Ferulic Acid and Salicylic Acid Foliar Treatments Reduce Short-Term Salt Stress in Chinese Cabbage by Increasing Phenolic Compounds Accumulation and Photosynthetic Performance
Abstract
:1. Introduction
2. Results
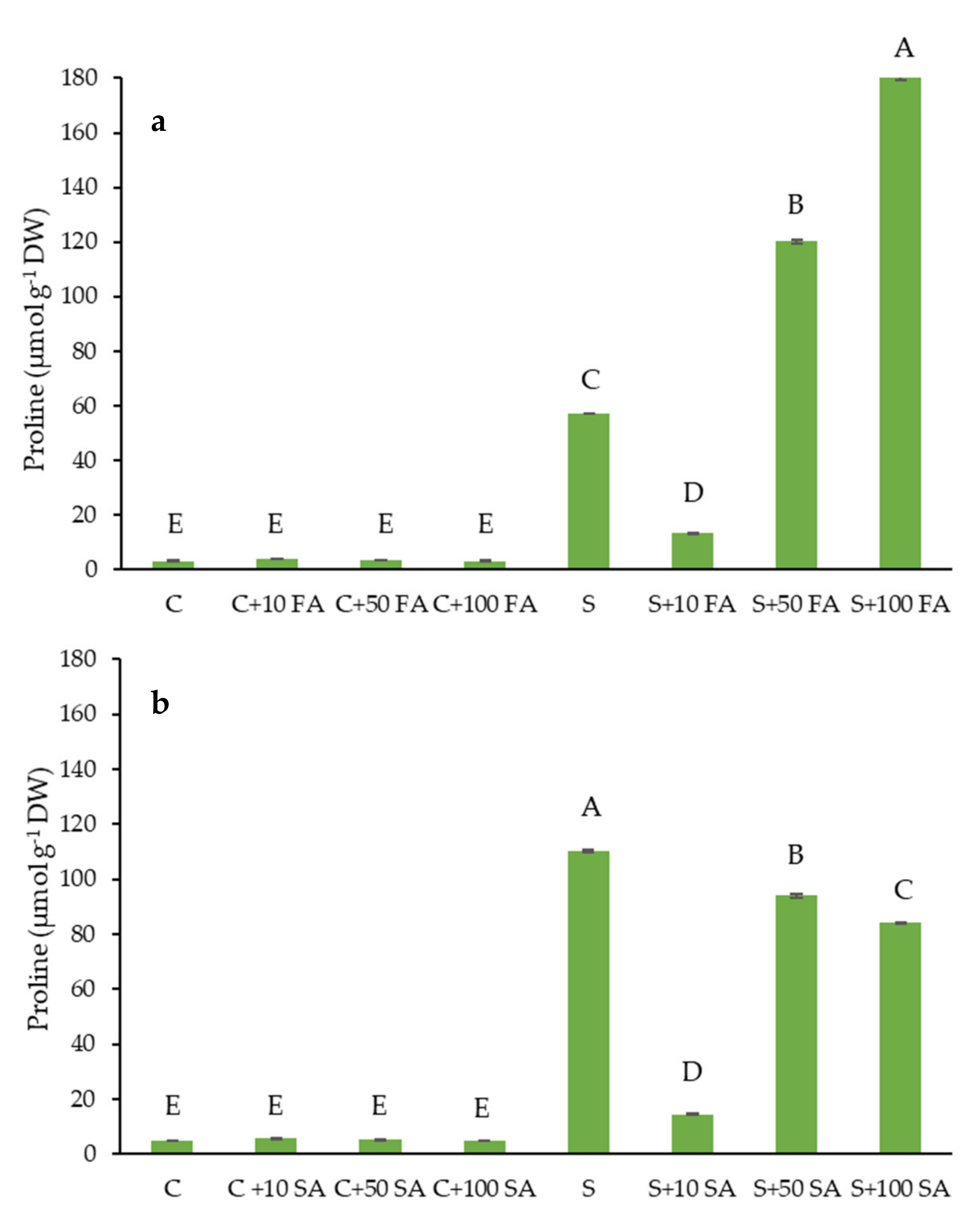
2.1. Proline Level
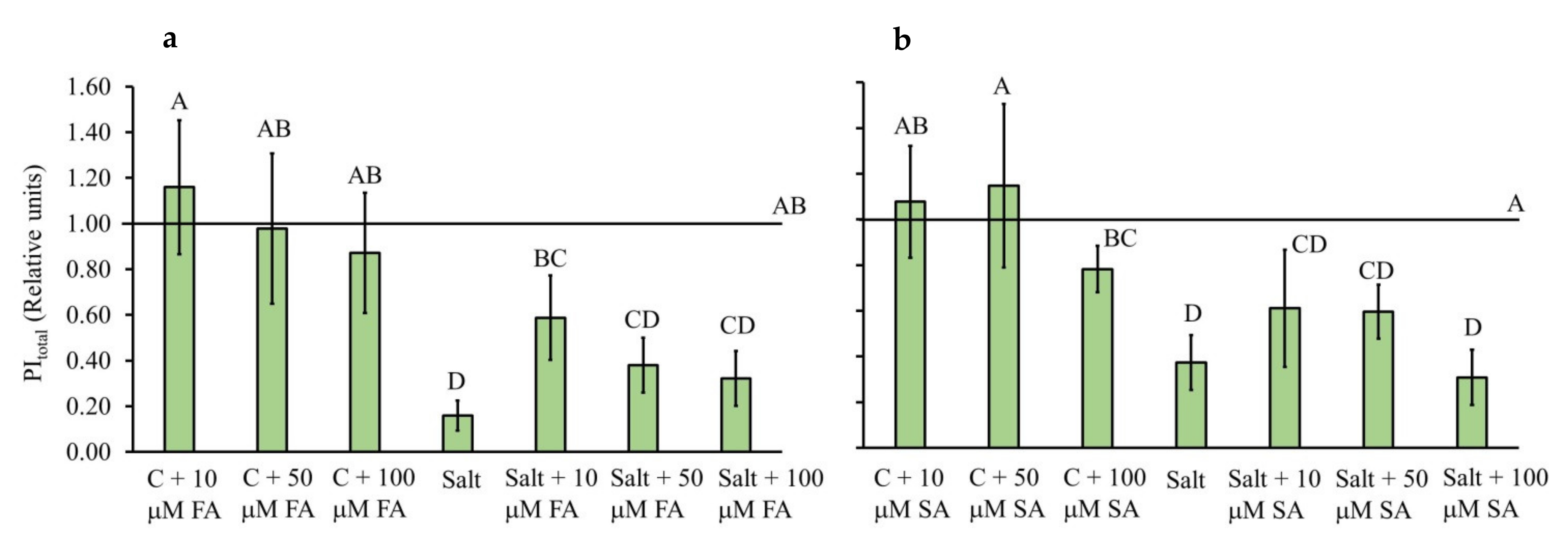
2.2. Chlorophyll a Fluorescence Parameters
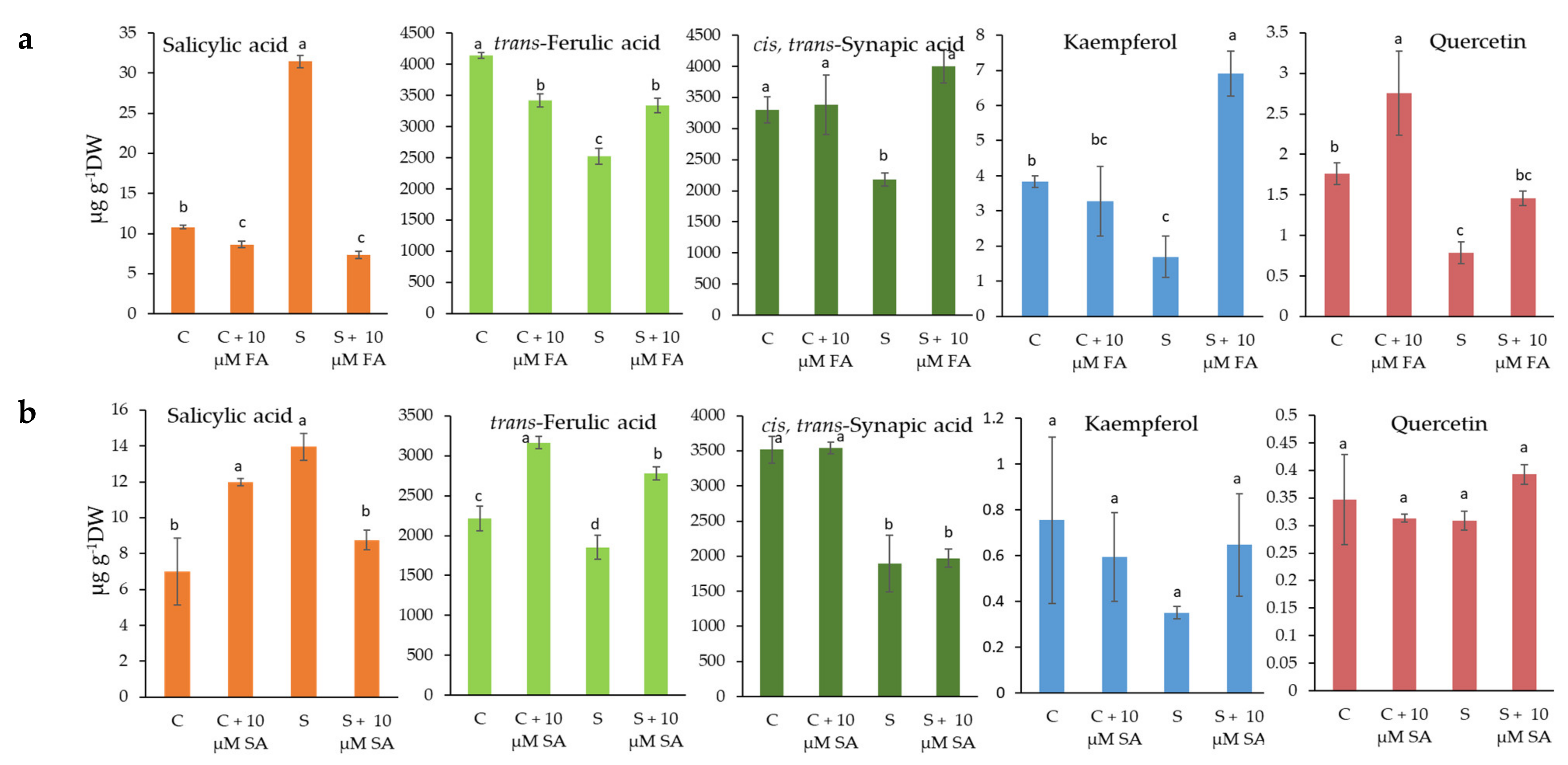
2.3. Phenolic Compounds and Antioxidative Activities
3. Discussion
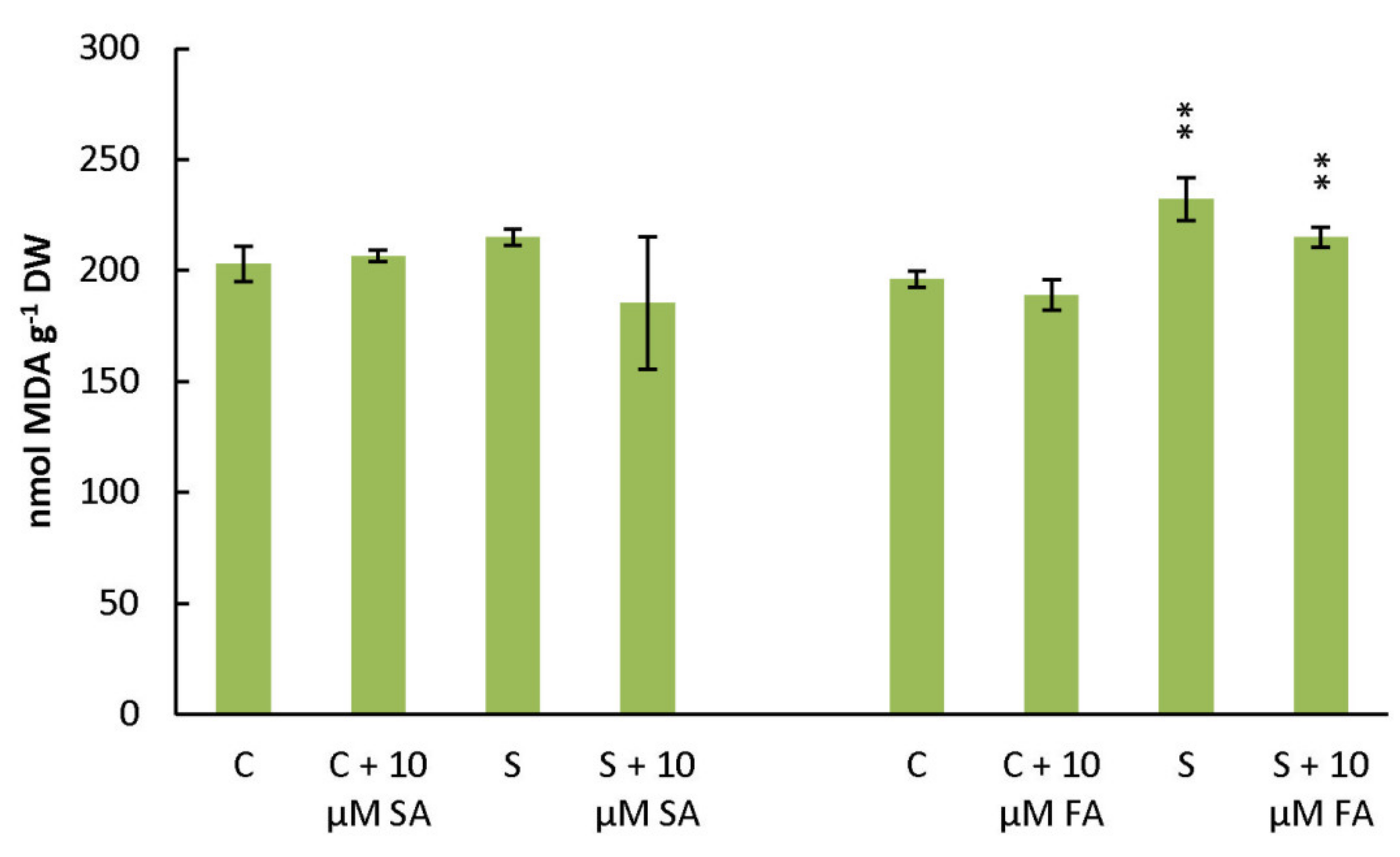
3.1. Attenuating Effects of FA and SA on Short-Term Salinity Stressed Plants: Stress Markers
3.2. FA and SA Treatments Stabilize Photosynthetic Parameters under Salinity Stress
3.3. FA and SA Treatments Increase an Accumulation of Phenolic Antioxidants and Antioxidant Activity under Stress Conditions
3.4. Conclusions
4. Materials and Methods
4.1. Plant Material and Experimental Conditions
4.2. Fast Chlorophyll a Fluorescence Kinetics
4.3. Proline Analysis
4.4. Determination of Phenolic Compounds and Antioxidant Activity
4.5. Extraction and LC-MS/MS Analysis of Selected Phenolic Compounds
4.6. Lipid Peroxidation Measurement
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, F.C.; Baldos, U.; Hertel, T.; Diaz, D. New science of climate change impacts on agriculture implies higher social cost of carbon. Nat. Commun. 2017, 8, 1607. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.N.; Tanveer, M.; Abbas, A.; Fahad, S.; Baloch, M.S.; Ahmad, M.I.; Saud, S.; Song, Y. Targeting salt stress coping mechanisms for stress tolerance in Brassica: A research perspective. Plant Physiol. Biochem. 2021, 158, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Patel, M.K.; Kumar, M.; Li, W.; Luo, Y.; Burritt, D.J.; Alkan, N.; Tran, L.-S.P. Enhancing salt tolerance of plants: From metabolic reprogramming to exogenous chemical treatments and molecular approaches. Cells 2020, 9, 2492. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M. Relationships between growth and gas exchange characteristics in some salt-tolerant amphidiploid Brassica species in relation to their diploid parents. Environ. Exp. Bot. 2001, 45, 155–163. [Google Scholar] [CrossRef]
