Biochemical and Metabolic Plant Responses toward Polycyclic Aromatic Hydrocarbons and Heavy Metals Present in Atmospheric Pollution
Abstract
1. Introduction
2. PAHs and HMs Affect Seed Germination and Plant Growth
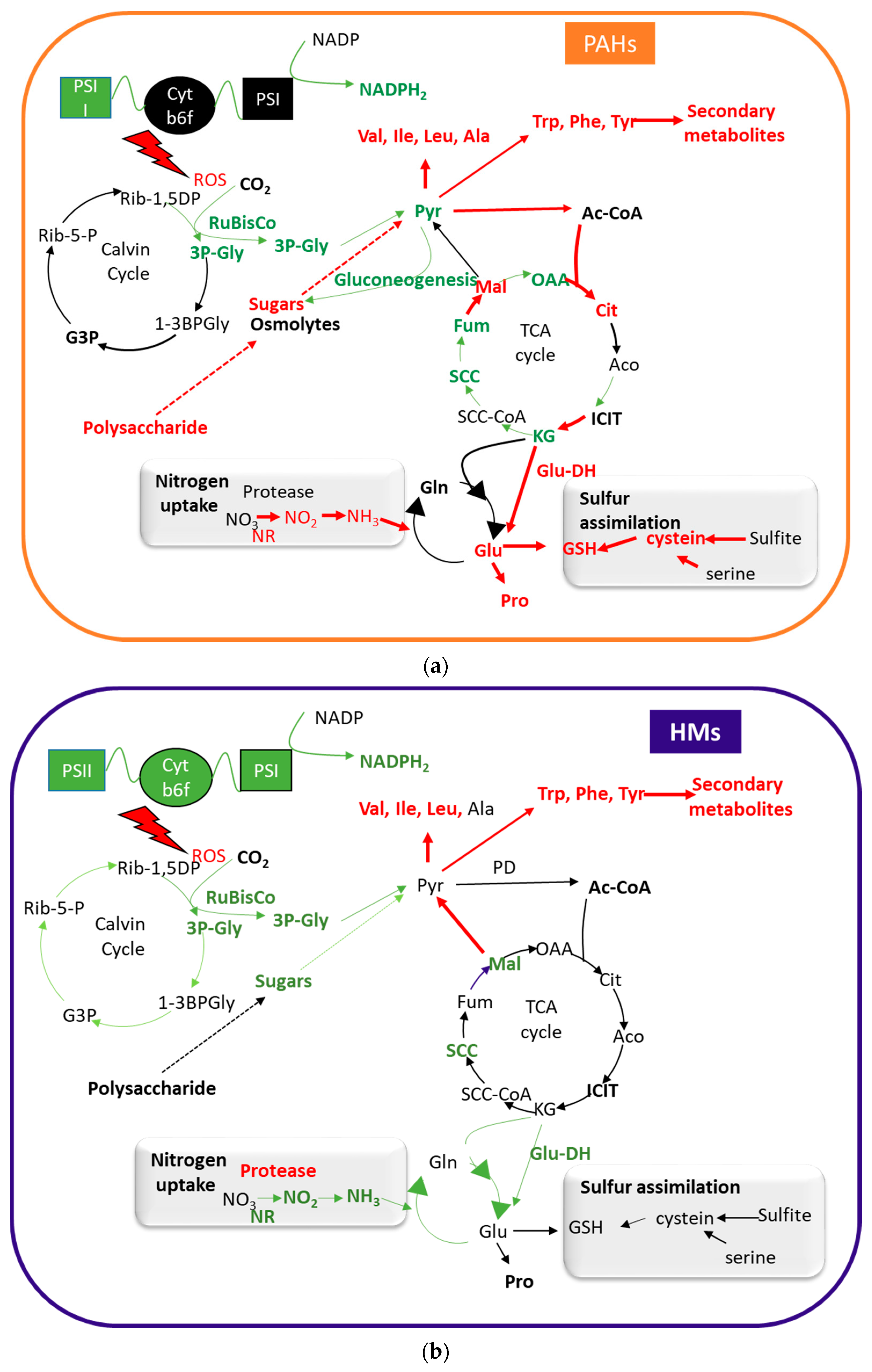
3. PAHs and HMs Affect Plant Metabolism
3.1. Effects on the Photosynthetic System
3.2. Effects on Carbon Metabolism
3.3. Effect on Amino-Acid and Nitrogen Metabolism
3.4. Effects on Secondary Metabolism
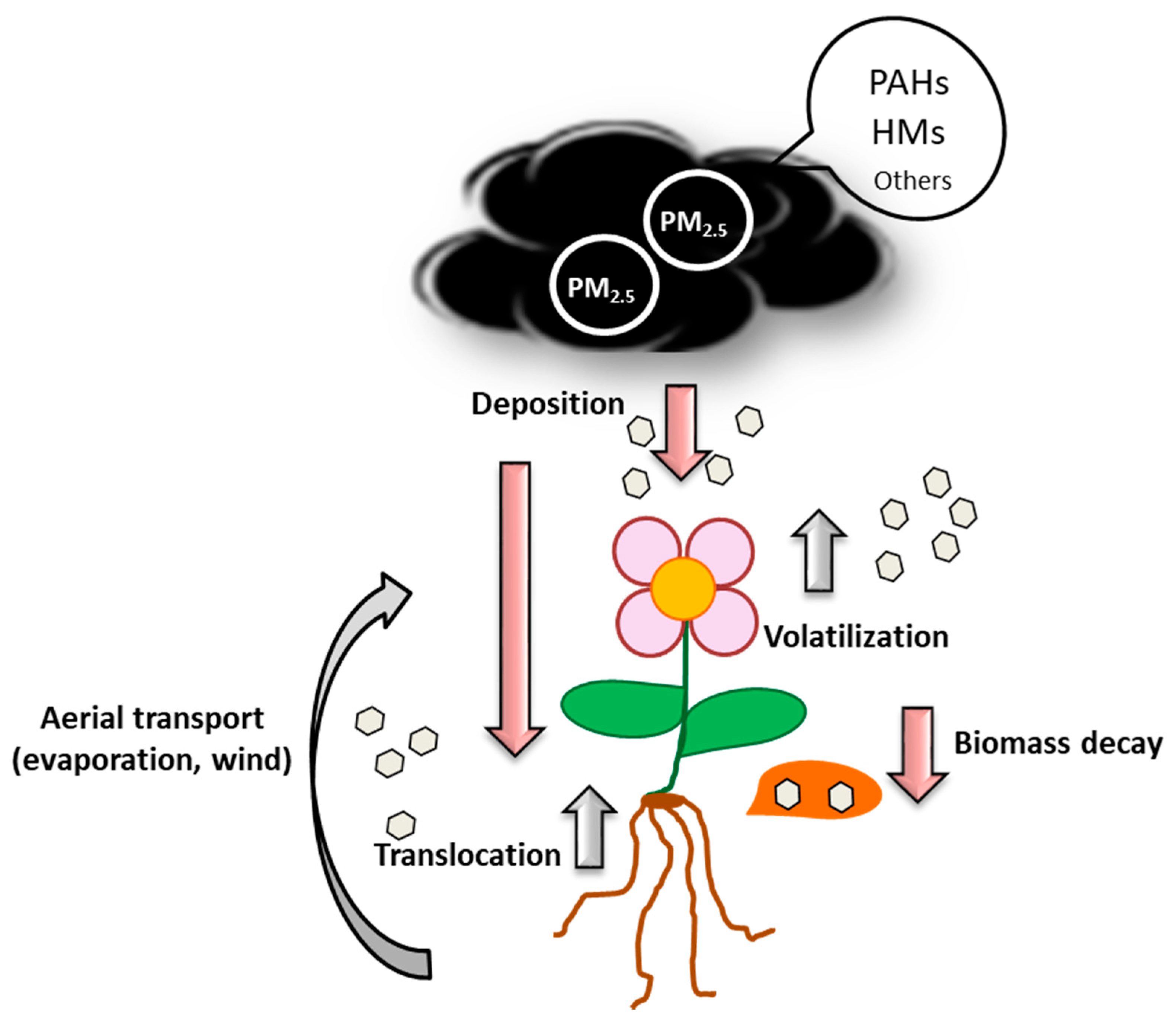
4. Adsorption, Absorption and Accumulation of PAHs and HMs by Plants
4.1. Adsorption
4.2. Absorption
4.3. Accumulation
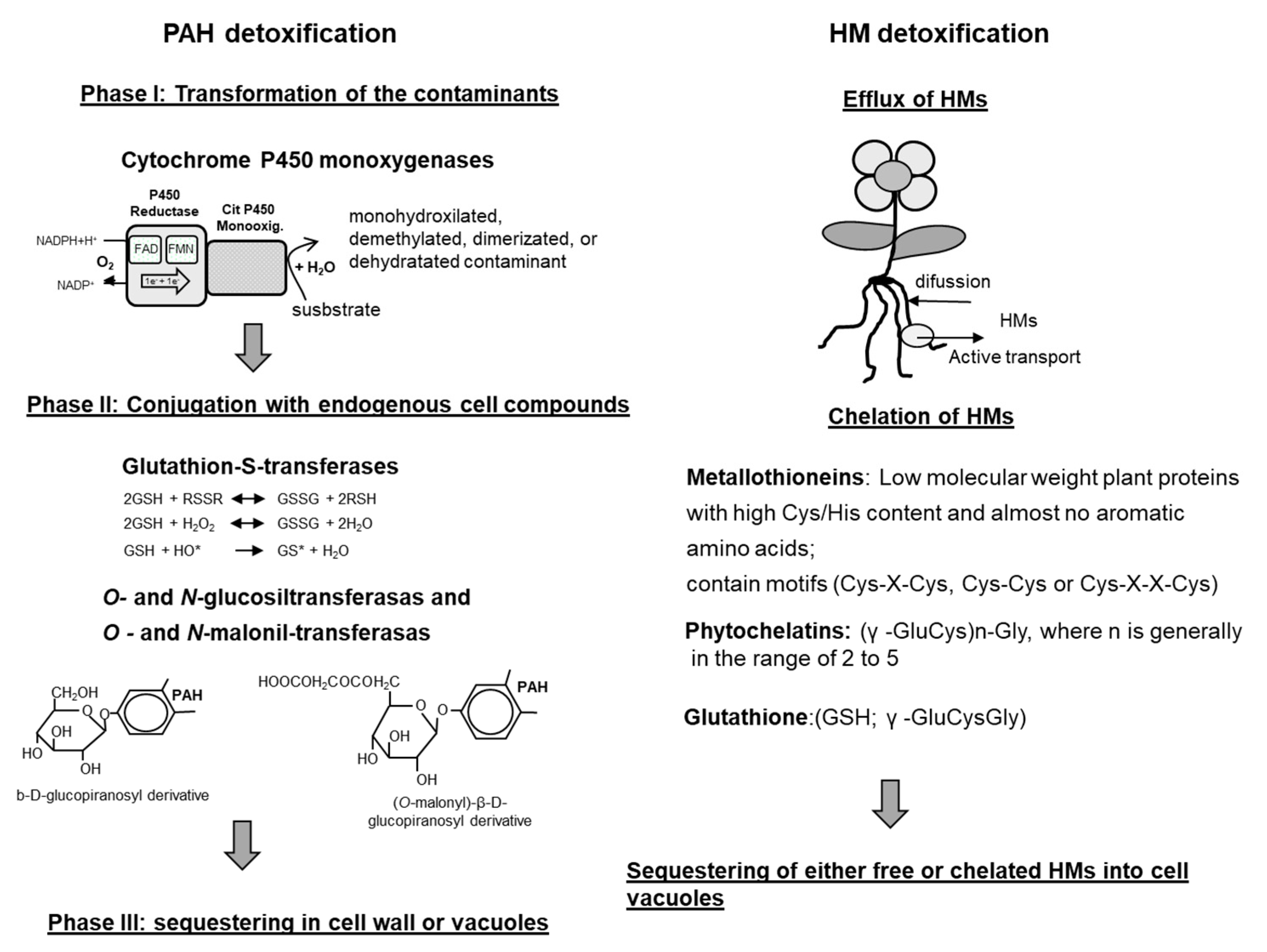
5. Detoxification of PAHs and HMs by Plants
5.1. Detoxification of Organic Compounds
5.2. Detoxification of HMs
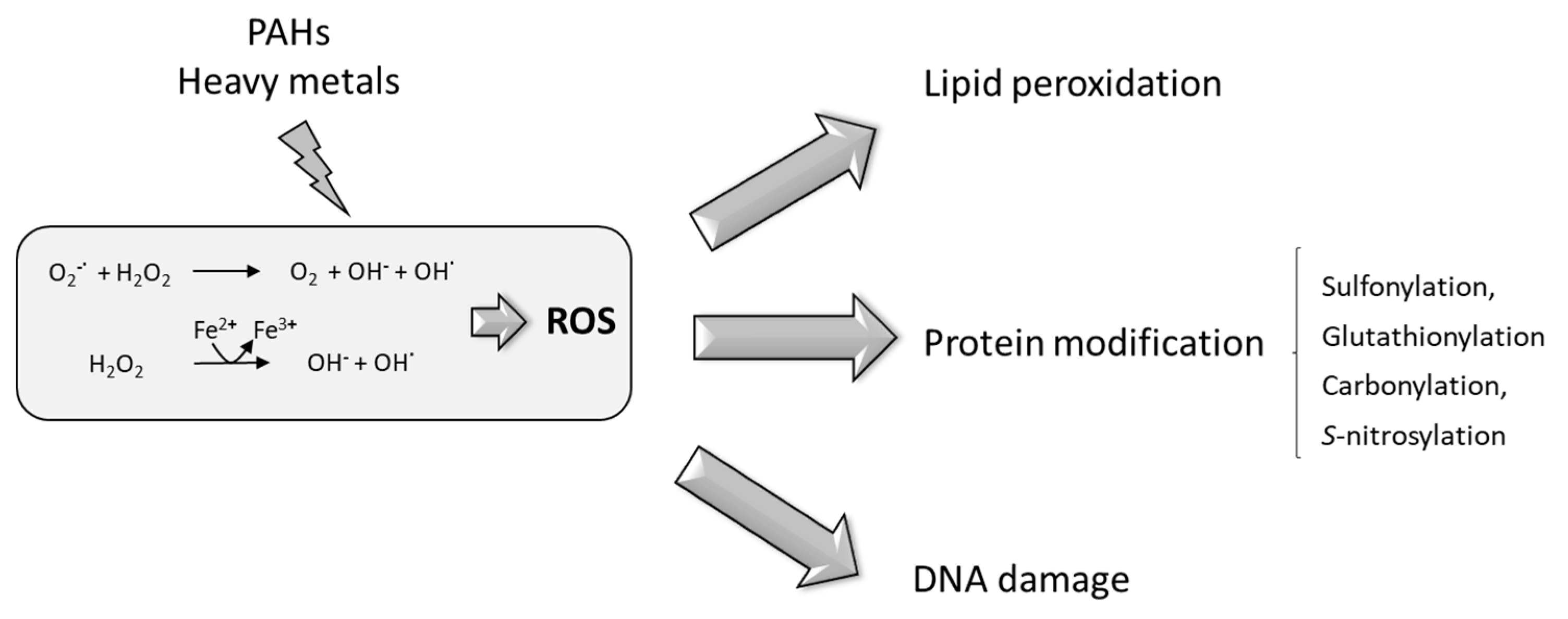
6. PAHs and HMs Produce Oxidative Stress in Plants
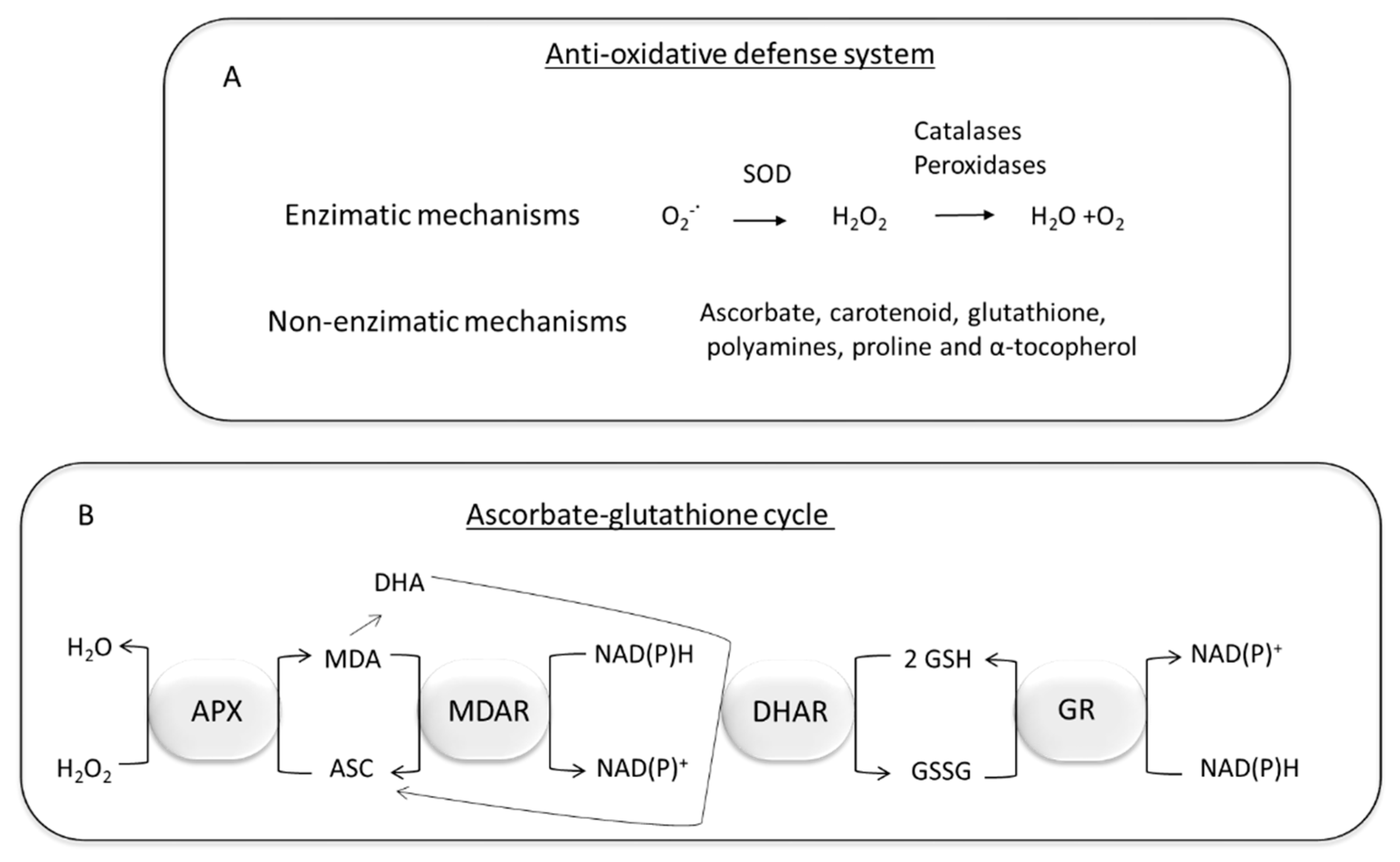
7. Plant Detoxification of Oxidative Stress Produced by PAHs and HMs
8. Phytohormone Signalling Cascades in Plants in Response to PAHs and HMs
9. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bai, L.; Wang, J.; Ma, X.; Lu, H. Air Pollution Forecasts: An Overview. Int. J. Environ. Res. Public Health 2018, 15, 780. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Kumar, P.; Van Poppel, M.; Bleux, N.; Frijns, E.; Reggente, M.; Berghmans, P.; Int Panis, L.; Samson, R. Wintertime Spatio-Temporal Variation of Ultrafine Particles in a Belgian City. Sci. Total Environ. 2012, 431, 307–313. [Google Scholar] [CrossRef]
- Bućko, M.S.; Magiera, T.; Johanson, B.; Petrovský, E.; Pesonen, L.J. Identification of Magnetic Particulates in Road Dust Accumulated on Roadside Snow Using Magnetic, Geochemical and Micro-Morphological Analyses. Environ. Pollut. 2011, 159, 1266–1276. [Google Scholar] [CrossRef]
- Dat, N.-D.; Chang, M.B. Review on Characteristics of PAHs in Atmosphere, Anthropogenic Sources and Control Technologies. Sci. Total Environ. 2017, 609, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.W.F.; Takeda, K.; Sakugawa, H. Polycyclic Aromatic Hydrocarbons (PAHs) Associated with Atmospheric Particles in Higashi Hiroshima, Japan: Influence of Meteorological Conditions and Seasonal Variations. Atmos. Res. 2008, 88, 224–233. [Google Scholar] [CrossRef]
- Zhang, K.; Chai, F.; Zheng, Z.; Yang, Q.; Zhong, X.; Fomba, K.W.; Zhou, G. Size Distribution and Source of Heavy Metals in Particulate Matter on the Lead and Zinc Smelting Affected Area. J. Environ. Sci. 2018, 71, 188–196. [Google Scholar] [CrossRef]
- Luo, J.; Han, Y.; Zhao, Y.; Huang, Y.; Liu, X.; Tao, S.; Liu, J.; Huang, T.; Wang, L.; Chen, K.; et al. Effect of Northern Boreal Forest Fires on PAH Fluctuations across the Arctic. Environ. Pollut. 2020, 261, 114186. [Google Scholar] [CrossRef] [PubMed]
- Remizovschi, A.; Carpa, R.; Forray, F.L.; Chiriac, C.; Roba, C.-A.; Beldean-Galea, S.; Andrei, A.-Ș.; Szekeres, E.; Baricz, A.; Lupan, I.; et al. Mud Volcanoes and the Presence of PAHs. Sci. Rep. 2020, 10, 1253. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Singh, V.P. The Relative Impact of Toxic Heavy Metals (THMs) (Arsenic (As), Cadmium (Cd), Chromium (Cr)(VI), Mercury (Hg), and Lead (Pb)) on the Total Environment: An Overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A Review of Airborne Polycyclic Aromatic Hydrocarbons (PAHs) and Their Human Health Effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Bermudez, G.M.A.; Jasan, R.; Plá, R.; Pignata, M.L. Heavy Metal and Trace Element Concentrations in Wheat Grains: Assessment of Potential Non-Carcinogenic Health Hazard through Their Consumption. J. Hazard. Mater. 2011, 193, 264–271. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation Approaches for Polycyclic Aromatic Hydrocarbons (PAHs) Contaminated Soils: Technological Constraints, Emerging Trends and Future Directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef]
- Henner, P.; Schiavon, M.; Druelle, V.; Lichtfouse, E. Phytotoxicity of Ancient Gaswork Soils. Effect of Polycyclic Aromatic Hydrocarbons (PAHs) on Plant Germination. Org. Geochem. 1999, 30, 963–969. [Google Scholar] [CrossRef]
- Reed, M.L.E.; Glick, B.R. Growth of Canola (Brassica napus) in the Presence of Plant Growth-Promoting Bacteria and Either Copper or Polycyclic Aromatic Hydrocarbons. Can. J. Microbiol. 2005, 51, 1061–1069. [Google Scholar] [CrossRef]
- Ren, L.; Zeiler, L.F.; Dixon, D.G.; Greenberg, B.M. Photoinduced Effects of Polycyclic Aromatic Hydrocarbons OnBrassica Napus(Canola) during Germination and Early Seedling Development. Ecotoxicol. Environ. Saf. 1996, 33, 73–80. [Google Scholar] [CrossRef]
- Czerniawska-Kusza, I.; Ciesielczuk, T.; Kusza, G.; Cichoń, A. Comparison of the Phytotoxkit Microbiotest and Chemical Variables for Toxicity Evaluation of Sediments. Environ. Toxicol. 2006, 21, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Korade, D.L.; Fulekar, M.H. Effect of Organic Contaminants on Seed Germination of Lolium Multiflrum in Soil. Biol. Med. 2009, 1, 28–34. [Google Scholar]
- Gong, P.; Wilke, B.-M.; Strozzi, E.; Fleischmann, S. Evaluation and Refinement of a Continuous Seed Germination and Early Seedling Growth Test for the Use in the Ecotoxicological Assessment of Soils. Chemosphere 2001, 44, 491–500. [Google Scholar] [CrossRef]
- Tang, L.; Tang, X.-Y.; Zhu, Y.-G.; Zheng, M.-H.; Miao, Q.-L. Contamination of Polycyclic Aromatic Hydrocarbons (PAHs) in Urban Soils in Beijing, China. Environ. Int. 2005, 31, 822–828. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Influence of Diesel Fuel on Seed Germination. Environ. Pollut. 2002, 120, 363–370. [Google Scholar] [CrossRef]
