Fungicide Difenoconazole Induced Biochemical and Developmental Toxicity in Wheat (Triticum aestivum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture Conditions
2.2. Morphology Parameters and Biomass
2.3. Chlorophyll Content and Chlorophyll a Fluorescence Characteristics
2.4. Lipid Peroxidation and Relative Electrolytic Leakage
2.5. Detection of ROS
2.6. Activities of Antioxidant Enzymes
2.7. Statistical Analysis
3. Results
3.1. Effects of Difenoconazole on the Growth of Wheat Seedlings
3.2. Effects of Difenoconazole on Root Development of Wheat Seedlings
3.3. Effects of Difenoconazole on Plant Height and Leaf Development of Wheat Seedlings
3.4. Effects of Difenoconazole on the Chlorophyll Content and Chlorophyll a Florescence
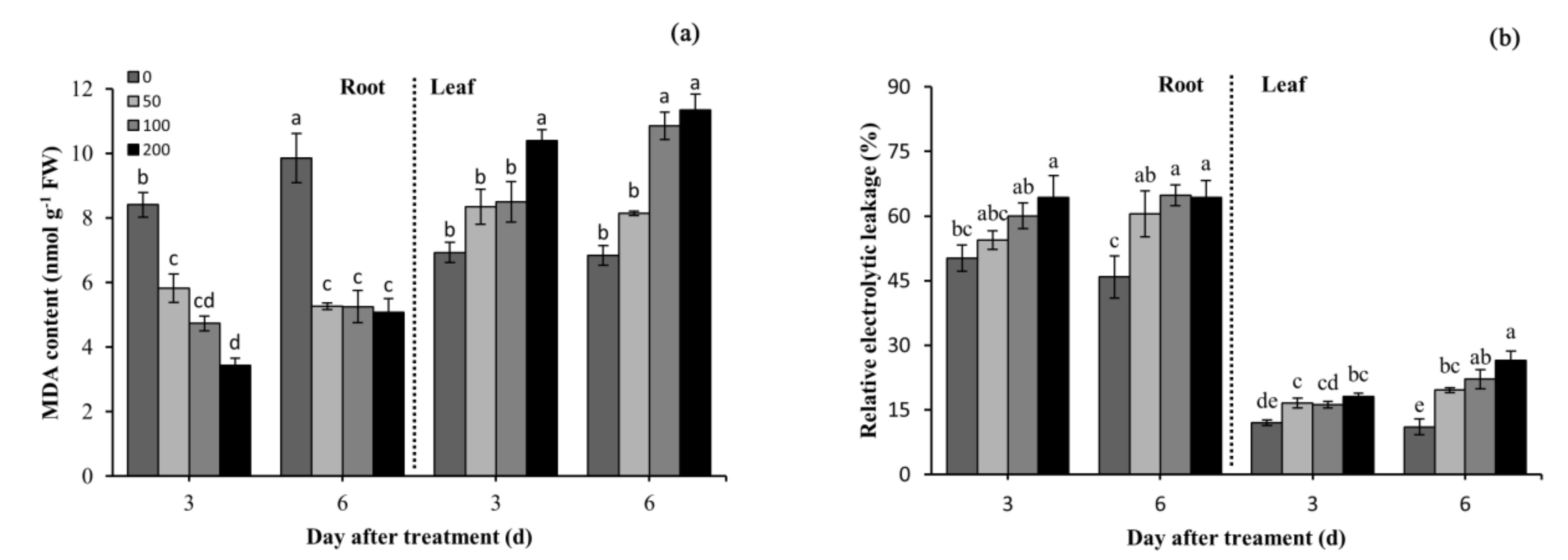
3.5. Effects of Difenoconazole on Lipid Peroxidation and Electrolyte Leakage
3.6. Effects of Difenoconazole on ROS Biosynthesis
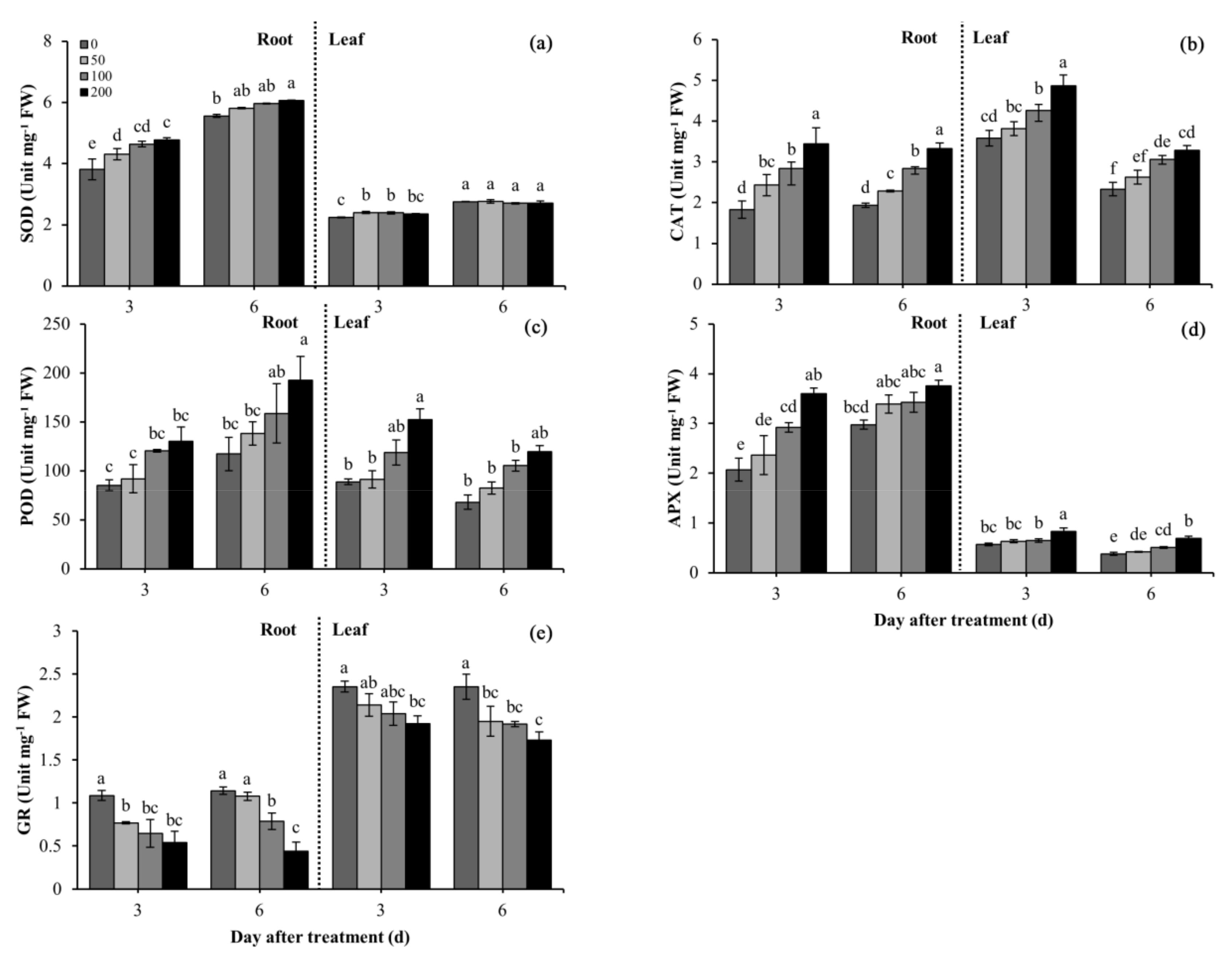
3.7. Effects of Difenoconazole on Antioxidant Enzyme Activities
4. Discussion
4.1. Growth Inhibition in Relation to Oxidative Stress
4.2. Difenoconazole Affects the Balance of Oxidation–Reduction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Gullino, M.L.; Leroux, P.; Smith, C.M. Uses and challenges of novel compounds for plant disease control. Crop. Prot. 2000, 19, 1–11. [Google Scholar] [CrossRef]
- Xiao, J.X.; Yue, Y.H.; Li, W.; Li, F.H.; Yun, L.Y.; Yan, H.Z.; Jing, Q.Y. Pesticides-induced depression of photosynthesis was alleviated by 24-epibrassinolide pretreatment in Cucumis sativus L. Pestic. Biochem. Physiol. 2006, 86, 42–48. [Google Scholar] [CrossRef]
- Untiedt, R.; Blanke, M. Effects of fruit thinning agents on apple tree canopy photosynthesis and dark respiration. Plant Growth Regul. 2001, 35, 1–9. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Akladious, S.A. Changes in antioxidants potential, secondary metabolites and plant hormones induced by different fungicides treatment in cotton plants. Pestic. Biochem. Physiol. 2017, 142, 117–122. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q.; Zhang, B.; Zhang, F.; Liu, P.; Zhou, S.; Liu, X. Hormonal and enzymatic responses of maize seedlings to chilling stress as affected by triazoles seed treatments. Plant Physiol. Biochem. 2020, 148, 220–227. [Google Scholar] [CrossRef]