- Chakraborty, K.; Bose, J.; Shabala, L.; Eyles, A.; Shabala, S. Evaluating relative contribution of osmotolerance and tissue tolerance mechanisms toward salinity stress tolerance in three Brassica species. Physiol. Plant. 2016, 158, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, I.; Mlinarić, S.; Tarkowská, D.; Oklestková, J.; Novák, O.; Lepeduš, H.; Vujčić Bok, V.; Radić Brkanac, S.; Strnad, M.; Salopek-Sondi, B. Early Brassica crops responses to salinity stress: A comparative analysis between Chinese cabbage, White cabbage and Kale. Front. Plant Sci. 2019, 10, 450. [Google Scholar] [CrossRef] [Green Version]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Ann. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M., Prasad, M., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany; LLC: New York, NY, USA, 2013; pp. 25–87. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, H.; Bhardwaj, R.D.; Grewal, S.K. Mitigation of salinity-induced oxidative damage in wheat (Triticum aestivum L.) seedlings by exogenous application of phenolic acids. Acta Physiol. Plant. 2017, 39, 221–236. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y.; Zhang, Y.; Zhang, L.; Zhou, Z.Q.; Li, X.; Shi, Q.; Wang, X.J.; Bai, J.G. Caffeic acid protects cucumber against chilling stress by regulating antioxidant enzyme activity and proline and soluble sugar contents. Acta Physiol. Plant. 2015, 37, 1706–1715. [Google Scholar] [CrossRef]
- El-Soud, W.A.; Hegab, M.M.; Elgawad, H.A.; Zinta, G.; Asard, H. Ability of ellagic acid to alleviate osmotic stress on chickpea seedlings. Plant Physiol. Biochem. 2013, 71, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Linić, I.; Šamec, D.; Grúz, J.; Vujčić Bok, V.; Strnad, M.; Salopek-Sondi, B. Involvement of phenolic acids in short-term adaptation to salinity stress is species specific among Brassicaceae. Plants 2019, 8, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of g-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta 2010, 1797, 1428–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Karmowska, K. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Kalaji, H.M.; Goltsev, V.; Paunov, M.; Rapacz, M.; Wójcik-Jagła, M.; Pawluśkiewicz, B.; Bąba, W.; Brestic, M. Exploration of chlorophyll a fluorescence and plant gas exchange parameters as indicators of drought tolerance in perennial ryegrass. Sensors 2019, 19, 2736. [Google Scholar] [CrossRef] [Green Version]