- Smith, M.J.; Flowers, T.H.; Duncan, H.J.; Alder, J. Effects of Polycyclic Aromatic Hydrocarbons on Germination and Subsequent Growth of Grasses and Legumes in Freshly Contaminated Soil and Soil with Aged PAHs Residues. Environ. Pollut. 2006, 141, 519–525. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, S.; Wang, Z.; Christie, P. Predicting Bioavailability of PAHs in Field-Contaminated Soils by Passive Sampling with Triolein Embedded Cellulose Acetate Membranes. Environ. Pollut. 2009, 157, 545–551. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Blain, R.B. Hormesis and Plant Biology. Environ. Pollut. 2009, 157, 42–48. [Google Scholar] [CrossRef]
- Calabrese, E. Hormesis: Path and Progression to Significance. Int. J. Mol. Sci. 2018, 19, 2871. [Google Scholar] [CrossRef]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; dos Reis, A.R. Hormesis in Plants: Physiological and Biochemical Responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Morkunas, I.; Woźniak, A.; Mai, V.; Rucińska-Sobkowiak, R.; Jeandet, P. The Role of Heavy Metals in Plant Response to Biotic Stress. Molecules 2018, 23, 2320. [Google Scholar] [CrossRef]
- Rodriguez-Conde, S.; Molina, L.; González, P.; García-Puente, A.; Segura, A. Degradation of Phenanthrene by Novosphingobium Sp. HS2a Improved Plant Growth in PAHs-Contaminated Environments. Appl. Microbiol. Biotechnol. 2016, 100, 10627–10636. [Google Scholar] [CrossRef]
- Kaur, N.; Erickson, T.E.; Ball, A.S.; Ryan, M.H. A Review of Germination and Early Growth as a Proxy for Plant Fitness under Petrogenic Contamination—Knowledge Gaps and Recommendations. Sci. Total Environ. 2017, 603–604, 728–744. [Google Scholar] [CrossRef]
- Sethy, S.; Ghosh, S. Effect of Heavy Metals on Germination of Seeds. J. Nat. Sci. Biol. Med. 2013, 4, 272. [Google Scholar] [CrossRef]
- Maila, M.P.; Cloete, T.E. Germination of Lepidium Sativum as a Method to Evaluate Polycyclic Aromatic Hydrocarbons (PAHs) Removal from Contaminated Soil. Int. Biodeterior. Biodegrad. 2002, 50, 107–113. [Google Scholar] [CrossRef]
- Oguntimehin, I.; Eissa, F.; Sakugawa, H. Negative Effects of Fluoranthene on the Ecophysiology of Tomato Plants (Lycopersicon esculentum Mill.). Chemosphere 2010, 78, 877–884. [Google Scholar] [CrossRef]
- Achuba, F.I. The Effect of Sublethal Concentrations of Crude Oil on the Growth and Metabolism of Cowpea (Vigna unguiculata) Seedlings. Environmentalist 2006, 26, 17–20. [Google Scholar] [CrossRef]
- Bona, C.; de Rezende, I.M.; de Santos, G.O.; de Souza, L.A. Effect of Soil Contaminated by Diesel Oil on the Germination of Seeds and the Growth of Schinus Terebinthifolius Raddi (Anacardiaceae) Seedlings. Braz. Arch. Biol. Technol. 2011, 54, 1379–1387. [Google Scholar] [CrossRef]
- Wei, H.; Song, S.; Tian, H.; Liu, T. Effects of Phenanthrene on Seed Germination and Some Physiological Activities of Wheat Seedling. Comptes Rendus Biol. 2014, 337, 95–100. [Google Scholar] [CrossRef]
- Afegbua, S.L.; Batty, L.C. Effect of Single and Mixed Polycyclic Aromatic Hydrocarbon Contamination on Plant Biomass Yield and PAH Dissipation during Phytoremediation. Environ. Sci. Pollut. Res. 2018, 25, 18596–18603. [Google Scholar] [CrossRef]
- Kechavarzi, C.; Pettersson, K.; Leeds-Harrison, P.; Ritchie, L.; Ledin, S. Root Establishment of Perennial Ryegrass (L. Perenne) in Diesel Contaminated Subsurface Soil Layers. Environ. Pollut. 2007, 145, 68–74. [Google Scholar] [CrossRef]
- Hussain, I.; Puschenreiter, M.; Gerhard, S.; Schöftner, P.; Yousaf, S.; Wang, A.; Syed, J.H.; Reichenauer, T.G. Rhizoremediation of Petroleum Hydrocarbon-Contaminated Soils: Improvement Opportunities and Field Applications. Environ. Exp. Bot. 2018, 147, 202–219. [Google Scholar] [CrossRef]
- Martin, B.C.; George, S.J.; Price, C.A.; Ryan, M.H.; Tibbett, M. The Role of Root Exuded Low Molecular Weight Organic Anions in Facilitating Petroleum Hydrocarbon Degradation: Current Knowledge and Future Directions. Sci. Total Environ. 2014, 472, 642–653. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead Uptake, Toxicity, and Detoxification in Plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2011; Volume 213, pp. 113–136. [Google Scholar] [CrossRef]
- Ahmad, M.S.A.; Ashraf, M. Essential Roles and Hazardous Effects of Nickel in Plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2012; Volume 214, pp. 125–167. [Google Scholar] [CrossRef]
- Pena, L.B.; Azpilicueta, C.E.; Gallego, S.M. Sunflower Cotyledons Cope with Copper Stress by Inducing Catalase Subunits Less Sensitive to Oxidation. J. Trace Elem. Med. Biol. 2011, 25, 125–129. [Google Scholar] [CrossRef]
- Rahoui, S.; Chaoui, A.; El Ferjani, E. Membrane Damage and Solute Leakage from Germinating Pea Seed under Cadmium Stress. J. Hazard. Mater. 2010, 178, 1128–1131. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Z.; Zhang, Y.; Zhang, Z.; Cai, Z. PAHs and Heavy Metals in the Surrounding Soil of a Cement Plant Co-Processing Hazardous Waste. Chemosphere 2018, 210, 247–256. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Furini, A. An Overview of Heavy Metal Challenge in Plants: From Roots to Shoots. Metallomics 2013, 5, 1117. [Google Scholar] [CrossRef]
- Sivaram, A.K.; Subashchandrabose, S.R.; Logeshwaran, P.; Lockington, R.; Naidu, R.; Megharaj, M. Rhizodegradation of PAHs Differentially Altered by C3 and C4 Plants. Sci. Rep. 2020, 10, 16109. [Google Scholar] [CrossRef]
- Jin, L.; Che, X.; Zhang, Z.; Li, Y.; Gao, H.; Zhao, S. The Mechanisms by Which Phenanthrene Affects the Photosynthetic Apparatus of Cucumber Leaves. Chemosphere 2017, 168, 1498–1505. [Google Scholar] [CrossRef]