- Yüzbaşıoğlu, E.; Dalyan, E. Salicylic acid alleviates thiram toxicity by modulating antioxidant enzyme capacity and pesticide detoxification systems in the tomato (Solanum lycopersicum Mill.). Plant Physiol. Biochem. 2018, 135, 322–330. [Google Scholar] [CrossRef]
- Petit, A.N.; Fontaine, F.; Clément, C.; Vaillant-Gaveau, N. Photosynthesis Limitations of Grapevine after Treatment with the Fungicide Fludioxonil. J. Agric. Food Chem. 2008, 56, 6761–6767. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Thukral, A.; Bhardwaj, R. Responses of Plants to Pesticide Toxicity: An Overview. Planta Daninha 2019, 37, e019184291. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.J.; Moore, T.; Nesnow, S. Cytotoxic effects of propiconazole and its metabolites in mouse and human hepatoma cells and primary mouse hepatocytes. Toxicol. Vitr. Int. J. Publ. Assoc. Bibra 2008, 22, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, J.X.; Zhang, H.Y. Dissipation of difenoconazole in rice, paddy soil, and paddy water under field conditions. Ecotoxicol. Environ. Saf. 2012, 86, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.H.; Zhang, C.F.; Wang, Z.; Liu, S.; Li, X.S. Residues and Risk Assessment of Environmental Hormone Pesticide Triazole Fungicides in Soil. Agrochemicals 2019, 58, 660–663. [Google Scholar]
- Wang, X.; Liu, Y.; Gao, S.; Fan, Y.; Fei, W.; Liu, H. Residue Analysis of Tebuconazole and Difenoconazole in Lonicera japonica. Chin. Agric. Sci. Bull. 2017, 33, 143–147. [Google Scholar]
- Mourad, A.M.I.; Sallam, A.; Belamkar, V.; Wegulo, S.N.; Bai, G.; Mahdy, E.; Bakheit, B.R.; Elwafa, A.A.; Jin, Y.; Baenziger, P.S. Molecular marker dissection of stem rust resistance in Nebraska bread wheat germplasm. Sci. Rep. 2019, 9, 11694. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, T.E.; Liu, J.; Li, Q.; Xue, H.; Pan, X. Potassium Deficiency Significantly Affected Plant Growth and Development as Well as microRNA-Mediated Mechanism in Wheat (Triticum aestivum L.). Front Plant Sci. 2020, 11, 1219. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.E.; Wang, G.; Sun, R.R.; Xue, H.Y.; Li, Q.; Liu, J.; David, K.E.; Thornburg, T.E.; Zhang, B.H.; Zhang, Z.Y.; et al. Impact of potassium deficiency on cotton growth, development and potential microRNA-mediated mechanism. Plant Physiol. Biochem. 2020, 153, 72–80. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jiang, W.; Jian, Q.; Song, W.C.; Zheng, Z.T.; Wang, D.L.; Liu, X.J. Residues and dissipation kinetics of triazole fungicides difenoconazole and propiconazole in wheat and soil in Chinese fields. Food Chem. 2015, 168, 396–403. [Google Scholar] [CrossRef]