- Mageney, V.; Neugart, S.; Albach, D.C. A guide to the variability of flavonoids in Brassica oleracea. Molecules 2017, 22, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Gharsallah, C.; Fakhfakh, H.; Grubb, D.; Gorsane, F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AOB Plants 2016, 8, plw055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Mir, B.A.; Wani, A.S.; Hasan, S.A.; Irfan, M.; Ahmad, A. Screening of salt-tolerant genotypes of Brassica juncea based on photosynthetic attributes. J. Plant Interact. 2011, 6, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Maurya, D.K.; Devasagayam, T.P.A. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-M.; Nie, Y.-X.; Zhang, J.; Yin, J.-S.; Li, Q.; Wang, X.-J.; Bai, J.-G. Ferulic acid pretreatment enhances dehydration-stress tolerance of cucumber seedlings. Biol. Plant. 2013, 57, 711–717. [Google Scholar] [CrossRef]
- Cheng, Z.-Y.; Sun, L.; Wang, X.-J.; Sun, R.; An, Y.-Q.; An, B.-L.; Zhu, M.-X.; Zhao, C.-F.; Bai, J.-G. Ferulic acid pretreatment alleviates heat stress in blueberry seedlings by inducing antioxidant enzymes, proline, and soluble sugars. Biol. Plant. 2018, 62, 534–542. [Google Scholar] [CrossRef]
- Pavlović, I.; Petřík, I.; Tarkowska, D.; Lepeduš, H.; Vujčić, V.; Radić Brkanac, S.; Novák, O.; Salopek-Sondi, B. Correlations between phytohormones and drought tolerance in selected Brassica crops: Chinese cabbage, white cabbage and kale. Int. J. Mol. Sci. 2018, 19, 2866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, I.A.A.; Shalby, N.; Bai, C.; Qin, M.; Agami, R.A.; Jie, K.; Wang, B.; Zhou, G. Stomatal and photosynthetic traits are associated with investigating sodium chloride tolerance of Brassica napus L. cultivars. Plants 2020, 9, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hniličková, H.; Hnilička, F.; Martinková, J.; Kraus, K. Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant Soil Environ. 2017, 63, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Syeed, S.; Anjum, N.A.; Nazar, R.; Iqbal, N.; Masood, A.; Khan, N.A. Salicylic acid-mediated changes in photosynthesis, nutrients content and antioxidant metabolism in two mustard (Brassica juncea L.) cultivars differing in salt tolerance. Acta Physiol. Plant. 