- Sivaram, A.K.; Logeshwaran, P.; Lockington, R.; Naidu, R.; Megharaj, M. Impact of Plant Photosystems in the Remediation of Benzo[a]Pyrene and Pyrene Spiked Soils. Chemosphere 2018, 193, 625–634. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Brestic, M.; Zharmukhamedov, S.K.; Lyubimov, V.Y.; Lankin, A.V.; Jajoo, A.; Allakhverdiev, S.I. Mechanisms of Inhibitory Effects of Polycyclic Aromatic Hydrocarbons in Photosynthetic Primary Processes in Pea Leaves and Thylakoid Preparations. Plant Biol. J. 2017, 19, 683–688. [Google Scholar] [CrossRef]
- Hossain, Z.; Komatsu, S. Contribution of Proteomic Studies towards Understanding Plant Heavy Metal Stress Response. Front. Plant Sci. 2013, 3, 310. [Google Scholar] [CrossRef]
- Semane, B.; Dupae, J.; Cuypers, A.; Noben, J.-P.; Tuomainen, M.; Tervahauta, A.; Kärenlampi, S.; Van Belleghem, F.; Smeets, K.; Vangronsveld, J. Leaf Proteome Responses of Arabidopsis Thaliana Exposed to Mild Cadmium Stress. J. Plant Physiol. 2010, 167, 247–254. [Google Scholar] [CrossRef]
- Shen, Y.; Li, J.; He, F.; Zhu, J.; Han, Q.; Zhan, X.; Xing, B. Phenanthrene-Triggered Tricarboxylic Acid Cycle Response in Wheat Leaf. Sci. Total Environ. 2019, 665, 107–112. [Google Scholar] [CrossRef]
- Zhan, X.; Yi, X.; Yue, L.; Fan, X.; Xu, G.; Xing, B. Cytoplasmic PH-Stat during Phenanthrene Uptake by Wheat Roots: A Mechanistic Consideration. Environ. Sci. Technol. 2015, 49, 6037–6044. [Google Scholar] [CrossRef]
- Shen, Y.; Du, J.; Yue, L.; Zhan, X. Proteomic Analysis of Plasma Membrane Proteins in Wheat Roots Exposed to Phenanthrene. Env. Sci. Pollut. Res. 2016, 23, 10863–10871. [Google Scholar] [CrossRef]
- Le Lay, P.; Isaure, M.-P.; Sarry, J.-E.; Kuhn, L.; Fayard, B.; Le Bail, J.-L.; Bastien, O.; Garin, J.; Roby, C.; Bourguignon, J. Metabolomic, Proteomic and Biophysical Analyses of Arabidopsis Thaliana Cells Exposed to a Caesium Stress. Influence of Potassium Supply. Biochimie 2006, 88, 1533–1547. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Ji, C.; Wei, L.; Zhao, J.; Yu, J. An Integrated Proteomic and Metabolomic Study on the Chronic Effects of Mercury in Suaeda Salsa under an Environmentally Relevant Salinity. PLoS ONE 2013, 8, e64041. [Google Scholar] [CrossRef]
- Sivaram, A.K.; Subashchandrabose, S.R.; Logeshwaran, P.; Lockington, R.; Naidu, R.; Megharaj, M. Metabolomics Reveals Defensive Mechanisms Adapted by Maize on Exposure to High Molecular Weight Polycyclic Aromatic Hydrocarbons. Chemosphere 2019, 214, 771–780. [Google Scholar] [CrossRef]
- Xia, L.; Xiaodong, M.; Yunhe, C.; Junxiang, L.; Junzhu, Z.; Feifei, Z.; Zhenyuan, S.; Lei, H. Transcriptomic and Metabolomic Insights into the Adaptive Response of Salix Viminalis to Phenanthrene. Chemosphere 2021, 262, 127573. [Google Scholar] [CrossRef]
- Rodríguez-Celma, J.; Rellán-Alvarez, R.; Abadía, A.; Abadía, J.; López-Millán, A.-F. Changes Induced by Two Levels of Cadmium Toxicity in the 2-DE Protein Profile of Tomato Roots. J. Proteom. 2010, 73, 1694–1706. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, D.-G.; Kim, K.-H.; Alam, I.; Lee, S.-H.; Lee, K.-W.; Lee, H.; Lee, B.-H. Analysis of Arsenic Stress-Induced Differentially Expressed Proteins in Rice Leaves by Two-Dimensional Gel Electrophoresis Coupled with Mass Spectrometry. Chemosphere 2010, 78, 224–231. [Google Scholar] [CrossRef]
- Chaffei, C.; Gouia, H.; Masclaux, C.; Ghorbel, M.H. Réversibilité des effets du cadmium sur la croissance et l’assimilation de l’azote chez la tomate (Lycopersicon esculentum). Comptes Rendus Biol. 2003, 326, 401–412. [Google Scholar] [CrossRef]
- Singh, P.; Singh, I.; Shah, K. Reduced Activity of Nitrate Reductase under Heavy Metal Cadmium Stress in Rice: An in Silico Answer. Front. Plant Sci. 2019, 9, 1948. [Google Scholar] [CrossRef]
- Zafari, S.; Sharifi, M.; Ahmadian Chashmi, N.; Mur, L.A.J. Modulation of Pb-Induced Stress in Prosopis Shoots through an Interconnected Network of Signaling Molecules, Phenolic Compounds and Amino Acids. Plant Physiol. Biochem. 2016, 99, 11–20. [Google Scholar] [CrossRef]
- Yusuf, M.; Fariduddin, Q.; Varshney, P.; Ahmad, A. Salicylic Acid Minimizes Nickel and/or Salinity-Induced Toxicity in Indian Mustard (Brassica juncea) through an Improved Antioxidant System. Environ. Sci. Pollut. Res. Int. 2012, 19, 8–18. [Google Scholar] [CrossRef]
- Kim, J.I.; Zhang, X.; Pascuzzi, P.E.; Liu, C.-J.; Chapple, C. Glucosinolate and Phenylpropanoid Biosynthesis Are Linked by Proteasome-Dependent Degradation of PAL. New Phytol. 2020, 225, 154–168. [Google Scholar] [CrossRef]
- Erdal, S.; Turk, H. Cysteine-Induced Upregulation of Nitrogen Metabolism-Related Genes and Enzyme Activities Enhance Tolerance of Maize Seedlings to Cadmium Stress. Environ. Exp. Bot. 2016, 132, 92–99. [Google Scholar] [CrossRef]
- Kohlmeier, M. Cysteine. In Nutrient Metabolism; Elsevier: Amsterdam, The Netherlands, 2003; pp. 348–356. [Google Scholar] [CrossRef]
- Jiang, S.; Weng, B.; Liu, T.; Su, Y.; Liu, J.; Lu, H.; Yan, C. Response of Phenolic Metabolism to Cadmium and Phenanthrene and Its Influence on Pollutant Translocations in the Mangrove Plant Aegiceras Corniculatum (L.) Blanco (Ac). Ecotoxicol. Environ. Saf. 2017, 141, 290–297. [Google Scholar] [CrossRef]
- Jaskulak, M.; Rorat, A.; Grobelak, A.; Kacprzak, M. Antioxidative Enzymes and Expression of RbcL Gene as Tools to Monitor Heavy Metal-Related Stress in Plants. J. Environ. Manag. 2018, 218, 71–78. [Google Scholar] [CrossRef]
- Tandey, R.; Chouhan, K.B.S.; Sen, K.K.; Mehta, R.; Dubey, A.; Das, R.; Saha, P.; Mandal, V. Physiological and Biochemical Responses of Amaranthus Cruentus to Polycyclic Aromatic Hydrocarbon Pollution Caused by Thermal Power Units. Environ. Sci. Pollut. Res. 2020, 27, 14790–14806. [Google Scholar] [CrossRef]