- Jungklang, J.; Saengnil, K.; Uthaibutra, J. Effects of water-deficit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in Curcuma alismatifolia Gagnep. cv. Chiang Mai Pink. Saudi J. Biol. Sci. 2017, 24, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Elstner, E.F.; Wildner, G.F.; Heupel, A. Oxygen activation by isolated chloroplasts from Euglena gracilis. Ferredoxin-dependent function of a fluorescent compound and photosynthetic electron transport close to photosystem. Arch. Biochem. Biophys. 1976, 173, 623–630. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Pamela, P.D.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 1, 93–101. [Google Scholar] [CrossRef]
- Del Buono, D.; Ioli, G.; Nasini, L.; Proietti, P. A Comparative Study on the Interference of Two Herbicides in Wheat and Italian Ryegrass and on Their Antioxidant Activities and Detoxification Rates. J. Agric. Food Chem. 2011, 59, 12109–12115. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X.D.; Lu, Y.; Wang, X.R. Effects of Rare Earth Metal Ions and Their EDTA Complexes on Antioxidant Enzymes of Fish Liver. Bull. Environ. Contam. Toxicol. 2000, 65, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Koricheva, J.; Roy, S.; Vranjic, J.A.; Haukioja, E.; Hughes, P.R.; Hänninen, O. Antioxidant responses to simulated acid rain and heavy metal deposition in birch seedlings. Environ. Pollut. 1997, 95, 249–258. [Google Scholar] [CrossRef]
- Mandhania, S.; Madan, S.; Sawhney, V. Antioxidant defense mechanism under salt stress in wheat seedlings. Biol. Plant. 2006, 50, 227–231. [Google Scholar] [CrossRef]
- ALKahtani, M.D.F.; Attia, K.A.; Hafez, Y.M.; Khan, N.; Eid, A.M.; Ali, M.A.M.; Abdelaal, K.A.A. Chlorophyll Fluorescence Parameters and Antioxidant Defense System Can Display Salt Tolerance of Salt Acclimated Sweet Pepper Plants Treated with Chitosan and Plant Growth Promoting Rhizobacteria. Agronomy 2020, 10, 1180. [Google Scholar] [CrossRef]
- García, P.C.; Rivero, R.M.; Romero, R.L. The Role of Fungicides in the Physiology of Higher Plants: Implications for Defense Responses. Bot. Rev. 2003, 69, 162–172. [Google Scholar] [CrossRef]
