Effect of Soil Moisture Regimes on the Glyphosate Sensitivity and Morpho-Physiological Traits of Windmill Grass (Chloris truncata R.Br.), Common Sowthistle (Sonchus oleraceus L.), and Flaxleaf Fleabane [Conyza bonariensis (L.) Cronq.]
Abstract
1. Introduction
2. Results
2.1. Impact of Soil Moisture Variations on Weed Susceptibility to Glyphosate
2.2. Impact of Soil Moisture Variations on Weed Morpho-Physiology and Growth
3. Discussion
3.1. Impact of Soil Moisture Variations on Weed Susceptibility to Glyphosate
3.2. Impact of Soil Moisture Variations on Weed Morpho-Physiology and Growth
4. Materials and Methods
4.1. Seed Collection
4.2. Dose-Response Study to Determine LD50 and LD80 Values
4.3. Determination of Water Holding Capacity
4.4. Impact of Soil Moisture Variations on Weed Susceptibility to Glyphosate
4.5. Impact of Soil Moisture Variations on Weed Morpho-Physiology and Growth
4.6. Experimental Designs and Statistical Analysis
5. Conclusion and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Widderick, M.; Cook, T.; McLean, A.; Churchett, J.; Keenan, M.; Miller, B.; Davidson, B. Improved management of key northern region weeds: Diverse problems, diverse solutions. In Proceedings of the 19th Australasian Weeds Conference, Hobart, TAS, Australia; 1–4 September 2014; pp. 206–209. [Google Scholar]
- Fromm, G.; Grieger, V. The effect of summer weed management on subsequent grain yield and quality. In Proceedings of the 13th Australian Weeds Conference, Perth, WA, Australia; 8–13 September 2002; pp. 8–13. [Google Scholar]
- Hunt, J.; Browne, C.; McBeath, T.; Verburg, K.; Craig, S.; Whitbread, A. Summer fallow weed control and residue management impacts on winter crop yield though soil water and N accumulation in a winter-dominant, low rainfall region of southern Australia. Crop Pasture Sci. 2013, 64, 922–934. [Google Scholar] [CrossRef]
- Cameron, J.; Storrie, A. Summer Fallow Weed Management; Grains Research & Development Corporation: Canberra, Australia, 2014. [Google Scholar]
- Soares, C.; Pereira, R.; Spormann, S.; Fidalgo, F. Is soil contamination by a glyphosate commercial formulation truly harmless to non-target plants?—Evaluation of oxidative damage and antioxidant responses in tomato. Environ. Pollut. 2019, 247, 256–265. [Google Scholar] [CrossRef]
- De Moraes, C.P.; de Brito, I.P.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Hormetic effect of glyphosate on Urochloa decumbens plants. J. Environ. Sci. Health Part B 2020, 55, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Tanpipat, S.; Adkins, S.W.; Swarbrick, J.T.; Boersma, M. Influence of selected environmental factors on glyphosate efficacy when applied to awnless barnyard grass (Echinochloa colona (L.)). Aust. J. Agric. Res. 1997, 48, 695–702. [Google Scholar] [CrossRef]
- Walker, S.; Widderick, M.; Storrie, A.; Osten, V. Preventing glyphosate resistance in weeds of the northern grain region. In Proceedings of the 14th Australian weeds conference: Weed Management: Balancing People, Planet, Profit, Wagga Wagga, NSW, Australia; 6–9 September 2004; pp. 428–431. [Google Scholar]
