Persistence of the Effects of Se-Fertilization in Olive Trees over Time, Monitored with the Cytosolic Ca2+ and with the Germination of Pollen
Abstract
1. Introduction
2. Results
2.1. Scanning Electron Microscopy Images of Olive Pollen Grains
2.2. Speciation of Selenium in Olive Pollen
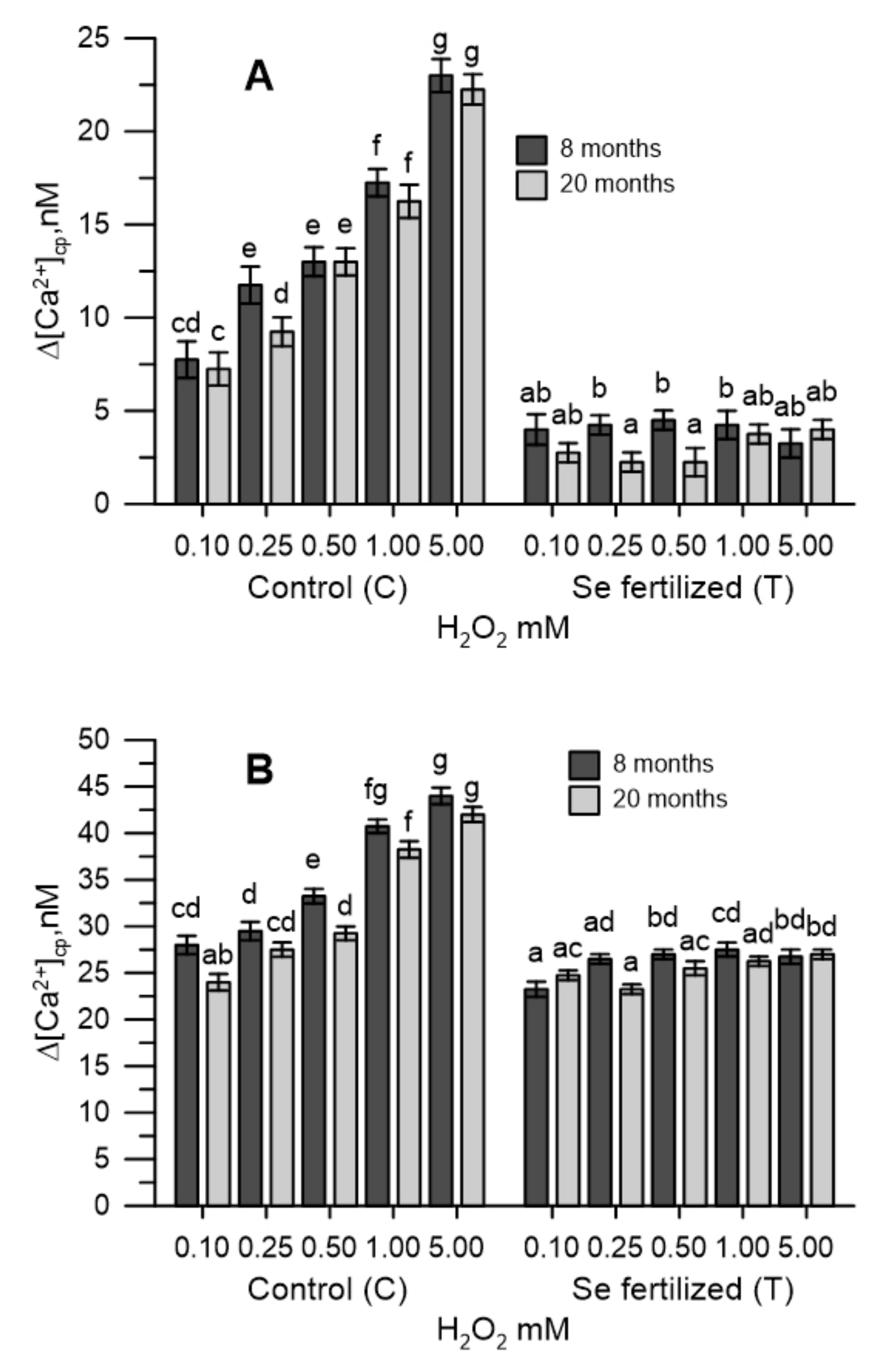
2.3. The Cytosolic Ca2+ Tested in Pollen during Oxidative Stress
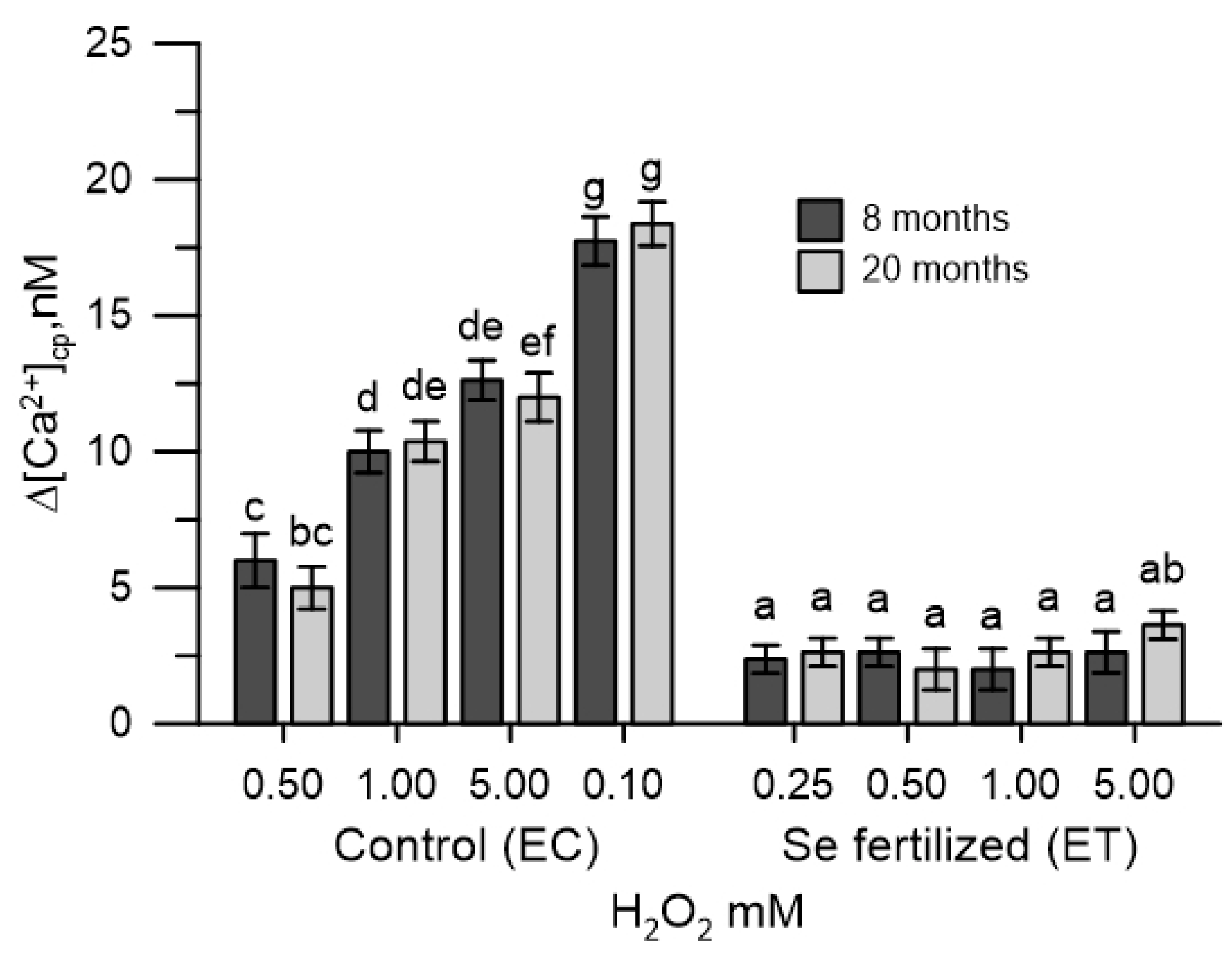
2.4. The Cytosolic Ca2+ Tested in Olive Pollen in the Presence of the Extracts of the Germinative Apexes
2.5. Germination of Olive Pollen Grains in Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Plant Material, Growing Conditions and Pollen and Vegetative Apexes Collection
4.3. Extracts of Vegetative Apexes of Olive Trees
4.4. Determination of Total Selenium in Olive Pollens and Vegetative Apexes
4.5. Se Speciation with HPLC ICPMS
4.6. Measurement of Cytosolic Ca2+
4.7. Pollen Germination
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Park, K.; Rimm, E.; Siscovick, D.; Spiegelman, D.; Morris, J.S.; Mozaffarian, D. Demographic and lifestyle factors and selenium levels in men and women in the U.S. Nutr. Res. Pract. 2011, 5, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, K.; Power, R.; Toborek, M. Biological activity of selenium: Revisited. IUBMB Life 2016, 68, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.-Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell. Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef]
- Wei, J.; Zeng, C.; Gong, Q.Y.; Li, X.X.; Lei, G.H.; Yang, T.B. Associations between dietary antioxidant intake and metabolic syndrome. PLoS ONE 2015, 10, e0130876. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.; Proietti, P.; Nasini, L.; Del Buono, D.; Tedeschini, E.; Businelli, D. Increase in the selenium content of extra virgin olive oil: Quantitative and qualitative implications. Grasas Aceites 2014, 65, e025. [Google Scholar] [CrossRef]
