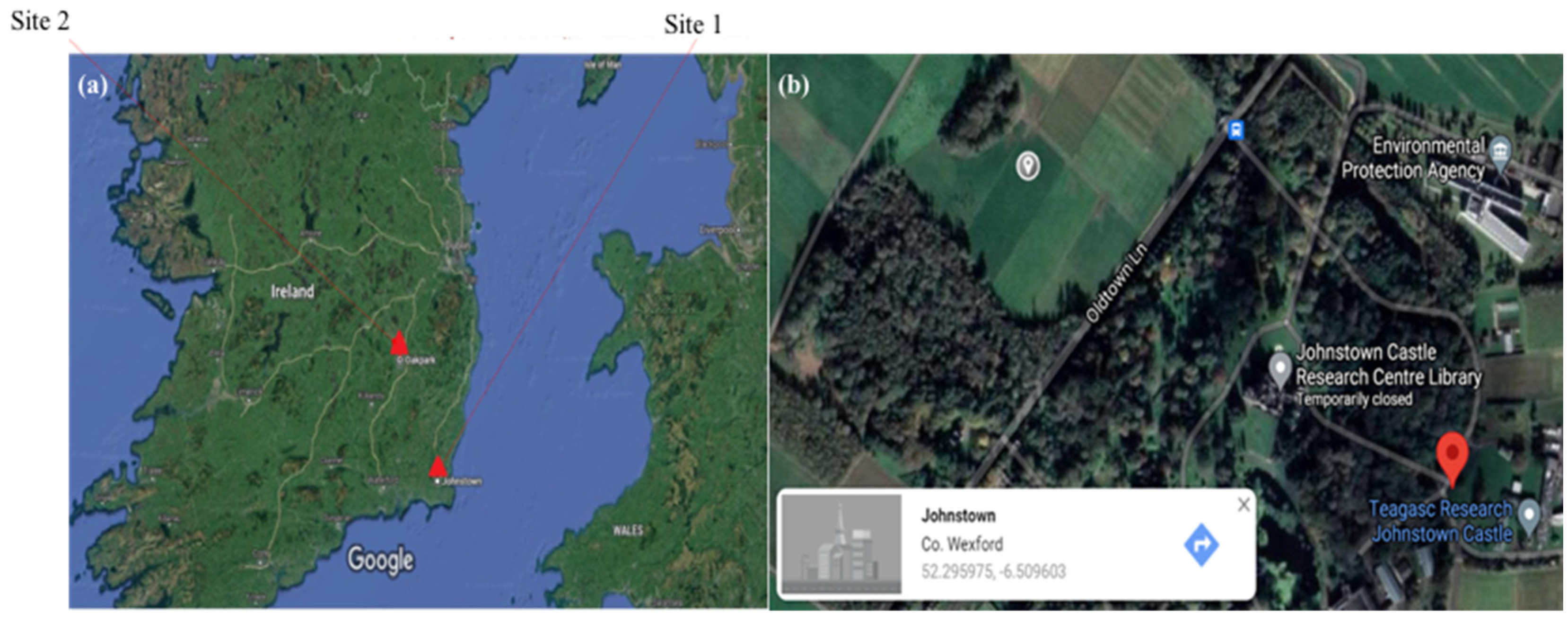
- Case study 1. Occurrence of PPN at Teagasc, Johnstown
- Case study 2. Occurrence of PPN taxa at Teagasc, Oakpark
4. Nematode Behaviour, Feeding and Host–Parasite Interactions
5. Plant Responses to Nematode Infection
6. Current Management Strategies and Their Limitations
7. Fermentation and Microbial-Based Products
8. Conclusions
9. Future Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicol, J.M.; Turner, S.J.; Coyne, D.; Nijs, L.D.; Hockland, S.; Maafi, Z.T. Current nematode threats to World Agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2011; pp. 21–43. [Google Scholar]
- Keating, B.A.; Carberry, P.S.; Bindraban, P.S.; Asseng, S.; Meinke, H.; Dixon, J. Eco-efficient Agriculture: Concepts, Challenges, and Opportunities. Crop. Sci. 2010, 50, S-109–S-119. [Google Scholar] [CrossRef]
- Mokrini, F.; Janati, S.; Houari, A.; Essarioui, A.; Bouharroud, R.; Mimouni, A. Management of Plant Parasitic Nematodes by means of organic amendments—A Review. Rev. Maroc. Sci. Agron. Vét. 2018, 6, 337–344. [Google Scholar]
- DEFRA (2010) Science Search. Available online: http://randd.defra.gov.uk/ (accessed on 26 April 2010).