- Jalaludin, A.; Widderick, M.J.; Broster, J.; Walsh, M.J. Glyphosate and 2, 4-D amine resistance in common sowthistle (Sonchus oleraceus) and fleabane (Conyza bonariensis) in the northern grain growing region of Australia. In Proceedings of the 21st Australasian Weeds Conference 2018: Weed Biosecurity-Protecting our future, Sydney, NSW, Australia; 9–13 September 2018; p. 139. [Google Scholar]
- Adkins, S.W.; Tanpipat, S.; Swarbrick, J.; Boersma, M. Influence of environmental factors on glyphosate efficacy when applied to Avena fatua or Urochloa panicoides. Weed Res. 1998, 38, 129–138. [Google Scholar] [CrossRef]
- Adkins, S.; Tanpipat, S.; Swarbrick, J.; Boersma, M. The influence of soil moisture content on glyphosate efficacy for the control of annual grasses in fallow land. Weed Res. 1998, 38, 119–127. [Google Scholar] [CrossRef]
- Varanasi, A.; Prasad, P.V.; Jugulam, M. Impact of climate change factors on weeds and herbicide efficacy. Adv. Agron. 2016, 135, 107–146. [Google Scholar]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Foreman, M.H.; Field, R.J. Drought induced tolerance to diclofop-methyl in cultivated oat. In Proceedings of the New Zealand Weed and Pest Control Conference, Palmerston North, New Zealand; 12–14 August 1986; pp. 267–271. [Google Scholar]
- Meeusen, R.; Lorey, S.; Yih, R. Effects of environmental factors on the herbicidal activity of acifluorfen. Asp. Appl. Biol. 1983, 4, 253–263. [Google Scholar]
- Ahmadi, M.; Haderlie, L.; Wicks, G. Effect of growth stage and water stress on barnyardgrass (Echinochloa crus-galli) control and on glyphosate absorption and translocation. Weed Sci. 1980, 28, 277–282. [Google Scholar] [CrossRef]
- Chase, R.L.; Appleby, A.P. Effects of humidity and moisture stress on glyphosate control of Cyperus rotundus L. Weed Res. 1979, 19, 241–246. [Google Scholar] [CrossRef]
- McWhorter, C.; Jordan, T.; Wills, G. Translocation of 14C-glyphosate in soybeans (Glycine max) and johnsongrass (Sorghum halepense). Weed Sci. 1980, 28, 113–118. [Google Scholar] [CrossRef]
- Moosavi-Nia, H.; Dore, J. Factors affecting glyphosate activity in Imperata cylindrica (L) Beauv. and Cyperus rotundas L. Effect of soil moisture. Weed Res. 1979, 19, 137–143. [Google Scholar] [CrossRef]
- Waldecker, M.A.; Wyse, D.L. Soil moisture effects on glyphosate absorption and translocation in common milkweed (Asclepias syriaca). Weed Sci. 1985, 33, 299–305. [Google Scholar] [CrossRef]
- Quiggin, J. Drought, climate change and food prices in Australia; The University of Queensland: St Lucia, QLD, Australia, 2007. [Google Scholar]
- Bajwa, A.A.; Chauhan, B.S.; Adkins, S. Morphological, physiological and biochemical responses of two Australian biotypes of Parthenium hysterophorus to different soil moisture regimes. Environ. Sci. Pollut. Res. 2017, 24, 16186–16194. [Google Scholar] [CrossRef]