- D’Amato, R.; Proietti, P.; Onofri, A.; Regni, L.; Esposto, S.; Servili, M.; Businelli, D.; Selvaggini, R. Biofortification (Se): Does it increase the content of phenolic compounds in virgin olive oil (VOO)? PLoS ONE 2017, 12, e0176580. [Google Scholar] [CrossRef]
- D’Amato, R.; De Feudis, M.; Hasuoka, P.E.; Regni, L.; Pacheco, P.H.; Onofri, A.; Businelli, D.; Proietti, P. The selenium supplementation influences olive tree production and oil stability against oxidation and can alleviate the water deficiency effects. Front. Plant Sci. 2018, 9, 1191. [Google Scholar] [CrossRef]
- Fontanella, M.C.; D’Amato, R.; Regni, L.; Proietti, P.; Beone, G.M.; Businelli, D. Selenium speciation profiles in biofortified sangiovese wine. J. Trace Elem. Med. Biol. 2017, 43, 87–92. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.; Petrelli, M.; Proietti, P.; Onofri, A.; Regni, L.; Perugini, D.; Businelli, D. Determination of changes in the concentration and distribution of elements within olive drupes (cv. Leccino) from Se biofortified plants, using laser ablation inductively coupled plasma mass spectrometry. J. Sci. Food Agric. 2018, 98, 4971–4977. [Google Scholar] [CrossRef] [PubMed]
- Regni, L.; Palmerini, C.A.; Del Pino, A.M.; Businelli, D.; D’Amato, R.; Mairech, H.; Marmottini, F.; Micheli, M.; Pacheco, P.H.; Proietti, P. Effects of selenium supplementation on olive under salt stress conditions. Sci. Hortic. 2021, 278, 109866. [Google Scholar] [CrossRef]
- Proietti, P.; Nasini, L.; Del Buono, D.; D’Amato, R.; Tedeschini, E.; Businelli, D. Selenium protects olive (Olea europaea L.) from drought stress. Sci. Hortic. 2013, 164, 165–171. [Google Scholar] [CrossRef]