- Atkins, S.D.; Clark, I.M.; Sosnowska, D.; Hirsch, P.; Kerry, B.R. Detection and Quantification of Plectosphaerella cucumerina, a Potential Biological Control Agent of Potato Cyst Nematodes, by Using Conventional PCR, Real-Time PCR, Selective Media, and Baiting. Appl. Environ. Microbiol. 2003, 69, 4788–4793. [Google Scholar] [CrossRef]
- Lord, J.S.; Lazzeri, L.; Atkinson, H.J.; Urwin, P.E. Biofumigation for Control of Pale Potato Cyst Nematodes: Activity of Brassica Leaf Extracts and Green Manures on Globodera pallida In Vitro and in Soil. J. Agric. Food Chem. 2011, 59, 7882–7890. [Google Scholar] [CrossRef]
- Fleming, T.R.; McGowan, N.E.; Maule, A.; Fleming, C.C. Prevalence and diversity of plant parasitic nematodes in Northern Ireland grassland and cereals, and the influence of soils and rainfall. Plant Pathol. 2016, 65, 1539–1550. [Google Scholar] [CrossRef]
- Koenning, S.; Overstreet, C.; Noling, W.J.; Donald, A.; Becker, J.O.; Fortnum, A.B. Survey of Crop Losses in Response to Phytoparasitic Nematodes in the United States for 1994. J. Nematol. 1999, 31, 587–618. [Google Scholar] [PubMed]
- Peng, D.L.; Nicol, J.M.; Li, H.M.; Hou, S.Y.; Li, H.X.; Chen, S.L.; Ma, P.; Li, H.L.; Riley, I.T. Current knowledge of cereal cyst nematode (Heterodera avenae) on wheat in China. In Cereal Cyst Nematodes: Status, Research and Outlook; CIMMYT: Ankara, Turkey, 2011; pp. 29–34. [Google Scholar]
- Hockland, S.; Niere, B.; Grenier, E.; Blok, V.; Phillips, M.; Nijs, L.D.; Anthoine, G.; Pickup, J.; Viaene, N. An evaluation of the implications of virulence in non-European populations of Globodera pallida and G. rostochiensis for potato cultivation in Europe. Nematology 2012, 14, 1–13. [Google Scholar] [CrossRef]
- Nose, M.; Shiraishi, S. Breeding for Resistance to Pine Wilt Disease. Pine Wilt Dis. 2008, 334–350. [Google Scholar] [CrossRef]
- The Department of Agriculture, Food and the Marine (DAFM). List of Union Quarantine Pests and Their Respective Codes Table of Contents Part A: Pests Not Known to Occur in the Union Territory; The Department of Agriculture, Food and the Marine (DAFM): Dublin, Ireland, 2019.
- Keith, A.M.; Griffin, C.T.; Schmidt, O. Predatory soil nematodes (Nematoda: Mononchida) in major land-use types across Ireland. J. Nat. Hist. 2009, 43, 2571–2577. [Google Scholar] [CrossRef]
- Downes, M.; Moore, J.; Griffin, C. Occurrence of Insect-Parasitic Nematodes (Steinernematidae, Heterorhabditidae) in the Republic of Ireland. Nematologica 1991, 37, 92–100. [Google Scholar] [CrossRef]
- Bhadury, P.; Austen, M.; Bilton, D.; Lambshead, P.; Rogers, A.; Smerdon, G. Development and evaluation of a DNA-barcoding approach for the rapid identification of nematodes. Mar. Ecol. Prog. Ser. 2006, 320, 1–9. [Google Scholar] [CrossRef]
- Holovachov, O.; Camp, L.; Nadler, S.A. Sensitivity of Ribosomal RNA Character Sampling in the Phylogeny of Rhabditida. J. Nematol. 2015, 47, 337–355. [Google Scholar]
- Blaxter, M.L.; De Ley, P.; Garey, J.; Liu, L.X.; Scheldeman, P.; Vierstraete, A.; Vanfleteren, J.R.; Mackey, L.Y.; Dorris, M.; Frisse, L.M.; et al. A molecular evolutionary framework for the phylum Nematoda. Nature 1998, 392, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Aguinaldo, A.M.A.; Turbeville, J.M.; Linford, L.S.; Rivera, M.C.; Garey, J.R.; Raff, R.A.; Lake, J.A. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 1997, 387, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Foucher, A.L.; Bongers, T.; Noble, L.; Wilson, M.J. Assessment of nematode biodiversity using DGGE of 18S rDNA following extraction of nematodes from soil. Soil Biol. Biochem. 2004, 36, 2027–2032. [Google Scholar] [CrossRef]
- Egan, A. Biological Control Capabilities of Plant Growth Promoting Bacteria against Plant Parasitic Nematodes and Their Potential for Oilseed Rape Bioremediation, Assessed by Nematode Bioindicators. Ph.D. Thesis, Institute of Technology Carlow, Carlow, Ireland, 2019. [Google Scholar]
- Van Bezooijen, J.; Van Bezooijen, J. Methods and Techniques for Nematology; Wageningen University: Wageningen, The Netherlands, 2006; p. 20. [Google Scholar]