2011, 33, 877–886. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Secondary metabolites, ferulic acid and p-hydroxybenzoic acid induced toxic effects on photosynthetic process in Rumex acetosa L. Biomolecules 2021, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, E.; Fusaro, L.; Strasser, R.J.; Bussotti, F.; Manes, F. Effects of acute O3 stress on PSII and PSI photochemistry of sensitive and resistant snap bean genotypes (Phaseolus vulgaris L.), probed by prompt chlorophyll “a” fluorescence and 820 nm modulated reflectance. Plant Physiol. Biochem. 2015, 97, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Ceppi, M.G.; Oukarroum, A.; Çiçek, N.; Strasser, R.J.; Schansker, G. The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: A study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol. Plant. 2012, 144, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Horváth, E.; Csiszár, J.; Gallé, Á.; Poór, P.; Szepesi, Á.; Tari, I. Hardening with salicylic acid induces concentration-dependent changes in abscisic acid biosynthesis of tomato under salt stress. J. Plant Physiol. 2015, 183, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Husen, A.; Iqbal, M.; Sohrab, S.S.; Ansari, M.K.A. Salicylic acid alleviates salinity-caused damage to foliar functions, plant growth and antioxidant system in Ethiopian mustard (Brassica carinata A. Br.). Agric. Food Secur. 2018, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Hura, T.; Hura, K.; Grzesiak, S. Possible contribution of cell-wall-bound ferulic acid in drought resistance and recovery in triticale seedlings. J. Plant Physiol. 2009, 166, 1720–1733. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xia, H.; Liu, Y.; Wei, H.; Zheng, X.; Song, C.; Chen, L.; Liu, H.; Luo, L. Transcriptomic and metabolomic studies disclose key metabolism pathways contributing to well-maintained photosynthesis under the drought and the consequent drought-tolerance in rice. Front. Plant Sci. 2016, 7, 1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buhmann, A.; Papenbrock, J. An economic point of view of secondary compounds in halophytes. Funct. Plant Biol. 2013, 40, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Guy, A.; Galili, G.; Dor, E.; Schweitzer, R.; Amir, R.; Hacham, Y. Enhanced production of aromatic amino acids in tobacco plants leads to increased phenylpropanoid metabolites and tolerance to stresses. Front. Plant Sci. 2021, 11, 604349. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Zhao, S.; Bian, L.; Chen, X. Saline stress enhanced accumulation of leaf phenolics in honeysuckle (Lonicera japonica Thunb.) without induction of oxidative stress. Plant Physiol. Biochem. 2017, 112, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef] [PubMed]
- Minh, L.T.; Khang, D.T.; Ha, P.T.T.; Tuyen, P.T.; Minh, T.N.; Quan, N.V.; Xuan, T.D. Effects of salinity stress on growth and phenolics of rice (Oryza sativa L.). Int. Lett. Nat. Sci. 2016, 57, 1–10. [Google Scholar] [CrossRef]
- Santangeli, M.; Capo, C.; Beninati, S.; Pietrini, F.; Forni, C. Gradual exposure to salinity improves tolerance to salt stress in rapeseed (Brassica napus L.). Water 2019, 11, 1667. [Google Scholar] [CrossRef] [Green Version]
- Šamec, D.; Linić, I.; Salopek-Sondi, B. Salinity stress as an elicitor for phytochemicals and minerals accumulation in selected leafy vegetables of Brassicaceae. Agronomy 2021, 11, 361. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The Role of Quercetin in Plants. Authorea 2021. (preprint). [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mohsin, S.M.; Fujita, M. Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, M.; Zhang, Y.; Mu, G.; Cui, S.; Yang, X.; Liu, L. Molecular cloning and expression characterization of flavonol synthase genes in peanut (Arachis hypogaea). Sci. Rep. 2020, 10, 17717. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.-P.; Zhang, L.; Yan, P.; Ahammed, G.J.; Han, W.-Y. Methyl salicylate enhances flavonoid biosynthesis in tea leaves by stimulating the phenylpropanoid pathway. Molecules 2019, 24, 362. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics witphosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 4th ed.; Council of Europe: Strasbourg, France, 2004; pp. 2377–2378. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Bartoszek, A.; Wolska, L.; Drzewiecki, J.; Gorinstein, S.; Namiésnik, J. Partial characterization of white cabbages (Brassica oleracea var. capitata f. alba) from different regions by glucosinolates, bioactive compounds, total antioxidant activities and proteins. LWT-Food Sci. Technol. 