- Tedeschini, E.; Proietti, P.; Timorato, V.; D’Amato, R.; Nasini, L.; Del Buono, D.; Businelli, D.; Frenguelli, G. Selenium as stressor and antioxidant affects pollen performance in Olea europaea. Flora 2015, 215, 16–22. [Google Scholar] [CrossRef]
- Lyons, G.H.; Genc, Y.; Soole, K.; Stangoulis, J.C.R.; Liu, F.; Graham, R.D. Selenium increases seed production in Brassica. Plant Soil 2009, 318, 73–80. [Google Scholar] [CrossRef]
- Prins, C.N.; Hantzis, L.J.; Quinn, C.F.; Pilon-Smits, E.A.H. Effects of selenium accumulation on reproductive functions in Brassica juncea and Stanleya pinnata. J. Exp. Bot. 2011, 62, 5633–5640. [Google Scholar] [CrossRef]
- Kwak, J.M.; Nguyen, V.; Schroeder, J.I. The Role of Reactive Oxygen Species in Hormonal Responses. Plant Physiol. 2006, 141, 323–329. [Google Scholar] [CrossRef]
- Hancock, J.T.; Desikan, R.; Neill, S.I. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 2001, 29, 345–350. [Google Scholar] [CrossRef]
- Laloi, C.; Apel, K.; Danon, A. Reactive oxygen signalling: The latest news. Curr. Opin. Plant Biol. 2004, 7, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wei, C.L.; Zhang, W.R.; Cheng, H.P.; Liu, J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol. Sin. 2006, 27, 821–826. [Google Scholar] [CrossRef]
- Brini, M.; Calì, T.; Ottolini, D.; Carofoli, E. Intracellular calcium homeostasis and signaling. In Metallomics and the Cell; Banci, L., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 12, pp. 119–168. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 2015, 460, 72–81. [Google Scholar] [CrossRef]
- Proietti, P.; Trabalza Marinucci, M.; Del Pino, A.M.; D’Amato, R.; Regni, L.; Acuti, G.; Chiaradia, E.; Palmerini, C.A. Selenium maintains Ca2+ homeostasis in sheep lymphocytes challenged by oxidative stress. PLoS ONE 2018, 13, e0201523. [Google Scholar] [CrossRef]