- Yoder, M.; De Ley, I.T.; King, I.W.; Mundo-Ocampo, M.; Mann, J.; Blaxter, M.; Poiras, L.; De Ley, P. DESS: A versatile solution for preserving morphology and extractable DNA of nematodes. Nematology 2006, 8, 367–376. [Google Scholar] [CrossRef]
- Powers, T.O.; Szalanski, A.L.; Mullin, P.G.; Harris, T.S.; Bertozzi, T.; Griesbach, J.A. Identification of Seed Gall Nematodes of Agronomic and Regulatory Concern with PCR-RFLP of ITS1. J. Nematol. 2001, 33, 191–194. [Google Scholar]
- Yeates, G.W.; Bongers, T.D.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera-an outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Sieriebriennikov, B.; Ferris, H.; Goede, R. NINJA: An automated calculation system for nematode-based biological monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- Grymaszewska, G.; Golinowski, W. Structure of syncytia induced by Heterodera schachtii Schmidt in roots of susceptible and resistant radish (Raphanus sativus L., var. oleiformis). Acta Soc. Bot. Pol. 2014, 67, 207–216. [Google Scholar] [CrossRef][Green Version]
- Jacob, J.; Mitreva, M. Transcriptomes of Plant-Parasitic Nematodes. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Tokyo, Japan, 2011; pp. 119–138. [Google Scholar]
- Moens, M.; Perry, R.N.; Starr, J.L. Meloidogyne species—A diverse group of novel and important plant parasites. Root-Knot Nematodes 2009, 1, 483. [Google Scholar]
- Price, J.A.; Coyne, D.; Blok, V.C.; Jones, J.T. Potato cyst nematodes Globodera rostochiensis and G. pallida. Mol. Plant Pathol. 2021, 22, 495–507. [Google Scholar] [CrossRef] [PubMed]
- De Waele, D.; Elsen, A. Challenges in Tropical Plant Nematology. Annu. Rev. Phytopathol. 2007, 45, 457–485. [Google Scholar] [CrossRef]
- Padgham, J.L.; Duxbury, J.M.; Mazid, A.M.; Abawi, G.S.; Hossain, M. Yield Loss Caused by Meloidogyne graminicola on Lowland Rainfed Rice in Bangladesh. J. Nematol. 2004, 36, 42–48. [Google Scholar]
- Lilley, C.J.; Kyndt, T.; Gheysen, G. Nematode Resistant GM Crops in Industrialised and Developing Countries. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Dordrecht, The Netherlands, 2011; pp. 517–541. [Google Scholar]
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Danchin, E.G.J.; Rosso, M.-N.; Vieira, P.; de Almeida-Engler, J.; Coutinho, P.M.; Henrissat, B.; Abad, P. Multiple lateral gene transfers and duplications have promoted plant parasitism ability in nematodes. Proc. Natl. Acad. Sci. USA 2010, 107, 17651–17656. [Google Scholar] [CrossRef]
- Jaouannet, M.; Magliano, M.; Arguel, M.J.; Gourgues, M.; Evangelisti, E.; Abad, P.; Rosso, M.-N. The Root-Knot Nematode Calreticulin Mi-CRT Is a Key Effector in Plant Defense Suppression. Mol. Plant-Microbe Interact. 2013, 26, 97–105. [Google Scholar] [CrossRef]
- Manzanilla-López, R.H.; Starr, J.L. 10 Interactions with Other Pathogens. Root-Knot Nematodes 2009, 223. [Google Scholar]
- McCarter, J.P.; Mitreva, M.D.; Martin, J.; Dante, M.; Wylie, T.; Rao, U.; Pape, D.; Bowers, Y.; Theising, B.; Murphy, C.V.; et al. Analysis and functional classification of transcripts from the nematode Meloidogyne incognita. Genome Biol. 2003, 4, R26. [Google Scholar] [CrossRef]
- Weerasinghe, R.R. Rhizobial-Legume Symbiosis and Root Knot Nematode Parasitism: Common Signal Transduction Pathways in Legumes. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2004. [Google Scholar]
- Mburu, H.; Cortada, L.; Haukeland, S.; Ronno, W.; Nyongesa, M.; Kinyua, Z.; Bargul, J.; Coyne, D. Potato Cyst Nematodes: A New Threat to Potato Production in East Africa. Front. Plant Sci. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.H.C.; Robinson, A.F.; Perry, R.N. Hatch and host location. Root-Knot Nematodes 2009, 1, 139–162. [Google Scholar] [CrossRef]