- Kipopoulou, A.M.; Manoli, E.; Samara, C. Bioconcentration of Polycyclic Aromatic Hydrocarbons in Vegetables Grown in an Industrial Area. Environ. Pollut. 1999, 106, 369–380. [Google Scholar] [CrossRef]
- Wagrowski, D.M.; Hites, R.A. Polycyclic Aromatic Hydrocarbon Accumulation in Urban, Suburban, and Rural Vegetation. Environ. Sci. Technol. 1997, 31, 279–282. [Google Scholar] [CrossRef]
- Corada, K.; Woodward, H.; Alaraj, H.; Collins, C.M.; de Nazelle, A. A Systematic Review of the Leaf Traits Considered to Contribute to Removal of Airborne Particulate Matter Pollution in Urban Areas. Environ. Pollut. 2021, 269, 116104. [Google Scholar] [CrossRef]
- Muhammad, S.; Wuyts, K.; Samson, R. Immobilized Atmospheric Particulate Matter on Leaves of 96 Urban Plant Species. Environ. Sci. Pollut. Res. 2020, 27, 36920–36938. [Google Scholar] [CrossRef]
- Paull, N.J.; Krix, D.; Irga, P.J.; Torpy, F.R. Airborne Particulate Matter Accumulation on Common Green Wall Plants. Int. J. Phytoremediation 2020, 22, 594–606. [Google Scholar] [CrossRef]
- Rey-Salgueiro, L.; Martínez-Carballo, E.; García-Falcón, M.S.; Simal-Gándara, J. Effects of a Chemical Company Fire on the Occurrence of Polycyclic Aromatic Hydrocarbons in Plant Foods. Food Chem. 2008, 108, 347–353. [Google Scholar] [CrossRef]
- Holoubek, I.; Kořínek, P.; Šeda, Z.; Schneiderová, E.; Holoubková, I.; Pacl, A.; Tříska, J.; Cudlín, P.; Čáslavský, J. The Use of Mosses and Pine Needles to Detect Persistent Organic Pollutants at Local and Regional Scales. Environ. Pollut. 2000, 109, 283–292. [Google Scholar] [CrossRef]
- Kylin, H.; Grimvall, E.; Oestman, C. Environmental Monitoring of Polychlorinated Biphenyls Using Pine Needles as Passive Samplers. Environ. Sci. Technol. 1994, 28, 1320–1324. [Google Scholar] [CrossRef]
- Collins, C.D.; Finnegan, E. Modeling the Plant Uptake of Organic Chemicals, Including the Soil-Air-Plant Pathway. Environ. Sci. Technol. 2010, 44, 998–1003. [Google Scholar] [CrossRef]
- Takaki, K.; Wade, A.J.; Collins, C.D. Assessment of Plant Uptake Models Used in Exposure Assessment Tools for Soils Contaminated with Organic Pollutants. Environ. Sci. Technol. 2014, 48, 12073–12082. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, S.; Zheng, L.; Li, T.; Sun, Y.; Ma, L.; Lin, Z.; Grathwohl, P.; Lohmann, R. Air-Soil Diffusive Exchange of PAHs in an Urban Park of Shanghai Based on Polyethylene Passive Sampling: Vertical Distribution, Vegetation Influence and Diffusive Flux. Sci. Total Environ. 2019, 689, 734–742. [Google Scholar] [CrossRef]
- Pullagurala, V.L.R.; Rawat, S.; Adisa, I.O.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Plant Uptake and Translocation of Contaminants of Emerging Concern in Soil. Sci. Total Environ. 2018, 636, 1585–1596. [Google Scholar] [CrossRef]
- Kapustka, L.A. Establishing Eco-SSLs for PAHs: Lessons Revealed from a Review of Literature on Exposure and Effects to Terrestrial Receptors. Hum. Ecol. Risk Assess. Int. J. 2004, 10, 185–205. [Google Scholar] [CrossRef]
- Baur, P.; Schönherr, J. Temperature Dependence of the Diffusion of Organic Compounds across Plant Cuticles. Chemosphere 1995, 30, 1331–1340. [Google Scholar] [CrossRef]
- Desalme, D.; Binet, P.; Chiapusio, G. Challenges in Tracing the Fate and Effects of Atmospheric Polycyclic Aromatic Hydrocarbon Deposition in Vascular Plants. Environ. Sci. Technol. 2013, 47, 3967–3981. [Google Scholar] [CrossRef] [PubMed]
- Sims, R.C.; Overcash, M.R. Fate of Polynuclear Aromatic Compounds (PNAs) in Soil-Plant Systems. In Residue Reviews; Gunther, F.A., Ed.; Springer: New York, NY, USA, 1983; pp. 1–68. [Google Scholar] [CrossRef]
- Wild, E.; Dent, J.; Thomas, G.O.; Jones, K.C. Direct Observation of Organic Contaminant Uptake, Storage, and Metabolism within Plant Roots. Environ. Sci. Technol. 2005, 39, 3695–3702. [Google Scholar] [CrossRef]
- Dumas, A.-S.; Taconnat, L.; Barbas, E.; Rigaill, G.; Catrice, O.; Bernard, D.; Benamar, A.; Macherel, D.; El Amrani, A.; Berthomé, R. Unraveling the Early Molecular and Physiological Mechanisms Involved in Response to Phenanthrene Exposure. BMC Genom. 2016, 17, 818. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zhang, X.; Yin, X.; Ma, H.; Liang, J.; Zhou, L.; Jiang, T.; Xu, G. H +/Phenanthrene Symporter and Aquaglyceroporin Are Implicated in Phenanthrene Uptake by Wheat (Triticum aestivum L.) Roots. J. Environ. Qual. 2012, 41, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Fismes, J.; Perrin-Ganier, C.; Empereur-Bissonnet, P.; Morel, J.L. Soil-to-Root Transfer and Translocation of Polycyclic Aromatic Hydrocarbons by Vegetables Grown on Industrial Contaminated Soils. J. Environ. Qual. 2002, 31, 1649–1656. [Google Scholar] [CrossRef]
- Fernández, V.; Eichert, T. Uptake of Hydrophilic Solutes through Plant Leaves: Current State of Knowledge and Perspectives of Foliar Fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar Heavy Metal Uptake, Toxicity and Detoxification in Plants: A Comparison of Foliar and Root Metal Uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From Plant Surface to Plant Metabolism: The Uncertain Fate of Foliar-Applied Nutrients. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Schreiber, L. Polar Paths of Diffusion across Plant Cuticles: New Evidence for an Old Hypothesis. Ann. Bot. 2005, 95, 1069–1073. [Google Scholar] [CrossRef]
- Schreck, E.; Laplanche, C.; Le Guédard, M.; Bessoule, J.-J.; Austruy, A.; Xiong, T.; Foucault, Y.; Dumat, C. Influence of Fine Process Particles Enriched with Metals and Metalloids on Lactuca sativa L. Leaf Fatty Acid Composition Following Air and/or Soil-Plant Field Exposure. Environ. Pollut. 2013, 179, 242–249. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Jockwer, F. Accumulation of Heavy Metals in Plants Grown on Mineralised Soils of the Austrian Alps. Environ. Pollut. 1999, 104, 145–155. [Google Scholar] [CrossRef]