- Moretti, M.L. Determination of Multiple Herbicide Resistance in Populations of Hairy Fleabane (Conyza bonariensis) in the Central Valley of California and Evaluation of Alternative Chemical Control. Master’s Thesis, California State University, Fresno, CA, USA, 2011. [Google Scholar]
- Bagavathiannan, M.; Singh, V.; Govindasamy, P.; Abugho, S.B.; Liu, R. Impact of concurrent weed or herbicide stress with other biotic and abiotic stressors on crop production. In Plant Tolerance to Individual and Concurrent Stresses; Springer: New York, NY, USA, 2017; pp. 33–45. [Google Scholar]
- Irmak, S.; Haman, D.Z.; Irmak, A.; Jones, J.W.; Campbell, K.L.; Crisman, T.L. Measurement and analyses of growth and stress parameters of Viburnum odoratissimum (Ker-gawl) grown in a multi-pot box system. HortScience 2004, 39, 1445–1455. [Google Scholar] [CrossRef]
- Michalk, D.; Herbert, P. The effects of grazing and season on the stability of Chloris Spp. (Windmill grasses) in natural pasture at Trangie, New South Wales. Rangel. J. 1976, 1, 106–111. [Google Scholar] [CrossRef]
- Neckář, K.; Brant, V.; Nečasová, M.; Novakova, K.; Venclova, V. Germination of weed species from Asteraceae family under water deficit conditions. J. Plant Dis. Prot. 2008, 125, 271–276. [Google Scholar]
- Spencer, D.F.; Tan, W.; Liow, P.-S.; Ksander, G.G.; Whitehand, L.C.; Weaver, S.; Olson, J.; Newhouser, M. Evaluation of glyphosate for managing giant reed (Arundo donax). Invasive Plant Sci. Manag. 2008, 1, 248–254. [Google Scholar] [CrossRef]
- Tanpipat, S. Environmental Control of Glyphosate Efficacy: Fallow Weeds of the Australian North-East Grain Belt. Ph.D. Thesis, The University of Queensland, Gatton, QLD, Australia, 1995. [Google Scholar]
- Aves, C.S. Herbicide resistance in Conyza bonariensis (L.) Cronquist (flaxleaf fleabane) populations from northeast Victoria and its management in mixed farming systems. Ph.D. Thesis, The University of Adelaide, Adelaide, SA, Australia, 2018. [Google Scholar]
- Brito, I.P.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Hormetic effects of glyphosate on plants. Pest Manag. Sci. 2018, 74, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Ziska, L.H. The role of climate change and increasing atmospheric carbon dioxide on weed management: Herbicide efficacy. Agric. Ecosyst. Environ. 2016, 231, 304–309. [Google Scholar] [CrossRef]
- De Ruiter, H.; Meinen, E. Influence of water stress and surfactant on the efficacy, absorption, and translocation of glyphosate. Weed Sci. 1998, 46, 289–296. [Google Scholar] [CrossRef]
- Baucom, R.S. Evolutionary and ecological insights from herbicide-resistant weeds: What have we learned about plant adaptation, and what is left to uncover? New Phytol. 2019, 223, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Effects of temperature and watering regime on growth, gas exchange and abscisic acid content of canola (Brassica napus) seedlings. Environ. Exp. Bot. 2012, 75, 107–113. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: Temperature, carbon dioxide and drought. Physiol. Plant. 2006, 128, 710–721. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer-Verlag: Berlin, Germany, 2003. [Google Scholar]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Lynch, A.L.; Godin, V.J.; Reid, D.M. Single and interactive effects of temperature, carbon dioxide, and watering regime on the invasive weed black knapweed (Centaurea nigra). Ecoscience 2013, 20, 328–338. [Google Scholar] [CrossRef]