- Jones, J. (Ed.) Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Gartner, U.; Hein, I.; Brown, L.H.; Chen, X.; Mantelin, S.; Sharma, S.K.; Dandurand, L.-M.; Kuhl, J.C.; Jones, J.T.; Bryan, G.J.; et al. Resisting Potato Cyst Nematodes with Resistance. Front. Plant Sci. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Jones, J.; Haegeman, A.; Danchin, E.; Gaur, H.S.; Helder, J.; Jones, M.; Kikuchi, T.; Manzanilla-López, R.H.; Palomares-Rius, J.E.; Wesemael, W.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Postma, W.J.; Slootweg, E.J.; Rehman, S.; Finkers-Tomczak, A.; Tytgat, T.O.; van Gelderen, K.; Lozano-Torres, J.L.; Roosien, J.; Pomp, R.; van Schaik, C.; et al. The Effector SPRYSEC-19 of Globodera rostochiensis Suppresses CC-NB-LRR-Mediated Disease Resistance in Plants. Plant Physiol. 2012, 160, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mitchum, M.G.; Gao, B.; Li, C.; Diab, H.; Baum, T.J.; Hussey, R.S.; Davis, E.L. A parasitism gene from a plant-parasitic nematode with function similar toCLAVATA3/ESR (CLE) of Arabidopsis thaliana. Mol. Plant Pathol. 2005, 6, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chronis, D.; Kenning, C.; Peret, B.; Hewezi, T.; Davis, E.L.; Baum, T.J.; Hussey, R.; Bennett, M.; Mitchum, M.G. The Novel Cyst Nematode Effector Protein 19C07 Interacts with the Arabidopsis Auxin Influx Transporter LAX3 to Control Feeding Site Development. Plant Physiol. 2011, 155, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Ithal, N.; Recknor, J.; Nettleton, D.; Maier, T.; Baum, T.J.; Mitchum, M.G. Developmental Transcript Profiling of Cyst Nematode Feeding Cells in Soybean Roots. Mol. Plant-Microbe Interact. 2007, 20, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Haegeman, A.; Vanholme, B.; Gheysen, G. Characterization of a putative endoxylanase in the migratory plant-parasitic nematode Radopholus similis. Mol. Plant Pathol. 2009, 10, 389–401. [Google Scholar] [CrossRef]
- Maier, T.R.; Hewezi, T.; Peng, J.; Baum, T.J. Isolation of Whole Esophageal Gland Cells from Plant-Parasitic Nematodes for Transcriptome Analyses and Effector Identification. Mol. Plant-Microbe Interact. 2013, 26, 31–35. [Google Scholar] [CrossRef]
- Vovlas, N.; Troccoli, A.; Palomares-Rius, J.E.; De Luca, F.; Liébanas, G.; Landa, B.B.; Subbotin, S.A.; Castillo, P. Ditylenchus gigas n. sp. parasitizing broad bean: A new stem nematode singled out from the Ditylenchus dipsaci species complex using a polyphasic approach with molecular phylogeny. Plant Pathol. 2011, 60, 762–775. [Google Scholar] [CrossRef]
- Perry, R.N.; Moens, M. Survival of parasitic nematodes outside the host. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Van Megen, H.; van den Elsen, S.; Holterman, M.; Karssen, G.; Mooyman, P.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology 2009, 11, 927–950. [Google Scholar] [CrossRef]
- Dababat, A.A.; Imren, M.; Erginbas-Orakci, G.; Ashrafi, S.; Yavuzaslanoglu, E.; Toktay, H.; Pariyar, S.R.; Elekcioglu, H.I.; Morgounov, A.; Mekete, T. The importance and management strategies of cereal cyst nematodes, Heterodera spp., in Turkey. Euphytica 2014, 202, 173–188. [Google Scholar] [CrossRef]
- Duncan, L.W.; Moens, M. Migratory endoparasitic nematodes. Plant Nematol. 2006, 123–152. [Google Scholar]
- Hofmann, J.; Wieczorek, K.; Blöchl, A.; Grundler, F.M.W. Sucrose supply to nematode-induced syncytia depends on the apoplasmic and symplasmic pathways. J. Exp. Bot. 2007, 58, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yuen, G.; Wang, Y.; Wei, L.; Ji, G. Evaluation of bacterial biological control agents for control of root-knot nematode disease on tomato. Crop. Prot. 2016, 84, 8–13. [Google Scholar] [CrossRef]
- Collange, B.; Navarrete, M.; Peyre, G.; Mateille, T.; Tchamitchian, M. Root-knot nematode (Meloidogyne) management in vegetable crop production: The challenge of an agronomic system analysis. Crop. Prot. 2011, 30, 1251–1262. [Google Scholar] [CrossRef]
- Kearn, J.; Ludlow, E.; Dillon, J.; O’Connor, V.; Holden-Dye, L. Fluensulfone is a nematicide with a mode of action distinct from anticholinesterases and macrocyclic lactones. Pestic. Biochem. Physiol. 2014, 109, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Feist, E.; Kearn, J.; Gaihre, Y.; O’Connor, V.; Holden-Dye, L. The distinct profiles of the inhibitory effects of fluensulfone, abamectin, aldicarb and fluopyram on Globodera pallida hatching. Pestic. Biochem. Physiol. 2020, 165, 104541. [Google Scholar] [CrossRef]
- Dong, L.Q.; Zhang, K.Q. Microbial control of plant-parasitic nematodes: A five-party interaction. Plant Soil 2006, 288, 31–45. [Google Scholar] [CrossRef]