- Krishnamurti, G.S.R.; Huang, P.M.; Kozak, L.M. Sorption and Desorption Kinetics of Cadmium from Soils: Influence of Phosphate. Soil Sci. 1999, 164, 888–898. [Google Scholar] [CrossRef]
- Antoniadis, V.; Robinson, J.S.; Alloway, B.J. Effects of Short-Term PH Fluctuations on Cadmium, Nickel, Lead, and Zinc Availability to Ryegrass in a Sewage Sludge-Amended Field. Chemosphere 2008, 71, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The Influence of PH and Organic Matter Content in Paddy Soil on Heavy Metal Availability and Their Uptake by Rice Plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wei, C.; Xiao, Y.; Li, L.; Wu, D. Heavy Metals Uptake and Transport by Native Wild Plants: Implications for Phytoremediation and Restoration. Environ. Earth Sci. 2019, 78, 103. [Google Scholar] [CrossRef]
- Seregin, I.V.; Ivanov, V.B. Physiological Aspects of Cadmium and Lead Toxic Effects on Higher Plants. Russ. J. Plant Physiol. 2001, 48, 523–544. [Google Scholar] [CrossRef]
- Alagić, S.Č.; Jovanović, V.P.S.; Mitić, V.D.; Cvetković, J.S.; Petrović, G.M.; Stojanović, G.S. Bioaccumulation of HMW PAHs in the Roots of Wild Blackberry from the Bor Region (Serbia): Phytoremediation and Biomonitoring Aspects. Sci. Total Environ. 2016, 562, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Pan, L.; Tsang, D.C.W.; Zhan, Y.; Zhu, L.; Li, X. Organic Contamination and Remediation in the Agricultural Soils of China: A Critical Review. Sci. Total Environ. 2018, 615, 724–740. [Google Scholar] [CrossRef]
- Parrish, Z.D.; White, J.C.; Isleyen, M.; Gent, M.P.N.; Iannucci-Berger, W.; Eitzer, B.D.; Kelsey, J.W.; Mattina, M.I. Accumulation of Weathered Polycyclic Aromatic Hydrocarbons (PAHs) by Plant and Earthworm Species. Chemosphere 2006, 64, 609–618. [Google Scholar] [CrossRef]
- Collins, C.; Fryer, M.; Grosso, A. Plant Uptake of Non-Ionic Organic Chemicals. Environ. Sci. Technol. 2006, 40, 45–52. [Google Scholar] [CrossRef]
- Topp, E.; Scheunert, I.; Attar, A.; Korte, F. Factors Affecting the Uptake of 14C-Labeled Organic Chemicals by Plants from Soil. Ecotoxicol. Environ. Saf. 1986, 11, 219–228. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Role of Hyperaccumulators in Phytoextraction of Metals from Contaminated Mining Sites: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 41, 168–214. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarría, G.; van der Ent, A. A Global Database for Plants that Hyperaccumulate Metal and Metalloid Trace Elements. New Phytol. 2017, 218, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Alford, É.R.; Pilon-Smits, E.A.H.; Paschke, M.W. Metallophytes—A View from the Rhizosphere. Plant Soil 2010, 337, 33–50. [Google Scholar] [CrossRef]
- Chaney, R.L.; Angle, J.S.; Broadhurst, C.L.; Peters, C.A.; Tappero, R.V.; Sparks, D.L. Improved Understanding of Hyperaccumulation Yields Commercial Phytoextraction and Phytomining Technologies. J. Environ. Qual. 2007, 36, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.N.V. Nickelophilous Plants and Their Significance in Phytotechnologies. Braz. J. Plant Physiol. 2005, 17, 113–128. [Google Scholar] [CrossRef]
- De Caroli, M.; Furini, A.; DalCorso, G.; Rojas, M.; Di Sansebastiano, G.-P. Endomembrane Reorganization Induced by Heavy Metals. Plants 2020, 9, 482. [Google Scholar] [CrossRef]
- Zheng, Z.; Shetty, K. Azo Dye-Mediated Regulation of Total Phenolics and Peroxidase Activity in Thyme (Thymus vulgaris L.) and Rosemary (Rosmarinus officinalis L.) Clonal Lines. J. Agric. Food Chem. 2000, 48, 932–937. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, V.; Ghanem, A.; Senesi, N. Phytoremediation of Pyrene Contaminated Soils by Different Plant Species. Clean Soil Air Water 2013, 41, 377–382. [Google Scholar] [CrossRef]
- Molina, L.; Udaondo, Z.; Montero-Curiel, M.; Wittich, R.-M.; García-Puente, A.; Segura, A. Clover Root Exudates Favor Novosphingobium sp. HR1a Establishment in the Rhizosphere and Promote Phenanthrene Rhizoremediation. mSphere 2021, 6. [Google Scholar] [CrossRef]
- Kumar, S.; Jin, M.; Weemhoff, J.L. Cytochrome P450-Mediated Phytoremediation Using Transgenic Plants: A Need for Engineered Cytochrome P450 Enzymes. J. Pet. Environ. Biotechnol. 2012, 3, 1–5. [Google Scholar] [CrossRef]
- Mansuy, D. The Great Diversity of Reactions Catalyzed by Cytochromes P450. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 121, 5–14. [Google Scholar] [CrossRef]
- Hernández-Vega, J.C.; Cady, B.; Kayanja, G.; Mauriello, A.; Cervantes, N.; Gillespie, A.; Lavia, L.; Trujillo, J.; Alkio, M.; Colón-Carmona, A. Detoxification of Polycyclic Aromatic Hydrocarbons (PAHs) in Arabidopsis Thaliana Involves a Putative Flavonol Synthase. J. Hazard. Mater. 2017, 321, 268–280. [Google Scholar] [CrossRef]
- Peng, R.-H.; Xu, R.-R.; Fu, X.-Y.; Xiong, A.-S.; Zhao, W.; Tian, Y.-S.; Zhu, B.; Jin, X.-F.; Chen, C.; Han, H.-J.; et al. Microarray Analysis of the Phytoremediation and Phytosensing of Occupational Toxicant Naphthalene. J. Hazard. Mater. 2011, 189, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kathi, S. Phytoremediation Approaches to PAH Contaminated Soil. Indian J. Sci. Technol. 2011, 4, 56–63. [Google Scholar] [CrossRef]
- Kvesitadze, E.; Sadunishvili, T.; Kvesitadze, G. Mechanisms of Organic Contaminants Uptake and Degradation in Plants. 2009. Available online: https://zenodo.org/record/1060096 (accessed on 16 September 2021).