2008, 41, 1–9. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth. Enzymol. 1999, 299, 15–27. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Šamec, D.; Kruk, V.; Ivanišević, P. Influence of seed origin on morphological characteristics and phytochemicals levels in Brassica oleracea var. acephala. Agronomy 2019, 9, 502. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]


| Recorded Parameters | Description |
|---|---|
| F0 | Minimal fluorescence intensity (20 μs) |
| Fm | Maximal fluorescence intensity |
| VJ = (FJ − F0)/(Fm − F0) | Relative variable fluorescence at 2 ms |
| VI = (FI − F0)/(Fm − F0) | Relative variable fluorescence at 30 ms |
| FV = Fm − F0 | Maximal variable fluorescence |
| M0 = (ΔV/Δt)0 | Approximated initial slope of relative variable fluorescence Fv |
| φP0 = TR0/ABS = TR0/ABS | Maximum quantum yield of PSII |
| ψE0 = ET0/TR0 = 1 − VJ | Probability that trapped exciton moves an electron further than QA− |
| φE0 = ET0/ABS = [1 − (F0/Fm)](1 − VJ) | Quantum yield for electron transport |
| δR0 = RE0 − ET0 = (1 − VI)/(1 − VJ) | Probability with which an electron from the intersystem electron carriers moves to reduce end electron acceptors at the PSI acceptor side |
| φR0 = RE0/ABS = [1 − (F0/Fm)]ψE0 δR0 | Quantum yield for reduction of end electron acceptors at the PSI acceptor side |
| RC/CS0 = (ABS/CS)/(ABS/RC) | Density of active RC per excited cross section |
| ABS/RC = M0(1/VJ)[1/φP0] | Absorption flux per active RC |
| TR0/RC = M0(1/VJ) | Trapping flux per active RC |
| ET0/RC = M0(1/VJ)(1 − VJ) | Electron transport flux per active RC |
| DI0/RC = (ABS/RC) − (TR0/RC) | Dissipation flux per active RC |
| RE0/RC = M0(1/VJ)ψE0 δR0 | Electron flux reducing end electron acceptors at the PSI acceptor side, per RC |
| γRC = ChlRC/Chltotal = RC/(ABS + RC) | Probability that a PSII Chl molecule functions as RC |
| PIABS = [γRC/(1 − γRC)][φP0/(1 − φP0)][ψE0/(1 − ψE0)] | Performance index on absorption basis |
| PItotal = PIABS[φR0/(1 − φR0)] | Performance index for energy conservation from exciton to the reduction of PSI end acceptors |
| Groups of Specialized Metabolites | Antioxidant Activity | |||||
|---|---|---|---|---|---|---|