- Terefe, M.; Tefera, T.; Sakhuja, P. Effect of a formulation of Bacillus firmus on root-knot nematode Meloidogyne incognita infestation and the growth of tomato plants in the greenhouse and nursery. J. Invertebr. Pathol. 2009, 100, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Hajihassani, A. Chemical Nematicides for Control of Plant-Parasitic Nematodes in Georgia Vegetable Crops; College of Agriculture and Environment Sciences Cooperative Extension Service, University of Georgia: Athens, GA, USA, 2018; Bulletin 1502. [Google Scholar]
- Douda, O.; Manasova, M.; Zouhar, M.; Hnatek, J.; Stejskal, V. Field Validation of the Effect of Soil Fumigation of Ethanedinitrile (EDN) on the Mortality of Meloidogyne hapla and Carrot Yield Parameters. Agronomy 2021, 11, 208. [Google Scholar] [CrossRef]
- Youssef, M.M.; Eissa, M.F. Biofertilizers and their role in management of plant parasitic nematodes. A review. E3 J. Biotechnol. Pharm. Res. 2014, 5, 1–6. [Google Scholar]
- Velasco-Azorsa, R.; Cruz-Santiago, H.; del Prado-Vera, I.C.; Ramirez-Mares, M.; Gutiérrez-Ortiz, M.; Santos-Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; León, K.L.-D.; Hernández-Carlos, B. Chemical Characterization of Plant Extracts and Evaluation of their Nematicidal and Phytotoxic Potential. Molecules 2021, 26, 2216. [Google Scholar] [CrossRef]
- Gaur, V. Biofertilizer–necessity for sustainability. J. Adv. Dev. 2010, 1, 7–8. [Google Scholar]
- Abbasi, P.; Riga, E.; Conn, K.; Lazarovits, G. Effect of neem cake soil amendment on reduction of damping-off severity and population densities of plant-parasitic nematodes and soil borne plant pathogens. Can. J. Plant Pathol. 2005, 27, 38–45. [Google Scholar] [CrossRef]
- Galadima, I.B.; Auwal, H.M.; Abubakar, I.; Madu, J.; Joseph, P. Enhanced Nematicidal Effect of Cow dung Soil Amendment by Neem (Azadirachta indica). OALib 2015, 2, 1–4. [Google Scholar] [CrossRef]
- Oka, Y. Mechanisms of nematode suppression by organic soil amendments—A review. Appl. Soil Ecol. 2010, 44, 101–115. [Google Scholar] [CrossRef]
- McSorley, R. Overview of Organic Amendments for Management of Plant-Parasitic Nematodes, with Case Studies from Florida. J. Nematol. 2011, 43, 69–81. [Google Scholar]
- Aires, A.; Carvalho, R.; Barbosa, M.D.C.; Rosa, E. Suppressing Potato Cyst Nematode, Globodera rostochiensis, with Extracts of Brassicaceae Plants. Am. J. Potato Res. 2009, 86, 327–333. [Google Scholar] [CrossRef]
- Wang, K.; Juncea, B.Y.C.; Napus, B.; Erecta, T. Suppression of Rotylenchulus reniformis by Crotalaria juncea, Brassica napus, and Targetes erecta. Nematropica 2001, 31, 235–249. [Google Scholar]
- Akhtar, M.; Malik, A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: A review. Bioresour. Technol. 2000, 74, 35–47. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y. Effect of compost and chemical fertilizer on soil nematode community in a Chinese maize field. Eur. J. Soil Biol. 2010, 46, 230–236. [Google Scholar] [CrossRef]
- Thoden, T.C.; Korthals, G.W.; Termorshuizen, A.J. Organic amendments and their influences on plant-parasitic and free-living nematodes:A promising method for nematode management? Nematology 2011, 13, 133–153. [Google Scholar] [CrossRef]
- Korthals, G.; Thoden, T.; Berg, W.V.D.; Visser, J. Long-term effects of eight soil health treatments to control plant-parasitic nematodes and Verticillium dahliae in agro-ecosystems. Appl. Soil Ecol. 2014, 76, 112–123. [Google Scholar] [CrossRef]
- Mao, L.; Wang, Q.; Yan, D.; Li, Y.; Ouyang, C.; Guo, M.; Cao, A. Flame soil disinfestation: A novel, promising, non-chemical method to control soilborne nematodes, fungal and bacterial pathogens in China. Crop. Prot. 2016, 83, 90–94. [Google Scholar] [CrossRef]
- Giannakou, I.; Anastasiadis, I.; Gowen, S.; Prophetou-Athanasiadou, D. Effects of a non-chemical nematicide combined with soil solarization for the control of root-knot nematodes. Crop. Prot. 2007, 26, 1644–1654. [Google Scholar] [CrossRef]
- Alarcón, A.; Chávez, M.G.; Cerrato, R.F.; Monter, A.V. Effectiveness of Glomus fasciculatum and Glomus etunicatum in the growth of Vitis vinifera L. seedlings obtained by micropropagation. Terra Latinoam. 2001, 19, 29–35. [Google Scholar]