- Reichenauer, T.G.; Germida, J.J. Phytoremediation of Organic Contaminants in Soil and Groundwater. ChemSusChem 2008, 1, 708–717. [Google Scholar] [CrossRef]
- Kvesitadze, G.; Khatisashvili, G.; Sadunishvili, T.; Kvesitadze, E. Plants for Remediation: Uptake, Translocation and Transformation of Organic Pollutants. In Plants, Pollutants and Remediation; Öztürk, M., Ashraf, M., Aksoy, A., Ahmad, M.S.A., Hakeem, K.R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 241–308. [Google Scholar] [CrossRef]
- El Amrani, A.; Dumas, A.-S.; Wick, L.Y.; Yergeau, E.; Berthomé, R. “Omics” Insights into PAH Degradation toward Improved Green Remediation Biotechnologies. Environ. Sci. Technol. 2015, 49, 11281–11291. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Jamil, S.; Singh, N. Transgenic Plants for Enhanced Biodegradation and Phytoremediation of Organic Xenobiotics. Biotechnol. Adv. 2009, 27, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.; Blake-Kalff, M.; Davies, E. Detoxification of Xenobiotics by Plants: Chemical Modification and Vacuolar Compartmentation. Trends Plant Sci. 1997, 2, 144–151. [Google Scholar] [CrossRef]
- Martinoia, E.; Grill, E.; Tommasini, R.; Kreuz, K.; Amrhein, N. ATP-Dependent Glutathione S-Conjugate “export” Pump in the Vacuolar Membrane of Plants. Nature 1993, 364, 247–249. [Google Scholar] [CrossRef]
- Sánchez-Fernández, R.; Davies, T.G.E.; Coleman, J.O.D.; Rea, P.A. The Arabidopsis Thaliana ABC Protein Superfamily, a Complete Inventory. J. Biol. Chem. 2001, 276, 30231–30244. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.J.; Campanella, B.F.; Castro, P.M.L.; Harms, H.; Lichtfouse, E.; Schäffner, A.R.; Smrcek, S.; Werck-Reichhart, D. Phytoremediation of Polyaromatic Hydrocarbons, Anilines and Phenols. Environ. Sci. Pollut. Res. 2002, 9, 29–47. [Google Scholar] [CrossRef]
- Ohkawa, H.; Tsujii, H.; Shimoji, M.; Imajuku, Y.; Imaishi, H. Cytochrome P450 Biodiversity and Plant Protection. J. Pestic. Sci. 1999, 24, 197–203. [Google Scholar] [CrossRef][Green Version]
- Gaspar, T. Integrated Relationships of Biochemical and Physiological Peroxidase Activities. In Molecular and Physiological Aspects of Plant Peroxidases; Greppin, H., Penel, C., Gaspar, T., Eds.; Centre de Botanique, Université de Genève: Genève, Switzerland, 1986; pp. 455–468. [Google Scholar]
- Kolb, M.; Harms, H. Metabolism of Fluoranthene in Different Plant Cell Cultures and Intact Plants. Environ. Toxicol. Chem. 2000, 19, 1304–1310. [Google Scholar] [CrossRef]
- Alkio, M.; Tabuchi, T.M.; Wang, X.; Colón-Carmona, A. Stress Responses to Polycyclic Aromatic Hydrocarbons in Arabidopsis Include Growth Inhibition and Hypersensitive Response-like Symptoms. J. Exp. Bot. 2005, 56, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Alves, W.S.; Manoel, E.A.; Santos, N.S.; Nunes, R.O.; Domiciano, G.C.; Soares, M.R. Detection of Polycyclic Aromatic Hydrocarbons (PAHs) in Medicago sativa L. by Fluorescence Microscopy. Micron 2017, 95, 23–30. [Google Scholar] [CrossRef]
- Cavé-Radet, A.; Rabhi, M.; Gouttefangeas, F.; El Amrani, A. Do Specialized Cells Play a Major Role in Organic Xenobiotic Detoxification in Higher Plants? Front. Plant Sci. 2020, 11, 1037. [Google Scholar] [CrossRef]
- Shiri, M.; Rabhi, M.; Abdelly, C.; Amrani, A.E. The Halophytic Model Plant Thellungiella Salsuginea Exhibited Increased Tolerance to Phenanthrene-Induced Stress in Comparison with the Glycophitic One Arabidopsis thaliana: Application for Phytoremediation. Ecol. Eng. 2015, 74, 125–134. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-Y.; Wang, Y.-S. Expression and Characterization Analysis of Type 2 Metallothionein from Grey Mangrove Species (Avicennia Marina) in Response to Metal Stress. Aquat. Toxicol. 2010, 99, 86–92. [Google Scholar] [CrossRef]
- Hussain, S.; Slikker Jr, W.; Ali, S.F. Role of Metallothionein and other Antioxidants in Scavenging Superoxide Radicals and their Possible Role in Neuroprotection. Neurochem. Int. 1996, 29, 145–152. [Google Scholar] [CrossRef]
- Bratić, A.M.; Majić, D.B.; Samardžić, J.T.; Maksimović, V.R. Functional Analysis of the Buckwheat Metallothionein Promoter: Tissue Specificity Pattern and up-Regulation under Complex Stress Stimuli. J. Plant Physiol. 2009, 166, 996–1000. [Google Scholar] [CrossRef]
- Leszczyszyn, O.I.; Imam, H.T.; Blindauer, C.A. Diversity and Distribution of Plant Metallothioneins: A Review of Structure, Properties and Functions. Metallomics 2013, 5, 1146. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and Metallothionenins: Roles in Heavy Metal Detoxification and Homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Pal, R.; Rai, J.P.N. Phytochelatins: Peptides Involved in Heavy Metal Detoxification. Appl. Biochem. Biotechnol. 2010, 160, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Le Faucheur, S.; Behra, R.; Sigg, L. Phytochelatin Induction, Cadmium Accumulation, and Algal Sensitivity to Free Cadmiun Ion in Scenedesmus vacuolatus. Environ. Toxicol. Chem. 2005, 24, 1731. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Cózatl, D.G.; Butko, E.; Springer, F.; Torpey, J.W.; Komives, E.A.; Kehr, J.; Schroeder, J.I. Identification of High Levels of Phytochelatins, Glutathione and Cadmium in the Phloem Sap of Brassica Napus. A Role for Thiol-Peptides in the Long-Distance Transport of Cadmium and the Effect of Cadmium on Iron Translocation. Plant J. 2008, 54, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Shri, M.; Gupta, A.; Rani, V.; Chakrabarty, D. Toxicity and Detoxification of Heavy Metals during Plant Growth and Metabolism. Environ. Chem. Lett. 2018, 16, 1169–1192. [Google Scholar] [CrossRef]
- Ma, J.F.; Zheng, S.J.; Matsumoto, H.; Hiradate, S. Detoxifying Aluminium with Buckwheat. Nature 1997, 390, 569–570. [Google Scholar] [CrossRef]
- Richau, K.H.; Kozhevnikova, A.D.; Seregin, I.V.; Vooijs, R.; Koevoets, P.L.M.; Smith, J.A.C.; Ivanov, V.B.; Schat, H. Chelation by Histidine Inhibits the Vacuolar Sequestration of Nickel in Roots of the Hyperaccumulator Thlaspi Caerulescens. New Phytol. 2009, 183, 106–116. [Google Scholar] [CrossRef]
- Persans, M.W.; Nieman, K.; Salt, D.E. Functional Activity and Role of Cation-Efflux Family Members in Ni Hyperaccumulation in Thlaspi Goesingense. Proc. Natl. Acad. Sci. USA 2001, 98, 9995–10000. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.G.; Jobe, T.O.; Hauser, F.; Schroeder, J.I. Long-Distance Transport, Vacuolar Sequestration, Tolerance, and Transcriptional Responses Induced by Cadmium and Arsenic. Curr. Opin. Plant Biol. 2011, 14, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Hendrix, S.; Amaral dos Reis, R.; De Smet, S.; Deckers, J.; Gielen, H.; Jozefczak, M.; Loix, C.; Vercampt, H.; Vangronsveld, J.; et al. Hydrogen Peroxide, Signaling in Disguise during Metal Phytotoxicity. Front. Plant Sci. 2016, 7, 470. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and Redox Signalling in the Response of Plants to Abiotic Stress: ROS and Redox Signalling in Plants. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Oelmüller, R. Reactive Oxygen Species Generation and Signaling in Plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, J.; Gu, R.; Yue, L.; Wang, H.; Zhan, X.; Xing, B. Carotenoid and Superoxide Dismutase Are the Most Effective Antioxidants Participating in ROS Scavenging in Phenanthrene Accumulated Wheat Leaf. Chemosphere 2018, 197, 513–525. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The Oxidative Burst in Plant Disease Resistance. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Montillet, J.-L.; Chamnongpol, S.; Rustérucci, C.; Dat, J.; van de Cotte, B.; Agnel, J.-P.; Battesti, C.; Inzé, D.; Van Breusegem, F.; Triantaphylidès, C. Fatty Acid Hydroperoxides and H 2 O 2 in the Execution of Hypersensitive Cell Death in Tobacco Leaves. Plant Physiol. 2005, 138, 1516–1526. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Khedia, J.; Agarwal, P.; Agarwal, P.K. Deciphering Hydrogen Peroxide-Induced Signalling towards Stress Tolerance in Plants. 3 Biotech 2019, 9, 395. [Google Scholar] [CrossRef]
- Poole, L.B. The Basics of Thiols and Cysteines in Redox Biology and Chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Akter, S.; Eeckhout, D.; Persiau, G.; Wahni, K.; Bodra, N.; Van Molle, I.; De Smet, B.; Vertommen, D.; Gevaert, K.; et al. Sulfenome Mining in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA 2014, 111, 11545–11550. [Google Scholar] [CrossRef] [PubMed]
- Wages, P.A.; Lavrich, K.S.; Zhang, Z.; Cheng, W.-Y.; Corteselli, E.; Gold, A.; Bromberg, P.; Simmons, S.O.; Samet, J.M. Protein Sulfenylation: A Novel Readout of Environmental Oxidant Stress. Chem. Res. Toxicol. 2015, 28, 2411–2418. [Google Scholar] [CrossRef]
- Fratelli, M.; Gianazza, E.; Ghezzi, P. Redox Proteomics: Identification and Functional Role of Glutathionylated Proteins. Expert Rev. Proteom. 2004, 1, 365–376. [Google Scholar] [CrossRef]