- Karkanis, A.; Bilalis, D.; Efthimiadou, A. Architectural plasticity, photosynthesis and growth responses of velvetleaf (Abutilon theophrasti Medicus) plants to water stress in a semi-arid environment. Aust. J. Crop Sci. 2011, 5, 369–374. [Google Scholar]
- Lovelli, S.; Perniola, M.; Ferrara, A.; Amato, M.; Di Tommaso, T. Photosynthetic response to water stress of pigweed (Amaranthus retroflexus) in a Southern-Mediterranean area. Weed Sci. 2010, 58, 126–131. [Google Scholar] [CrossRef]
- Wu, L.-M.; Fang, Y.; Yang, H.-N.; Bai, L.-Y. Effects of drought-stress on seed germination and growth physiology of quinclorac-resistant Echinochloa crusgalli. PLoS ONE 2019, 14, e0214480. [Google Scholar] [CrossRef] [PubMed]
- Weller, S.; Florentine, S.; Mutti, N.; Jha, P.; Chauhan, B.S. Response of Chloris truncata to moisture stress, elevated carbon dioxide and herbicide application. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Boutraa, T.; Akhkha, A.; Al-Shoaibi, A.A.; Alhejeli, A.M. Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. J. Taibah Univ. Sci. 2010, 3, 39–48. [Google Scholar] [CrossRef]
- Sellers, B.A.; Smeda, R.J.; Johnson, W.G. Diurnal fluctuations and leaf angle reduce glufosinate efficacy. Weed Technol. 2003, 17, 302–306. [Google Scholar] [CrossRef]
- Da Silva Santos, R.T.; Della Vechia, J.F.; Dos Santos, C.A.M.; Almeida, D.P.; da Costa Ferreira, M. Relationship of contact angle of spray solution on leaf surfaces with weed control. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Oosterhuis, D.; Hampton, R.; Wullschleger, S. Water deficit effects on the cotton leaf cuticle and the efficiency of defoliants. J. Prod. Agric. 1991, 4, 260–265. [Google Scholar] [CrossRef]
- Rocha-Pereira, M.R.; Klar, A.E.; Martins, D.; Ferreira de Souza, G.S.; Villalba, J. Effect of water stress on herbicide efficiency applied to Urochloa decumbens. Cienc. Investig. Agrar. 2012, 39, 211–220. [Google Scholar] [CrossRef][Green Version]
- Polle, A. Protection from oxidative stress in trees as affected by elevated CO2 and environmental stress. In Carbon Dioxide and Terrestrial Ecosystems; Koch, G., Mooney, H., Eds.; Academic Press: New York, NY, USA, 1996; pp. 299–315. [Google Scholar]
- Ziska, L.H.; Teasdale, J.R. Sustained growth and increased tolerance to glyphosate observed in a C3 perennial weed, quackgrass (Elytrigia repens), grown at elevated carbon dioxide. Funct. Plant Biol. 2000, 27, 159–166. [Google Scholar] [CrossRef]
- Bollig, J.; Seiler, J.; Zedaker, S.; Thompson, J.; Lucero, D. Effect of plant moisture stress and application surface on uptake and translocation of triclopyr with organosilicone surfactant in red maple seedlings. Can. J. For. Res. 1995, 25, 425–429. [Google Scholar] [CrossRef]