- Zhang, K.-Q.; Hyde, K.D. Nematode-Trapping Fungi; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Brand, D.; Soccol, C.R.; Sabu, A.; Roussos, S. Production of fungal biological control agents through solid state fermentation: A case study on Paecilomyces lilacinus against root-knot nematodes. Micol. Apl. Int. 2010, 22, 31–48. [Google Scholar]
- Twamley, T.; Gaffney, M.; Feechan, A. A Microbial Fermentation Mixture Primes for Resistance Against Powdery Mildew in Wheat. Front. Plant Sci. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, L.Y.; Zhou, X.B. Effects of Myrothecium verrucaria ZW-2 fermentation filtrates on various plant-parasitic nematodes. J. Plant Dis. Prot. 2020, 127, 545–552. [Google Scholar] [CrossRef]
- Hallmann, J.; Sikora, R.A. Toxicity of fungal endophyte secondary metabolites to plant parasitic nematodes and soil-borne plant pathogenic fungi. Eur. J. Plant Pathol. 1996, 102, 155–162. [Google Scholar] [CrossRef]
- Molinari, S.; Leonetti, P. Bio-control agents activate plant immune response and prime susceptible tomato against root-knot nematodes. PLoS ONE 2019, 14, e0213230. [Google Scholar] [CrossRef]
- Sikora, R.A.; Schafer, K.; Dababat, A.A. Modes of action associated with microbially induced in planta suppression of plant-parasitic nematodes. Australas. Plant Pathol. 2007, 36, 124–134. [Google Scholar] [CrossRef]
- Zuckerman, B.M.; Dicklow, M.B.; Acosta, N. A Strain of Bacillus thuringiensis for the Control of Plant—Parasitic Nematodes. Biocontrol Sci. Technol. 1993, 3, 41–46. [Google Scholar] [CrossRef]
- El-Nagdi, W.M.A.; Youssef, M.M.A. Soaking faba bean seed in some bio-agents as prophylactic treatment for controlling Meloidogyne incognita root-knot nematode infection. J. Pest Sci. 2004, 77, 75–78. [Google Scholar] [CrossRef]
- Khalil, M. Abamectin and Azadirachtin as Eco-friendly Promising Biorational Tools in Integrated Nematodes Management Programs. J. Plant Pathol. Microbiol. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Spiegel, Y.; Chet, I. Evaluation of Trichoderma spp. as a Biocontrol Agent against Soil borne Fungi and Plant-parasitic Nematodes in Israel. Integr. Pest Manag. Rev. 1998, 3, 169–175. [Google Scholar] [CrossRef]
- Walters, T.W.; Pinkerton, J.N.; Riga, E.; Zasada, I.A.; Particka, M.; Yoshida, H.A.; Ishida, C. Managing Plant–Parasitic Nematodes in Established Red Raspberry Fields. Hort Technol. 2009, 19, 762–768. [Google Scholar] [CrossRef]
- Pulavarty, A.; Daly, R.; Horgan, K.; Kakouli-Duarte, T. Effect of Alltech® Crop Science products on root-knot nematode attraction and infestation in tomato plants. Acta Agric. Scand. Sect. B Plant Soil Sci. 2021, 1–10. [Google Scholar] [CrossRef]
- Pulavarty, A.; Horgan, K.; Kakouli-Duarte, T. Effect of an Alltech soil health product on entomopathogenic nematodes, root-knot nematodes and on the growth of tomato plants in the greenhouse. J. Nematol. 2020, 52, 1–10. [Google Scholar] [CrossRef]
- Miamoto, A.; Silva, M.T.R.; Dias-Arieira, C.R.; Puerari, H.H. Alternative products for Pratylenchus brachyurus and Meloidogyne javanica management in soya bean plants. J. Phytopathol. 2017, 165, 635–640. [Google Scholar] [CrossRef]
- Saikia, S.K.; Tiwari, S.; Pandey, R. Rhizospheric biological weapons for growth enhancement and Meloidogyne incognita management in Withania somnifera cv. poshita. Biol. Control 2013, 65, 225–234. [Google Scholar] [CrossRef]
- Anastasiadis, I.; Giannakou, I.; Prophetou-Athanasiadou, D.; Gowen, S. The combined effect of the application of a biocontrol agent Paecilomyces lilacinus, with various practices for the control of root-knot nematodes. Crop. Prot. 2008, 27, 352–361. [Google Scholar] [CrossRef]
| Nematode Taxa | Feeding Type | Mean Relative Abundance (±SD) | |
|---|---|---|---|
| Family | Genus | ||
| Dolichodoridae | Merlinius | Ectoparasites | 6.90 ± (0.47) |
| Tylenchidae | Aglenchus | Epidermal/root hair feeders | 6.05 ± (1.34) |
| Tylenchidae | Neopsilenchus | Epidermal/root hair feeders | 2.07 ± (0.51) |
| Longidoridae | Xiphinema | Ectoparasites | 1.39 ± (0.450) |
| Hoplolaimidae | Helicotylenchus | Semi-endoparasites | 1.01 ± (0.31) |
| Paratylenchidae | Pratylenchus | Migratory endoparasites | 0.98 ± (0.52) |
| Longidoridae | Longidorus | Ectoparasites | 0.62 ± (0.24) |