- Gallogly, M.M.; Mieyal, J.J. Mechanisms of Reversible Protein Glutathionylation in Redox Signaling and Oxidative Stress. Curr. Opin. Pharmacol. 2007, 7, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E. [38] Protein Oxidative Damage. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2000; Volume 319, pp. 428–436. [Google Scholar] [CrossRef]
- Madian, A.G.; Regnier, F.E. Proteomic Identification of Carbonylated Proteins and Their Oxidation Sites. J. Proteome Res. 2010, 9, 3766–3780. [Google Scholar] [CrossRef]
- Arnaiz, A.; Rosa-Diaz, I.; Romero-Puertas, M.C.; Sandalio, L.M.; Diaz, I. Nitric Oxide, an Essential Intermediate in the Plant–Herbivore Interaction. Front. Plant Sci. 2021, 11, 620086. [Google Scholar] [CrossRef]
- Htet Hlaing, K.; Clément, M.-V. Formation of Protein S-Nitrosylation by Reactive Oxygen Species. Free Radic. Res. 2014, 48, 996–1010. [Google Scholar] [CrossRef]
- Yun, B.-W.; Feechan, A.; Yin, M.; Saidi, N.B.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.-G.; Kwon, E.; Spoel, S.H.; et al. S-Nitrosylation of NADPH Oxidase Regulates Cell Death in Plant Immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef]
- Roldán-Arjona, T.; Ariza, R.R. Repair and Tolerance of Oxidative DNA Damage in Plants. Mutat. Res./Rev. Mutat. Res. 2009, 681, 169–179. [Google Scholar] [CrossRef]
- Babu, T.S.; Marder, J.B.; Tripuranthakam, S.; Dixon, D.G.; Greenberg, B.M. Synergistic Effects of a Photooxidized Polycyclic Aromatic Hydrocarbon and Copper on Photosynthesis and Plant Growth: Evidence That in Vivo Formation of Reactive Oxygen Species Is a Mechanism of Copper Toxicity. Environ. Toxicol. Chem. 2001, 20, 1351–1358. [Google Scholar] [CrossRef]
- Lin, Q.; Shen, K.-L.; Zhao, H.-M.; Li, W.-H. Growth Response of Zea mays L. in Pyrene–Copper Co-Contaminated Soil and the Fate of Pollutants. J. Hazard. Mater. 2008, 150, 515–521. [Google Scholar] [CrossRef]
- Sun, L.; Yan, X.; Liao, X.; Wen, Y.; Chong, Z.; Liang, T. Interactions of Arsenic and Phenanthrene on Their Uptake and Antioxidative Response in Pteris vittata L. Environ. Pollut. 2011, 159, 3398–3405. [Google Scholar] [CrossRef]
- Zhang, Z.; Rengel, Z.; Meney, K.; Pantelic, L.; Tomanovic, R. Polynuclear Aromatic Hydrocarbons (PAHs) Mediate Cadmium Toxicity to an Emergent Wetland Species. J. Hazard. Mater. 2011, 189, 119–126. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of Brassinosteroids in Alleviation of Phenanthrene–Cadmium Co-Contamination-Induced Photosynthetic Inhibition and Oxidative Stress in Tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef]
- Chen, J.; Xia, X.; Zhang, Z.; Wen, W.; Xi, N.; Zhang, Q. The Combination of Warming and Copper Decreased the Uptake of Polycyclic Aromatic Hydrocarbons by Spinach and Their Associated Cancer Risk. Sci. Total Environ. 2020, 727, 138732. [Google Scholar] [CrossRef] [PubMed]
- Babu, T.S.; Tripuranthakam, S.; Greenberg, B.M. Biochemical Responses of the Aquatic Higher Plant Lemna gibba to a Mixture of Copper and 1,2-Dihydroxyanthraquinone: Synergistic Toxicity via Reactive Oxygen Species. Environ. Toxicol. Chem. 2005, 24, 3030. [Google Scholar] [CrossRef]
- Fryzova, R.; Pohanka, M.; Martinkova, P.; Cihlarova, H.; Brtnicky, M.; Hladky, J.; Kynicky, J. Oxidative Stress and Heavy Metals in Plants. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 245, pp. 129–156. [Google Scholar] [CrossRef]
- Liu, H.; Weisman, D.; Tang, L.; Tan, L.; Zhang, W.; Wang, Z.; Huang, Y.; Lin, W.; Liu, X.; Colón-Carmona, A. Stress Signaling in Response to Polycyclic Aromatic Hydrocarbon Exposure in Arabidopsis Thaliana Involves a Nucleoside Diphosphate Kinase, NDPK-3. Planta 2015, 241, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Scandalios, J.G. Oxidative Stress: Molecular Perception and Transduction of Signals Triggering Antioxidant Gene Defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef] [PubMed]
- Mathé, C.; Barre, A.; Jourda, C.; Dunand, C. Evolution and Expression of Class III Peroxidases. Arch. Biochem. Biophys. 2010, 500, 58–65. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Cuypers, A.; Keunen, E.; Bohler, S.; Jozefczak, M.; Opdenakker, K.; Gielen, H.; Vercampt, H.; Bielen, A.; Schellingen, K.; Vangronsveld, J.; et al. Cadmium and Copper Stress Induce a Cellular Oxidative Challenge Leading to Damage Versus Signalling. In Metal Toxicity in Plants: Perception, Signaling and Remediation; Gupta, D.K., Sandalio, L.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 65–90. [Google Scholar] [CrossRef]
- Marrs, K.A. The Functions and Regulation of Glutathione S-transferases in Plants. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1996, 47, 127–158. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in Plants: An Integrated Overview: Glutathione Status and Functions. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Burritt, D.J. The Polycyclic Aromatic Hydrocarbon Phenanthrene Causes Oxidative Stress and Alters Polyamine Metabolism in the Aquatic Liverwort Riccia fluitans L. Plant Cell Environ. 2008, 31, 1416–1431. [Google Scholar] [CrossRef]
- Ali, I.; Alothman, Z.A.; Alwarthan, A. Uptake of Propranolol on Ionic Liquid Iron Nanocomposite Adsorbent: Kinetic, Thermodynamics and Mechanism of Adsorption. J. Mol. Liq. 2017, 236, 205–213. [Google Scholar] [CrossRef]
- Król, A.; Amarowicz, R.; Weidner, S. The Effects of Cold Stress on the Phenolic Compounds and Antioxidant Capacity of Grapevine (Vitis vinifera L.) Leaves. J. Plant Physiol. 2015, 189, 97–104. [Google Scholar] [CrossRef]
- Mélida, H.; Caparrós-Ruiz, D.; Álvarez, J.; Acebes, J.L.; Encina, A. Deepening into the Proteome of Maize Cells Habituated to the Cellulose Biosynthesis Inhibitor Dichlobenil. Plant Signal. Behav. 2011, 6, 143–146. [Google Scholar] [CrossRef]
- Manquián-Cerda, K.; Cruces, E.; Escudey, M.; Zúñiga, G.; Calderón, R. Interactive Effects of Aluminum and Cadmium on Phenolic Compounds, Antioxidant Enzyme Activity and Oxidative Stress in Blueberry (Vaccinium corymbosum L.) Plantlets Cultivated in Vitro. Ecotoxicol. Environ. Saf. 2018, 150, 320–326. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive Oxygen Gene Network of Plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive Oxygen Signaling and Abiotic Stress. Physiol. Plant 2008, 133, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kang, M.-I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and Electrophilic Stresses Activate Nrf2 through Inhibition of Ubiquitination Activity of Keap1. Mol. Cell. Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B. The Role of Ethylene and ROS in Salinity, Heavy Metal, and Flooding Responses in Rice. Front. Plant Sci. 2014, 5, 685. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive Oxygen Species in Plant Development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Stress-Triggered Redox Signalling: What’s in PROSpect?: What’s in PROSpect? Plant Cell Environ. 2016, 39, 951–964. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Gautam, V.; Bali, S.; Sharma, A.; Khanna, K.; Arora, S.; Thukral, A.K.; Ohri, P.; Karpets, Y.V.; et al. ROS Signaling in Plants Under Heavy Metal Stress. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Khan, M.I.R., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 185–214. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Heavy Metal Stress Signaling in Plants. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 585–603. [Google Scholar] [CrossRef]
- Palmieri, M.C.; Sell, S.; Huang, X.; Scherf, M.; Werner, T.; Durner, J.; Lindermayr, C. Nitric Oxide-Responsive Genes and Promoters in Arabidopsis Thaliana: A Bioinformatics Approach. J. Exp. Bot. 2008, 59, 177–186. [Google Scholar] [CrossRef]
- Sinha, A.K.; Jaggi, M.; Raghuram, B.; Tuteja, N. Mitogen-Activated Protein Kinase Signaling in Plants under Abiotic Stress. Plant Signal. Behav. 2011, 6, 196–203. [Google Scholar] [CrossRef]
- Taj, G.; Agarwal, P.; Grant, M.; Kumar, A. MAPK Machinery in Plants: Recognition and Response to Different Stresses through Multiple Signal Transduction Pathways. Plant Signal. Behav. 2010, 5, 1370–1378. [Google Scholar] [CrossRef]
- Jonak, C.; Nakagami, H.; Hirt, H. Heavy Metal Stress. Activation of Distinct Mitogen-Activated Protein Kinase Pathways by Copper and Cadmium. Plant Physiol. 2004, 136, 3276–3283. [Google Scholar] [CrossRef]
- Rao, K.P.; Vani, G.; Kumar, K.; Wankhede, D.P.; Misra, M.; Gupta, M.; Sinha, A.K. Arsenic Stress Activates MAP Kinase in Rice Roots and Leaves. Arch. Biochem. Biophys. 2011, 506, 73–82. [Google Scholar] [CrossRef]
- Šamajová, O.; Plíhal, O.; Al-Yousif, M.; Hirt, H.; Šamaj, J. Improvement of Stress Tolerance in Plants by Genetic Manipulation of Mitogen-Activated Protein Kinases. Biotechnol. Adv. 2013, 31, 118–128. [Google Scholar] [CrossRef]
- Fusco, N.; Micheletto, L.; Dal Corso, G.; Borgato, L.; Furini, A. Identification of Cadmium-Regulated Genes by CDNA-AFLP in the Heavy Metal Accumulator Brassica juncea L. J. Exp. Bot. 2005, 56, 3017–3027. [Google Scholar] [CrossRef]