- Saladin, G.; Magné, C.; Clément, C. Effects of fludioxonil and pyrimethanil, two fungicides used against Botrytis cinerea, on carbohydrate physiology in Vitis vinifera L. Pest Manag. Sci. 2003, 59, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Srivastava, G.; Prasad, S.M. Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Sci. Hortic. 2009, 120, 373–378. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xiao, Z.; Chen, C.J.; Zhou, M.G.; Wang, H.C. Effects of fungicides JS399-19, azoxystrobin, tebuconazloe, and carbendazim on the physiological and biochemical indices and grain yield of winter wheat. Pestic. Biochem. Physiol. 2010, 98, 151–157. [Google Scholar] [CrossRef]
- Sharma, I.; Bhardwaj, R.; Pati, P.K. Exogenous Application of 28-Homobrassinolide Modulates the Dynamics of Salt and Pesticides Induced Stress Responses in an Elite Rice Variety Pusa Basmati-1. J. Plant Growth Regul. 2015, 34, 509–518. [Google Scholar] [CrossRef]
- Moldes, C.A.; Medici, L.O.; Abrahao, O.S.; Tsai, S.M.; Azevedo, R.A. Biochemical responses of glyphosate resistant and susceptible soybean plants exposed to glyphosate. Acta Physiol. Plant. 2008, 30, 469–479. [Google Scholar] [CrossRef]
- Kate, M.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar]
- Iersel, M.V.; Bugbee, B. Phytotoxic effects of benzimidazole fungicides on bedding plants. J. Am. Soc. Hortic. Sci. Am. Soc. Hortic. Sci. 1996, 121, 1095. [Google Scholar] [CrossRef]
- Nason, M.A.; Farrar, J.; Bartlett, D. Strobilurin fungicides induce changes in photosynthetic gas exchange that do not improve water use efficiency of plants grown under conditions of water stress. Pest Manag. Sci. 2010, 63, 1191–1200. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Els, V.N.; Ec, V.H.D.; Boelens, P.G.; Klaske, V.N.; Am, V.L.P. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ma, L.; Sui, Y.; Han, S.Q.; Wu, Z.Y.; Feng, Y.X.; Yang, H. Effect of manure compost on the herbicide prometryne bioavailability to wheat plants. J. Hazard. Mater. 2010, 184, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.L.; Cui, J.; Tao, L.; Yang, H. Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 2010, 19, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Parween, T.; Mahmooduzzafar, J.S.; Fatma, T. Evaluation of oxidative stress in Vigna radiata L. in response to chlorpyrifos. Int. J. Environ. Sci. Technol. 