| Meloidogynidae | Meloidogyne | Sedentary parasites | 0.33 ± (0.03) |
| Tylenchidae | Boleodorus | Epidermal/root hair feeders | 0.12 ± (0.01) |
| Meloidogynidae | Meloidogyne | Sedentary parasites | 0.11 ± (0.01) |
| Tylenchidae | Basiria | Epidermal/root hair feeders | 0.021 ± (0.00) |
| Paratylenchidae | Pratylenchus | Migratory endoparasites | 0.015 ± (0.00) |
| Tylenchidae | Tylenchus | Epidermal/root hair feeders | 0.008 ± (0.00) |
| Tylenchidae | Lelenchus | Epidermal/root hair feeders | 0.002 ± (0.00) |
| Nematode Taxa | Feeding Type | Mean Relative Abundance (±SD) | |
|---|---|---|---|
| Family | Genus | ||
| Telotylenchidae | Amplimerlinius | Herbivores–ectoparasites | 1.00 ± (0.00) |
| Telotylenchidae | Bitylenchus | Herbivores–ectoparasites | 0.50 ± (0.70) |
| Tylenchidae | Cephalenchus | Herbivores–epidermal/root hair feeders | 1.00 ± (1.00) |
| Dolichodoridae | Dolichodorus | Herbivores–ectoparasites | 1.00 ± (0.00) |
| Dolichodoridae | Dolichorhynchus | Herbivores–ectoparasites | 1.00 ± (0.00) |
| Hoplolaimidae | Helicotylenchus | Herbivores–semi-endoparasites | 1.25 ± (0.22) |
| Tylenchidae | Malenchus | Herbivores–epidermal/root hair feeders | 1.33 ± (0.29) |
| Heteroderidae | Meloidogyne | Herbivores–sedentary parasites | 1.00 ± (0.00) |
| Ironidae | Ironus | Predators | 1.00 ± (0.00) |
| Pratylenchidae | Pratylenchoides | Herbivores–semi-endoparasites | 1.00 ± (0.00) |
| Pratylenchidae | Pratylenchus | Herbivores–migratory endoparasites | 9.40 ± (1.32) |
| Hoplolaimidae | Rotylenchus | Herbivores–semi-endoparasites | 2.00 ± (0.26) |
| Trichodoridae | Trichodorus | Herbivores–ectoparasites | 1.00 ± (0.00) |
| Belonolaimidae | Tylenchorhynchus | Herbivores–ectoparasites | 4.00 ± (0.00) |
| Tylenchidae | Tylenchus | Herbivores–epidermal/root hair feeders | 7.33 ± (1.11) |
| Longidoridae | Xiphinema | Herbivores–ectoparasites | 1.33 ± (0.29) |
| Nematode Groups | Genus | Feeding Type | Physical Manifestations | Mode of Action |
|---|---|---|---|---|
| Root-Knot | Meloidogyne spp. | Obligate | Forms galls (root-knots) on infected roots | Feeds on giant cells of the root and suppresses the host defence mechanisms |
| Cyst | Heterodera and Globodera spp. | Obligate biotrophs | Forms cysts (enclosing eggs) due to a large multinucleate feeding structure called the syncytium | Dissolves plant cell walls and fuses protoplasts Effectors target the host cell nucleus and suppress plant defence mechanisms. |
| Root lesion | Pratylenchus spp. | Polyphagous, migratory, intercellular root endoparasites | Formation of lesions, necrotic areas, browning and plant cell death, often followed by root rotting. | Secretions from pharyngeal glands have effectors that degrade plant cell walls. |
| Burrowing | Radopholus similis | Migratory endoparasite | Weakens the root system, forms dark lesions, root rotting and causes toppling disease | The effectors contain plant-cell-wall-degrading enzymes like cellulases, pectate lyases and xylanases. |
| Stem and bulb | Ditylenchus dipsaci | Migratory endoparasite | Causes stunted growth, twisted stems and the discoloration of bulbs | Feeds on the parenchymatous cells of the cortex and produces cell-wall-softening enzymes and effectors such as expansins |
| Pine wilt | Bursaphelencus xylophilus | Migratory endoparasite | Completely infects and kills all the pine trees growing in an area | Parasitizes plants with the help of cellulose-degrading proteins like glycoside hydrolase It is carried with the help of insect vector, Monochamus beetles. |
| Reniform | Rotylenchulus reniformis | Sedentary Semi-endoparasite | Leads to moisture and nutrient deficiency in infected host along with root necrosis, chlorosis and stunted growth | Feeds on pericycle and endodermal root cells by inserting 1/3 of their anterior body Cell walls break to form a two-cell-deep syncytium. |
| Large plant parasitic species | Xiphinema index | Ectoparasite | Infection retards root extension, causes swelling and gall formation | Have feeding mechanisms similar to root-knot nematodes |
| False-root knot | Nacobbus aberrans | Migratory juveniles Sedentary adult female | Causes cavities and lesions on root tissues; root galls are formed around feeding sites | Induces the partial dissolution the of cell wall and the fusion of protoplasts to form a syncytium |