- Van De Mortel, J.E.; Schat, H.; Moerland, P.D.; Van Themaat, E.V.L.; Van Der Ent, S.; Blankestijn, H.; Ghandilyan, A.; Tsiatsiani, S.; Aarts, M.G.M. Expression Differences for Genes Involved in Lignin, Glutathione and Sulphate Metabolism in Response to Cadmium in Arabidopsis Thaliana and the Related Zn/Cd-Hyperaccumulator Thlaspi Caerulescens. Plant Cell Environ. 2008, 31, 301–324. [Google Scholar] [CrossRef]
- Weisman, D.; Alkio, M.; Colón-Carmona, A. Transcriptional Responses to Polycyclic Aromatic Hydrocarbon-Induced Stress in Arabidopsis Thaliana Reveal the Involvement of Hormone and Defense Signaling Pathways. BMC Plant Biol. 2010, 10, 59. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C.; et al. Potential Role of Phytohormones and Plant Growth-Promoting Rhizobacteria in Abiotic Stresses: Consequences for Changing Environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef] [PubMed]
- Langebartels, C.; Wohlgemuth, H.; Kschieschan, S.; Grün, S.; Sandermann, H. Oxidative Burst and Cell Death in Ozone-Exposed Plants. Plant Physiol. Biochem. 2002, 40, 567–575. [Google Scholar] [CrossRef]
- Penninckx, I.A.; Eggermont, K.; Terras, F.R.; Thomma, B.P.; De Samblanx, G.W.; Buchala, A.; Métraux, J.P.; Manners, J.M.; Broekaert, W.F. Pathogen-Induced Systemic Activation of a Plant Defensin Gene in Arabidopsis Follows a Salicylic Acid-Independent Pathway. Plant Cell 1996, 8, 2309–2323. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Considine, M.J.; María Sandalio, L.; Helen Foyer, C. Unravelling How Plants Benefit from ROS and NO Reactions, While Resisting Oxidative Stress. Ann. Bot. 2015, 116, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Vivancos, P.; de Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione–Linking Cell Proliferation to Oxidative Stress. Free Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Lelarge-Trouverie, C.; Mhamdi, A. The Metabolomics of Oxidative Stress. Phytochemistry 2015, 112, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.B.; Bielach, A.; Hrtyan, M. Redox Regulation at the Site of Primary Growth: Auxin, Cytokinin and ROS Crosstalk: Apical Meristems Plasticity in Response to Stress. Plant Cell Environ. 2017, 40, 2586–2605. [Google Scholar] [CrossRef] [PubMed]
- Potter, S. Regulation of a Hevein-like Gene in Arabidopsis. MPMI 1993, 6, 680. [Google Scholar] [CrossRef]
- Cabot, C.; Gallego, B.; Martos, S.; Barceló, J.; Poschenrieder, C. Signal Cross Talk in Arabidopsis Exposed to Cadmium, Silicon, and Botrytis Cinerea. Planta 2013, 237, 337–349. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Sesin, V.; Kisiala, A.; Emery, R.J.N. The Role of Phytohormones in Enhancing Metal Remediation Capacity of Algae. Bull. Environ. Contam Toxicol. 2020, 105, 671–678. [Google Scholar] [CrossRef]
- Maggio, A.; Barbieri, G.; Raimondi, G.; De Pascale, S. Contrasting Effects of GA3 Treatments on Tomato Plants Exposed to Increasing Salinity. J. Plant Growth Regul. 2010, 29, 63–72. [Google Scholar] [CrossRef]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between Plant Hormones and Heavy Metals Responses. Genet. Mol. Biol. 2017, 40 (Suppl. 1), 373–386. [Google Scholar] [CrossRef]
- Abozeid, A.; Ying, Z.; Lin, Y.; Liu, J.; Zhang, Z.; Tang, Z. Ethylene Improves Root System Development under Cadmium Stress by Modulating Superoxide Anion Concentration in Arabidopsis Thaliana. Front. Plant Sci. 2017, 8, 253. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Rehman, S.; Zhaorong, D. Role of 24-Epibrassinolide (EBL) in Mediating Heavy Metal and Pesticide Induced Oxidative Stress in Plants: A Review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. Jasmonates: Mechanisms and Functions in Abiotic Stress Tolerance of Plants. Biocatal. Agric. Biotechnol. 2019, 20, 101210. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Cytokinins: Metabolism and Function in Plant Adaptation to Environmental Stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different Plant Hormones Regulate Similar Processes through Largely Nonoverlapping Transcriptional Responses. Cell 2006, 126, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and Their Metabolic Engineering for Abiotic Stress Tolerance in Crop Plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Sharma, S.S.; Kumar, V. Responses of Wild Type and Abscisic Acid Mutants OfArabidopsis Thaliana to Cadmium. J. Plant Physiol. 2002, 159, 1323–1327. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of Brassinosteroids on the Plant Responses to Environmental Stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic Metabolism and Related Heavy Metal Tolerance Mechanism in Kandelia Obovata under Cd and Zn Stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. MicroRNA Metabolism in Plants. In RNA Interference; Paddison, P.J., Vogt, P.K., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 320, pp. 117–136. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K.; Suprasanna, P.; D’Souza, S.F. Identification and Profiling of Arsenic Stress-Induced MicroRNAs in Brassica Juncea. J. Exp. Bot. 2013, 64, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Shiri, M.; Rabhi, M.; Abdelly, C.; Bouchereau, A.; El Amrani, A. Moderate Salinity Reduced Phenanthrene-Induced Stress in the Halophyte Plant Model Thellungiella Salsuginea Compared to Its Glycophyte Relative Arabidopsis Thaliana: Cross Talk and Metabolite Profiling. Chemosphere 2016, 155, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl Radical Scavenging Activity of Compatible Solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Gangola, M.P.; Ramadoss, B.R. Sugars Play a Critical Role in Abiotic Stress Tolerance in Plants. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2018; pp. 17–38. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble Sugars: Metabolism, Sensing and Abiotic Stress: A Complex Network in the Life of Plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Edwards, R.; Buono, D.D.; Fordham, M.; Skipsey, M.; Brazier, M.; Dixon, D.P.; Cummins, I. Differential Induction of Glutathione Transferases and Glucosyltransferases in Wheat, Maize and Arabidopsis Thaliana by Herbicide Safeners. Z. Für Nat. C 2005, 60, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Pagé, A.P.; Yergeau, É.; Greer, C.W. Salix Purpurea Stimulates the Expression of Specific Bacterial Xenobiotic Degradation Genes in a Soil Contaminated with Hydrocarbons. PLoS ONE 2015, 10, e0132062. [Google Scholar] [CrossRef]
- Korenblum, E.; Dong, Y.; Szymanski, J.; Panda, S.; Jozwiak, A.; Massalha, H.; Meir, S.; Rogachev, I.; Aharoni, A. Rhizosphere Microbiome Mediates Systemic Root Metabolite Exudation by Root-to-Root Signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 3874–3883. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wei, G. Resilience and Assemblage of Soil Microbiome in Response to Chemical Contamination Combined with Plant Growth. Appl. Environ. Microbiol. 2019, 85, e02523-18. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.; Dai, X.; Li, S.; Lu, G.; Zhou, Y. Structure and Function of the Bacterial Communities during Rhizoremediation of Hexachlorobenzene in Constructed Wetlands. Environ. Sci. Pollut. Res. Int. 2017, 24, 11483–11492. [Google Scholar] [CrossRef]
- Leveau, J.H. A Brief from the Leaf: Latest Research to Inform Our Understanding of the Phyllosphere Microbiome. Curr. Opin. Microbiol. 2019, 49, 41–49. [Google Scholar] [CrossRef]
- Espenshade, J.; Thijs, S.; Gawronski, S.; Bové, H.; Weyens, N.; Vangronsveld, J. Influence of Urbanization on Epiphytic Bacterial Communities of the Platanus × Hispanica Tree Leaves in a Biennial Study. Front. Microbiol. 2019, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Asgari Lajayer, B.; Ghorbanpour, M.; Nikabadi, S. Heavy Metals in Contaminated Environment: Destiny of Secondary Metabolite Biosynthesis, Oxidative Status and Phytoextraction in Medicinal Plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Hojati, M.; Modarres-Sanavy, S.A.M.; Enferadi, S.T.; Majdi, M.; Ghanati, F.; Farzadfar, S.; Pazoki, A. Cadmium and Copper Induced Changes in Growth, Oxidative Metabolism and Terpenoids of Tanacetum Parthenium. Environ. Sci. Pollut. Res. 2017, 24, 12261–12272. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, L.; Segura, A. Biochemical and Metabolic Plant Responses toward Polycyclic Aromatic Hydrocarbons and Heavy Metals Present in Atmospheric Pollution. Plants 2021, 10, 2305. https://doi.org/10.3390/plants10112305
Molina L, Segura A. Biochemical and Metabolic Plant Responses toward Polycyclic Aromatic Hydrocarbons and Heavy Metals Present in Atmospheric Pollution. Plants. 2021; 10(11):2305. https://doi.org/10.3390/plants10112305
Chicago/Turabian StyleMolina, Lázaro, and Ana Segura. 2021. "Biochemical and Metabolic Plant Responses toward Polycyclic Aromatic Hydrocarbons and Heavy Metals Present in Atmospheric Pollution" Plants 10, no. 11: 2305. https://doi.org/10.3390/plants10112305
APA StyleMolina, L., & Segura, A. (2021). Biochemical and Metabolic Plant Responses toward Polycyclic Aromatic Hydrocarbons and Heavy Metals Present in Atmospheric Pollution. Plants, 10(11), 2305. https://doi.org/10.3390/plants10112305