2012, 9, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhang, Z.Y.; Unver, T.; Zhang, B.H. CRISPR/Cas: A powerful tool for gene function study and crop improvement. J. Adv. Res. 2021, 29, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Brant, E.; Budak, H.; Zhang, B.H. CRISPR/Cas: A Nobel Prize award-winning precise genome editing technology for gene therapy and crop improvement. J. Zhejiang Univ. Sci. B 2021, 22, 253–284. [Google Scholar] [CrossRef] [PubMed]

| DAT * (Days) | Treatment (mg/L) | Fresh Weight (mg) | Dry Weight (mg) | ||||
|---|---|---|---|---|---|---|---|
| Root | Shoot | Total Weight | Root | Shoot | Total Weight | ||
| 3 | 0 | 1388.3 ± 31.4 b | 1154.0 ± 20.1 b | 2542.3 ± 28.1 b | 98.7 ± 1.9 b | 135.3 ± 7.9 b | 234.0 ± 6.1 b |
| 50 | 805.0 ± 18.7 d | 591.3 ± 10.7 d | 1396.3 ± 29.4 d | 67.3 ± 3.9 d | 102.7 ± 4.3 c | 170.0 ± 5.3 d | |
| 100 | 679.3 ± 5.2 e | 523.0 ± 9.8 ef | 1202.3 ± 9.1 e | 56.7 ± 1.8 e | 75.7 ± 1.8 d | 132.3 ± 1.0 f | |
| 200 | 548.0 ± 6.1 f | 489.5 ± 18.2 f | 1037.5 ± 21.1 f | 48.0 ± 1.5 f | 72.3 ± 0.7 d | 120.3 ± 1.0 f | |
| 6 | 0 | 2142.7 ± 33.7 a | 2168.7 ± 51.3 a | 4311.3 ± 79.8 a | 169.0 ± 4.0 a | 329.7 ± 10.9 a | 498.7 ± 10.3 a |
| 50 | 971.3 ± 35.0 c | 721.0 ± 4.2 c | 1692.3 ± 33.8 c | 81.3 ± 3.3 c | 131.3 ± 8.7 b | 212.7 ± 6.7 c | |
| 100 | 771.3 ± 17.9 d | 677.0 ± 7.1 c | 1448.3 ± 22.3 d | 70.0 ± 3.2 d | 112.7 ± 3.0 c | 182.7 ± 2.0 d | |
| 200 | 645.5 ± 3.9 e | 579.8 ± 7.2 de | 1225.2 ± 6.5 e | 54.5 ± 1.0 ef | 100.2 ± 1.6 c | 154.8 ± 1.5 e | |
| DAT * (Days) | Treatment (mg/L) | Total Root Length (cm) | Total Root Surface Area (cm2) | Total Root Volume (cm3) | Average Root Diameter (mm) |
|---|---|---|---|---|---|
| 3 | 0 | 1266.86 ± 45.63 b | 115.75 ± 2.71 b | 0.84 ± 0.04 bc | 0.29 ± 0.01 de |
| 50 | 847.20 ± 27.29 c | 93.71 ± 4.10 bc | 0.74 ± 0.04 c | 0.35 ± 0.01 b | |
| 100 | 811.49 ± 13.11 c | 79.48 ± 2.11 c | 0.68 ± 0.06 cd | 0.36 ± 0.01 ab | |
| 200 | 774.37 ± 15.24 c | 74.82 ± 3.38 c | 0.54 ± 0.05 d | 0.38 ± 0.01 a | |
| 6 | 0 | 2857.22 ± 211.82 a | 240.79 ± 20.77 a | 1.74 ± 0.13 a | 0.27 ± 0.01 e |
| 50 | 1111.28 ± 34.66 bc | 106.70 ± 5.47 bc | 0.93 ± 0.02 b | 0.30 ± 0.00 cd | |
| 100 | 939.24 ± 89.24 bc | 89.84 ± 8.46 bc | 0.82 ± 0.03 bc | 0.33 ± 0.01 bc | |
| 200 | 854.92 ± 142.46 c | 80.35 ± 2.11 c | 0.75 ± 0.02 c | 0.34 ± 0.01 b |