- Ohashi, Y.; Saneoka, H.; Fujita, K. Effect of water stress on growth, photosynthesis, and photoassimilate translocation in soybean and tropical pasture legume siratro. Soil Sci. Plant Nutr. 2000, 46, 417–425. [Google Scholar]
- Yanniccari, M.; Istilart, C.; Gimenez, D.O.; Castro, A.M. Glyphosate resistance in perennial ryegrass (Lolium perenne L.) from Argentina. Crop Prot. 2012, 32, 12–16. [Google Scholar] [CrossRef]
- Okada, M.; Hanson, B.D.; Hembree, K.J.; Peng, Y.; Shrestha, A.; Stewart Jr, C.N.; Wright, S.D.; Jasieniuk, M. Evolution and spread of glyphosate resistance in Conyza canadensis in California. Evol. Appl. 2013, 6, 761–777. [Google Scholar] [CrossRef]
- Darwish, M.; Vidal, V.; Lopez-Lauri, F.; Alnaser, O.; Junglee, S.; El Maataoui, M.; Sallanon, H. Tolerance to clomazone herbicide is linked to the state of LHC, PQ-pool and ROS detoxification in tobacco (Nicotiana tabacum L.). J. Plant Physiol. 2015, 175, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Gardner, W. Water retention: Field methods. In Methods of Soil Analysis. Part 1: Physical and Mineralogical Methods; Klute, A., Ed.; Southern Weed Science Society: Westminster, CO, USA, 1986; pp. 29–46. [Google Scholar]
- Peerzada, A.M. Glyphosate Efficacy under Projected Climate Change: Impact on Three Summer Fallow Weeds of Australian Northern Grain Region. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2020. [Google Scholar]
- Frans, R. (Ed.) Experimental Design and Techniques for Measuring and Analyzing Plant Responses to Weed Control Practices; American Society of Agronomy, Inc.; Soil Science Society of America, Inc.: Madison, WI, USA, 1986; Volume 5.1, pp. 29–46. [Google Scholar]
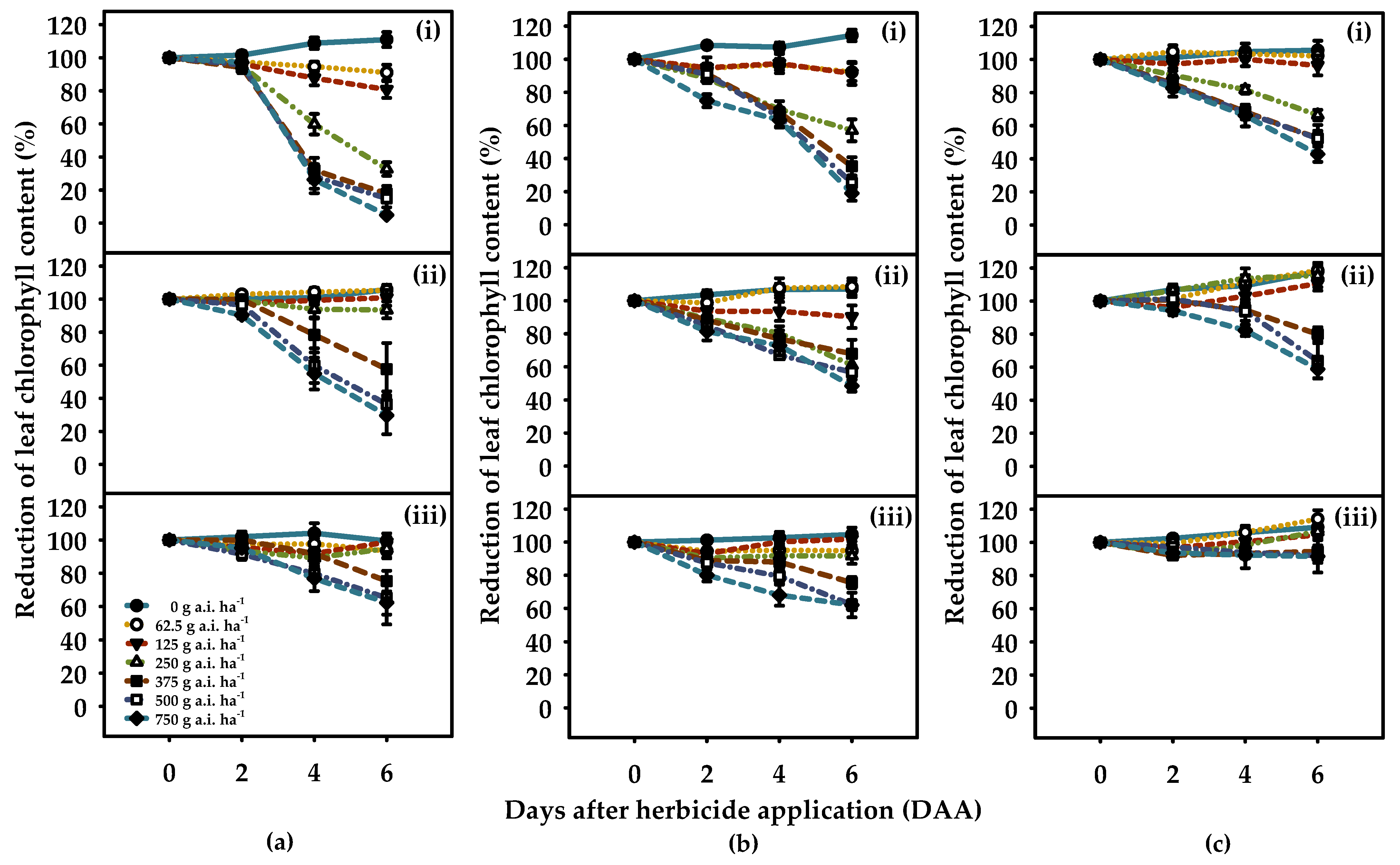
 ) 90–100% of the WHC = high soil moisture availability (HSM), (
) 90–100% of the WHC = high soil moisture availability (HSM), ( ) 50–60% of the WHC = moderate soil moisture availability (MSM), and (
) 50–60% of the WHC = moderate soil moisture availability (MSM), and ( ) 20–30% of the WHC = reduced soil moisture availability (RSM)] at 21 days after spraying. The figures show the observed data fitted to Equations (1) and (2). Error bars represent the ± two standard errors of the mean (n = 8) from repeated experiments.
) 20–30% of the WHC = reduced soil moisture availability (RSM)] at 21 days after spraying. The figures show the observed data fitted to Equations (1) and (2). Error bars represent the ± two standard errors of the mean (n = 8) from repeated experiments.
 ) 90–100% of the WHC = high soil moisture availability (HSM), (
) 90–100% of the WHC = high soil moisture availability (HSM), ( ) 50–60% of the WHC = moderate soil moisture availability (MSM), and (
) 50–60% of the WHC = moderate soil moisture availability (MSM), and ( ) 20–30% of the WHC = reduced soil moisture availability (RSM)] at 21 days after spraying. The figures show the observed data fitted to Equations (1) and (2). Error bars represent the ± two standard errors of the mean (n = 8) from repeated experiments.