| Treatment | Total Polyphenols | Total Phenolic Acids | Total Flavonoids | Total Flavanols | DPPH Assay | FRAP Assay |
| mg GAE g− 1 DW (%) | mg CAE g−1 DW (%) | mg CE g−1 DW (%) | µg CE g−1 DW (%) | µmol TE g−1 DW (%) | µmol Fe2+ g−1 DW (%) | |
| C | 32.66 ± 0.40 c (100) | 6.91 ± 0.08 a (100) | 5.63 ± 0.03 a (100) | 66.46 ± 0.19 bc (100) | 41.76 ± 0.60 b (100) | 41.07 ± 0.10 b (100) |
| C + 10 µM FA | 35.03 ± 0.20 a (107.26) | 6.01 ± 0.02 b (86.98) | 5.69 ± 0.04 a (101.07) | 75.57 ± 0.79 a (113.71) | 40.23 ± 0.17 c (96.34) | 44.73 ± 0.54 a (108.91) |
| C + 50 µM FA | 25.52 ± 0.04 d (78.14) | 4.10 ± 0.04 g (59.33) | 4.12 ± 0.01 d (73.18) | 65.38 ± 0.55 c (98.37) | 34.66 ± 0.23 f (83.00) | 32.42 ± 0.21 e (78.94) |
| C + 100 µM FA | 32.45 ± 0.11 c (99.36) | 5.36 ± 0.03 c (77.57) | 5.24 ± 0.04 b (93.07) | 58.27 ± 0.93 d (87.68) | 37.00 ± 0.23 e (88.60) | 37.02 ± 0.56 d (90.14) |
| S | 23.57 ± 0.04 e (72.17) | 4.32 ± 0.01 f (62.52) | 3.81 ± 0.01 f (67.67) | 51.02 ± 0.67 f (76.77) | 38.98 ± 0.06 d (93.34) | 25.99 ± 0.18 fg (63.28) |
| S + 10 µM FA | 34.22 ± 0.12 b (104.78) | 5.25 ± 0.03 c (75.98) | 4.85 ± 0.01 c (86.15) | 67.97 ± 0.93 b (102.27) | 51.39 ± 0.23 a (123.06) | 39.74 ± 0.46 c (96.76) |
| S + 50 µM FA | 23.60 ± 0.18 e (72.26) | 4.84 ± 0.04 d (70.04) | 3.71 ± 0.03 g (65.90) | 44.04 ± 0.57 g (66.27) | 39.85 ± 0.13 cd (95.43) | 25.27 ± 0.29 g (61.53) |
| S + 100 µM FA | 24.07 ± 0.13 e (73.70) | 4.52 ± 0.03 e (65.41) | 3.97 ± 0.03 e (70.52) | 53.08 ± 0.54 e (79.87) | 37.75 ± 0.50 e (90.40) | 26.76 ± 0.07 f (65.16) |
| Groups of Specialized Metabolites | Antioxidant Activity | |||||
|---|---|---|---|---|---|---|
| Treatment | Total Polyphenols | Total Phenolic Acids | Total Flavonoids | Total Flavanols | DPPH Assay | FRAP Assay |
| mg GAE g−1 DW (%) | mg CAE g−1 DW (%) | mg CE g−1 DW (%) | µg CE g−1 DW (%) | µmol TE g−1 DW (%) | µmol Fe2+ g−1 DW (%) | |
| C | 27.48 ± 0.17 b (100) | 4.89 ± 0.02 d (100) | 7.88 ± 0.04 c (100) | 58.47 ± 0.58 b (100) | 31.92 ± 0.47 b (100) | 56.98 ± 0.15 c (100) |
| C + 10 µM SA | 29.79 ± 0.21 a (108.40) | 5.81 ± 0.06 c (118.81) | 8.84 ± 0.09 b (112.18) | 69.07 ± 0.86 a (118.13) | 31.56 ± 0.35 b (98.87) | 58.40 ± 0.61 b (102.49) |
| C + 50 µM SA | 25.78 ± 0.25 c (93.81) | 6.16 ± 0.06 b (125.97) | 8.94 ± 0.12 b (113.45) | 58.88 ± 1.11 b (100.7) | 28.63 ± 0.58 c (89.69) | 57.13 ± 0.20 c (100.26) |