| DAT * (Days) | Treatment (mg/L) | Leaf Area (cm2) | Plant Height (cm) |
|---|---|---|---|
| 3 | 0 | 32.26 ± 1.70 b | 26.68 ± 1.74 a |
| 50 | 16.03 ± 1.01 de | 20.70 ± 0.63 b | |
| 100 | 14.18 ± 0.60 de | 20.32 ± 0.44 b | |
| 200 | 13.56 ± 0.59 e | 20.42 ± 0.25 b | |
| 6 | 0 | 43.81 ± 0.69 a | 28.26 ± 0.67 a |
| 50 | 20.66 ± 0.65 c | 22.46 ± 0.42 b | |
| 100 | 17.41 ± 0.69 d | 21.30 ± 0.38 b | |
| 200 | 16.68 ± 0.32 de | 21.68 ± 0.54 b |
| DAT * (Days) | Treatment (mg/L) | Chl (μg/cm−2) | Flav (Dualex Units) | NBI (Dualex Units) | Fv/Fm | Fv/Fo |
|---|---|---|---|---|---|---|
| 3 | 0 | 41.19 ± 1.56 a | 0.79 ± 0.05 e | 53.01 ± 2.22 a | 0.81 ± 0.00 a | 4.03 ± 0.23 a |
| 50 | 30.54 ± 1.32 b | 0.89 ± 0.03 de | 34.90 ± 2.36 b | 0.77 ± 0.01 b | 3.40 ± 0.25 b | |
| 100 | 28.08 ± 1.92 bc | 0.97 ± 0.03 cd | 29.48 ± 2.48 bc | 0.75 ± 0.02 bc | 3.12 ± 0.16 bc | |
| 200 | 25.75 ± 0.65 bcd | 1.02 ± 0.05 bc | 26.01 ± 1.80 cd | 0.76 ± 0.01 bc | 3.20 ± 0.12 bc | |
| 6 | 0 | 41.88 ± 0.94 a | 0.90 ± 0.02 d | 47.78 ± 2.09 a | 0.81 ± 0.00 a | 4.18 ± 0.06 a |
| 50 | 27.76 ± 3.48 bc | 1.09 ± 0.03 ab | 22.97 ± 1.32 d | 0.77 ± 0.00 b | 3.33 ± 0.06 bc | |
| 100 | 22.38 ± 1.48 d | 1.11 ± 0.03 ab | 20.41 ± 1.68 d | 0.76 ± 0.01 bc | 3.12 ± 0.16 bc | |
| 200 | 24.29 ± 1.30 cd | 1.17 ± 0.04 a | 20.88 ± 1.19 d | 0.73 ± 0.02 c | 2.80 ± 0.23 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Li, J.; Zhang, L.; Feng, T.; Zhang, Z.; Zhang, B. Fungicide Difenoconazole Induced Biochemical and Developmental Toxicity in Wheat (Triticum aestivum L.). Plants 2021, 10, 2304. https://doi.org/10.3390/plants10112304
Liu R, Li J, Zhang L, Feng T, Zhang Z, Zhang B. Fungicide Difenoconazole Induced Biochemical and Developmental Toxicity in Wheat (Triticum aestivum L.). Plants. 2021; 10(11):2304. https://doi.org/10.3390/plants10112304
Chicago/Turabian StyleLiu, Runqiang, Jingchong Li, Lei Zhang, Ta Feng, Zhiyong Zhang, and Baohong Zhang. 2021. "Fungicide Difenoconazole Induced Biochemical and Developmental Toxicity in Wheat (Triticum aestivum L.)" Plants 10, no. 11: 2304. https://doi.org/10.3390/plants10112304
APA StyleLiu, R., Li, J., Zhang, L., Feng, T., Zhang, Z., & Zhang, B. (2021). Fungicide Difenoconazole Induced Biochemical and Developmental Toxicity in Wheat (Triticum aestivum L.). Plants, 10(11), 2304. https://doi.org/10.3390/plants10112304