) 20–30% of the WHC = reduced soil moisture availability (RSM)] at 21 days after spraying. The figures show the observed data fitted to Equations (1) and (2). Error bars represent the ± two standard errors of the mean (n = 8) from repeated experiments.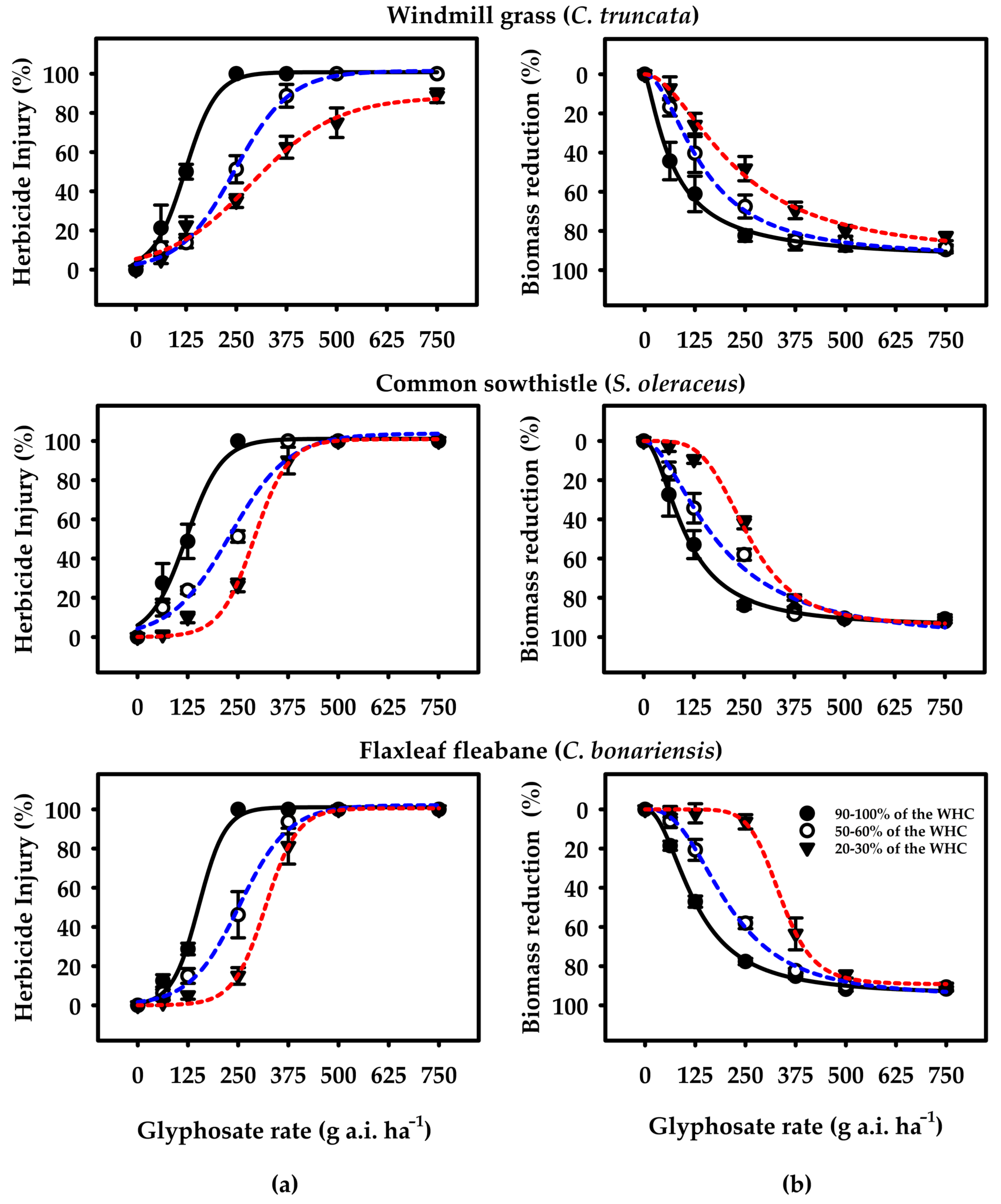
| Species | Parameters | Soil Moisture | a | b | LD50 * | TF ** | LD80 ** | R2 | |
|---|---|---|---|---|---|---|---|---|---|
| % of the WHC | (%) | g a.i. ha−1 | CI 95% ** Upper–Lower Limit | g a.i. ha−1 | |||||
| C. truncata | Injury (%) | 90–100% | 100.8 ± 2.5 | 38.9 ± 7.0 | 122.2 | 115.7–128.6 | 1.0 | 175.2 | 0.91 |
| 50–60% | 101.5 ± 2.8 | 68.8 ± 7.7 | 244.5 | 235.2–253.7 | 2.0 | 337.0 | 0.94 | ||
| 20–30% | 88.2 ± 4.6 | 105.8 ± 14.9 | 316.7 | 297.0–336.4 | 2.6 | 528.7 | 0.87 | ||
| Biomass reduction (%) | 90–100% | 94.6 ± 8.4 | −1.3 ± 0.5 | 77.8 | 66.2–89.4 | 1.0 | 236.1 | 0.81 | |
| 50–60% | 93.8 ± 6.3 | −1.9 ± 0.4 | 153.9 | 137.5–170.3 | 2.0 | 338.8 | 0.87 | ||
| 20–30% | 93.7 ± 9.1 | −1.8 ± 0.4 | 239.4 | 208.5–270.3 | 2.6 | 506.0 | 0.87 | ||
| S. oleraceus | Injury (%) | 90–100% | 101.1 ± 2.8 | 44.1 ± 8.1 | 120.7 | 112.8–128.5 | 1.0 | 180.4 | 0.88 |
| 50–60% | 103.8 ± 2.2 | 73.7 ± 5.8 | 225.3 | 218.1–232.6 | 1.9 | 320.2 | 0.96 | ||
| 20–30% | 100.9 ± 2.4 | 42.7 ± 5.3 | 290.4 | 283.7–297.0 | 2.4 | 348.5 | 0.96 | ||
| Biomass reduction (%) | 90–100% | 94.7 ± 5.1 | −1.9 ± 0.4 | 110.1 | 99.5–120.7 | 1.0 | 234.6 | 0.85 | |
| 50–60% | 102.7 ± 6.9 | −1.8 ± 0.3 | 178.9 | 159.4–198.4 | 1.6 | 340.6 | 0.91 | ||
| 20–30% | 94.1 ± 2.0 | −4.2 ± 0.4 | 264.9 | 259.7–270.1 | 2.4 | 373.9 | 0.98 | ||
| C. bonariensis | Injury (%) | 90–100% | 101.1 ± 1.1 | 32.9 ± 3.2 | 150.9 | 147.2–154.6 | 1.0 | 195.6 | 0.98 |
| 50–60% | 102.1 ± 3.9 | 61.6 ± 10.6 | 250.9 | 239.0–262.7 | 1.7 | 332.7 | 0.89 | ||
| 20–30% | 100.6 ± 2.8 | 39.9 ± 5.1 | 317.6 | 309.8–325.4 | 2.1 | 372.3 | 0.94 | ||