| C + 100 µM SA | 30.10 ± 0.24 a (109.53) | 6.47 ± 0.05 a (132.31) | 9.59 ± 0.04 a (121.70) | 43.30 ± 1.14 d (74.06) | 35.31 ± 0.57 a (110.62) | 62.29 ± 0.28 a (109.32) |
| S | 22.43 ± 0.15 e (81.62) | 3.99 ± 0.07 g (81.60) | 5.74 ± 0.06 g (72.84) | 31.69 ± 0.59 f (54.20) | 23.21 ± 0.44 e (72.71) | 32.84 ± 0.02 g (57.63) |
| S + 10 µM SA | 21.64 ± 0.22 f (78.75) | 4.23 ± 0.03 f (86.50) | 7.16 ± 0.07 d (90.86) | 34.91 ± 0.77 e (59.71) | 23.55 ± 0.34 de (73.78) | 35.73 ± 0.40 f (62.70) |
| S + 50 µM SA | 24.79 ± 0.26 d (90.21) | 3.80 ± 0.01 h (77.71) | 6.10 ± 0.03 f (77.41) | 46.93 ± 0.30 c (80.26) | 24.77 ± 0.41 d (77.60) | 42.86 ± 0.09 e (75.22) |
| S + 100 µM SA | 22.70 ± 0.35 e (82.61) | 4.54 ± 0.05 e (92.84) | 6.66 ± 0.13 e (84.52) | 42.16 ± 0.66 d (72.11) | 21.58 ± 0.49 f (67.61) | 44.43 ± 0.18 d (77.97) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linić, I.; Mlinarić, S.; Brkljačić, L.; Pavlović, I.; Smolko, A.; Salopek-Sondi, B. Ferulic Acid and Salicylic Acid Foliar Treatments Reduce Short-Term Salt Stress in Chinese Cabbage by Increasing Phenolic Compounds Accumulation and Photosynthetic Performance. Plants 2021, 10, 2346. https://doi.org/10.3390/plants10112346
Linić I, Mlinarić S, Brkljačić L, Pavlović I, Smolko A, Salopek-Sondi B. Ferulic Acid and Salicylic Acid Foliar Treatments Reduce Short-Term Salt Stress in Chinese Cabbage by Increasing Phenolic Compounds Accumulation and Photosynthetic Performance. Plants. 2021; 10(11):2346. https://doi.org/10.3390/plants10112346
Chicago/Turabian StyleLinić, Ida, Selma Mlinarić, Lidija Brkljačić, Iva Pavlović, Ana Smolko, and Branka Salopek-Sondi. 2021. "Ferulic Acid and Salicylic Acid Foliar Treatments Reduce Short-Term Salt Stress in Chinese Cabbage by Increasing Phenolic Compounds Accumulation and Photosynthetic Performance" Plants 10, no. 11: 2346. https://doi.org/10.3390/plants10112346
APA StyleLinić, I., Mlinarić, S., Brkljačić, L., Pavlović, I., Smolko, A., & Salopek-Sondi, B. (2021). Ferulic Acid and Salicylic Acid Foliar Treatments Reduce Short-Term Salt Stress in Chinese Cabbage by Increasing Phenolic Compounds Accumulation and Photosynthetic Performance. Plants, 10(11), 2346. https://doi.org/10.3390/plants10112346