| White tip disease variety | Aphelenchoides besseyi | Ecto/endo parasite Fungivorous | Infected plants have stunted growth, and other symptoms include chlorotic white tips on leaves, leaf necrosis, reduction in rice grain size and number | Does not induce re-differentiation of plant cells Local cell damage, tissue disintegration and browning in epidermal cells and palisade parenchyma |
| Fermentation Extracts/Microbial Based Products | Target Nematode | Plants | Reference |
|---|---|---|---|
| Fermentation filtrate of Myrothecium verrucaria | M. incognita, H. glycines, B. xylophilus, Hirschmanniella spp. | Soybean and tomato | [83] |
| Paecilomyces lilacinus produced through the solid-state fermentation | Meloidogyne spp. | Tomato | [79] |
| Bacillus firmus, commercial WP formulation (BioNem) (*WP:Wettable powder) | M. incognita | Tomato | [61] |
| Isolate of Bacillus thuringiensis | R. reniformis M. incognita P. penetrans | Tomatoes, pepper and strawberry | [87] |
| Abamectin (a fermentation product from Streptomyces avermitilis), a strain of B. thuringiensis, Nemaless (containing strains of Serratia marcescens) | M. incognita | Faba beans | [88] |
| Abamectin (a fermentation product from Streptomyces avermitilis) | M. incognita, M. javanica, Radopholus similis, D. dipsaci | Tomato, banana, garlic, cucumber | [89] |
| Bacillus methylotrophicus strain R2-2 and Lysobacter antibioticus strain 13-6 | M. incognita | Tomato | [56] |
| Strains of F. oxysporum | M. incognita | Lettuce | [84] |
| Trichoderma harzianum and Trichoderma lignorum isolates | M. javanica | Tomato | [90] |
| DiTera (a fermentation product of the fungus Myrothecium verrucaria) | P. penetrans | Red raspberry (Rubus idaeus) | [91] |
| ACS5075 (proprietary blend of fermentation and plant extracts with micronutrients, Alltech, Inc., Nicholasville, KY USA) and ACS3048 (a microbial-based product) | M. javanica, M. incognita | Tomato | [92] [93] |
| Compost- Aid (Lactobacillus plantarum, Bacillus subtilis and Enterococcus faecium) | Pratylenchus brachyurus and M. javanica | Soyabean | [94] |
| Bacillus megaterium (ATCC No. 14581), Pseudomonas fluorescens (ATCC No. 13525), Trichoderma viride (MTCC No. 167), Paecilomyces lilacinus (PDBC PL55) and Glomus intraradices | M. incognita | Withania somnifera L. | [95] |
| BioNem (containing lyophilised bacteria spores of Bacillus firmus) | Meloidogyne spp. | Tomatoes | [78] |
| Micosat (by C.C.S. Aosta, Italy) (constituted by 40% roots hosting arbuscular mycorrhiza forming fungi of Glomus spp. and a mixture of antagonistic fungi, rhizobacteria and yeasts) | M. incognita | Tomatoes | [85] |
| Bioact (P. lilacinus and Bacillus firmus) | Meloidogyne spp. | Tomatoes | [96] |
| Bacillus sphaericus B43 | M. incognita | Tomatoes | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulavarty, A.; Egan, A.; Karpinska, A.; Horgan, K.; Kakouli-Duarte, T. Plant Parasitic Nematodes: A Review on Their Behaviour, Host Interaction, Management Approaches and Their Occurrence in Two Sites in the Republic of Ireland. Plants 2021, 10, 2352. https://doi.org/10.3390/plants10112352
Pulavarty A, Egan A, Karpinska A, Horgan K, Kakouli-Duarte T. Plant Parasitic Nematodes: A Review on Their Behaviour, Host Interaction, Management Approaches and Their Occurrence in Two Sites in the Republic of Ireland. Plants. 2021; 10(11):2352. https://doi.org/10.3390/plants10112352
Chicago/Turabian StylePulavarty, Anusha, Aoife Egan, Anna Karpinska, Karina Horgan, and Thomais Kakouli-Duarte. 2021. "Plant Parasitic Nematodes: A Review on Their Behaviour, Host Interaction, Management Approaches and Their Occurrence in Two Sites in the Republic of Ireland" Plants 10, no. 11: 2352. https://doi.org/10.3390/plants10112352
APA StylePulavarty, A., Egan, A., Karpinska, A., Horgan, K., & Kakouli-Duarte, T. (2021). Plant Parasitic Nematodes: A Review on Their Behaviour, Host Interaction, Management Approaches and Their Occurrence in Two Sites in the Republic of Ireland. Plants, 10(11), 2352. https://doi.org/10.3390/plants10112352