| Biomass reduction (%) | 90–100% | 94.9 ± 1.8 | −2.1 ± 0.1 | 131.1 | 126.9–135.3 | 1.0 | 289.0 | 0.98 | |
| 50–60% | 96.1 ± 3.3 | −2.7 ± 0.3 | 211.6 | 201.9–221.3 | 1.6 | 363.1 | 0.95 | ||
| 20–30% | 89.3 ± 3.3 | −8.3 ± 1.6 | 347.2 | 343.2–361.6 | 2.6 | 474.2 | 0.92 | ||
| Soil Moisture | Plant Height | No. of Leaves | Chlorophyll Content | Stomatal Conductance | Leaf Thickness | Leaf Area | Dry Weight |
|---|---|---|---|---|---|---|---|
| % of the WHC | cm | - | SPAD Units | mmol m−2 s−1 | µm | cm2 | g |
| 90–100% | 47.8 ± 2.5 | 94 ± 3.4 | 28.2 ± 0.4 | 163.8 ± 5.2 | 95.8 ± 2.1 | 267.1 ± 6.1 | 3.3 ± 0.1 |
| 50–60% | 35.3 ± 1.3 | 71 ± 3.0 | 36.2 ± 0.5 | 127.4 ± 4.8 | 105.8 ± 1.9 | 174.2 ± 4.5 | 2.2 ± 0.1 |
| 20–30% | 25.8 ± 1.7 | 47 ± 2.6 | 46.2 ± 0.5 | 80.6 ± 2.4 | 114.6 ± 1.2 | 129.7 ± 3.6 | 1.4 ± 0.05 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| LSD (p < 0.05) | 5.7 | 8.8 | 1.3 | 12.6 | 5.3 | 14.2 | 0.3 |
| Soil Moisture | Plant Height | No. of Leaves | Chlorophyll Content | Stomatal Conductance | Leaf Thickness | Leaf Area | Dry Weight |
|---|---|---|---|---|---|---|---|
| % of the WHC | cm | - | SPAD Units | mmol m−2 s−1 | µm | cm2 | g |
| 90–100% | 64.8 ± 1.9 | 27.6 ± 1.0 | 36.0 ± 1.1 | 288.9 ± 6.1 | 308.5 ± 5.1 | 423.5 ± 15.5 | 3.1 ± 0.06 |
| 50–60% | 49.6 ± 1.4 | 18.0 ± 0.9 | 42.1 ± 1.3 | 211.5 ± 4.4 | 327.4 ± 6.0 | 245.5 ± 14.0 | 2.1 ± 0.09 |
| 20–30% | 20.5 ± 0.8 | 14.5 ± 1.1 | 47.2 ± 1.1 | 108.2 ± 5.1 | 371.2 ± 9.3 | 166.2 ± 8.7 | 1.2 ± 0.03 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| LSD (p < 0.05) | 4.3 | 2.9 | 3.4 | 15.5 | 20.72 | 38.4 | 0.2 |
| Soil Moisture | Plant Height | No. of Leaves | Chlorophyll Content | Stomatal Conductance | Leaf Thickness | Leaf Area | Dry Weight |
|---|---|---|---|---|---|---|---|
| % of the WHC | cm | - | SPAD Units | mmol m−2 s−1 | µm | cm2 | g |
| 90–100% | 39.3 ± 1.2 | 97.8 ± 2.5 | 35.6 ± 2.0 | 360.5 ± 11.4 | 293.0 ± 8.3 | 250.2 ± 4.1 | 2.8 ± 0.06 |
| 50–60% | 16.5 ± 0.9 | 55.6 ± 2.3 | 41.5 ± 1.4 | 188.8 ± 9.0 | 335.4 ± 9.2 | 160.9 ± 3.9 | 2.1 ± 0.05 |
| 20–30% | 12.6 ± 0.2 | 25.9 ± 0.5 | 50.2 ± 1.5 | 96.2 ± 6.8 | 367.7 ± 7.8 | 87.7 ± 2.9 | 0.9 ± 0.02 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| LSD (p < 0.05) | 3.4 | 5.8 | 4.9 | 27.3 | 24.8 | 10.9 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peerzada, A.M.; Williams, A.; O’Donnell, C.; Adkins, S. Effect of Soil Moisture Regimes on the Glyphosate Sensitivity and Morpho-Physiological Traits of Windmill Grass (Chloris truncata R.Br.), Common Sowthistle (Sonchus oleraceus L.), and Flaxleaf Fleabane [Conyza bonariensis (L.) Cronq.]. Plants 2021, 10, 2345. https://doi.org/10.3390/plants10112345
Peerzada AM, Williams A, O’Donnell C, Adkins S. Effect of Soil Moisture Regimes on the Glyphosate Sensitivity and Morpho-Physiological Traits of Windmill Grass (Chloris truncata R.Br.), Common Sowthistle (Sonchus oleraceus L.), and Flaxleaf Fleabane [Conyza bonariensis (L.) Cronq.]. Plants. 2021; 10(11):2345. https://doi.org/10.3390/plants10112345
Chicago/Turabian StylePeerzada, Arslan Masood, Alwyn Williams, Chris O’Donnell, and Steve Adkins. 2021. "Effect of Soil Moisture Regimes on the Glyphosate Sensitivity and Morpho-Physiological Traits of Windmill Grass (Chloris truncata R.Br.), Common Sowthistle (Sonchus oleraceus L.), and Flaxleaf Fleabane [Conyza bonariensis (L.) Cronq.]" Plants 10, no. 11: 2345. https://doi.org/10.3390/plants10112345
APA StylePeerzada, A. M., Williams, A., O’Donnell, C., & Adkins, S. (2021). Effect of Soil Moisture Regimes on the Glyphosate Sensitivity and Morpho-Physiological Traits of Windmill Grass (Chloris truncata R.Br.), Common Sowthistle (Sonchus oleraceus L.), and Flaxleaf Fleabane [Conyza bonariensis (L.) Cronq.]. Plants, 10(11), 2345. https://doi.org/10.3390/plants10112345