- Del Pino, A.M.; Guiducci, M.; D’Amato, R.; Di Michele, A.; Tosti, G.; Datti, A.; Palmerini, C.A. Selenium maintains cytosolic Ca2+ homeostasis and preserves germination rates of maize pollen under H2O2-induced oxidative stress. Sci. Rep. 2019, 9, 1350. [Google Scholar] [CrossRef]
- Del Pino, A.M.; Regni, L.; D’Amato, R.; Tedeschini, E.; Businelli, D.; Proietti, P.; Palmerini, C.A. Selenium-Enriched Pollen Grains of Olea europaea L.: Ca2+ Signaling and Germination Under Oxidative Stress. Front. Plant Sci. 2019, 10, 1611. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Shakeel, A.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Lazzaro, M.D.; Cardenas, L.; Bhatt, A.P.; Justus, C.D.; Phillips, M.S.; Holdaway-Clarke, T.L.; Hepler, P.K. Calcium gradients in conifer pollen tubes; dynamic properties differ from those seen in angiosperms. J. Exp. Bot. 2005, 56, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Rejo’n, J.D.; Zienkiewicz, A.; Rodríguez-García, M.I.; Castro, A.J. Profiling and functional classification of esterases in olive (Olea europaea) pollen during germination. Ann. Bot. 2012, 110, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.S.; Davide, L.M.C.; Miranda, G.J.; Barizon, J.O.; de Assis, F., Jr.; de Carvalho, R.P.; Gonçalves, M.C. In vitro pollen viability of maize cultivars at different times of collection. Ciênc. Rural 2017, 47, e20151077. [Google Scholar] [CrossRef][Green Version]


| MeSeCys ppb | Se Met ppb | Se (IV) ppb | Se (VI) ppb | Se Total ppb | |
|---|---|---|---|---|---|
| C8 | 23 ± 3 a | 858.2 ± 15 a | 300.5 ± 8 a | 1725.0 ± 32 a | 3210 ± 30 a |
| C20 | 41 ± 4 a | 778.7 ± 14 a | 460.7 ± 9 b | 1883.2 ± 33 a | 3600 ± 29 a |
| T8 | 153 ± 10 b | 4895.2 ± 23 c | 218.4 ± 7 a | 15,860.0 ± 34 b | 28,370 ± 51 b |
| T20 | 211.8 ± 12 b | 2541.2 ± 22 b | 582.4 ± 11 b | 14,563.0 ± 31 b | 17,900 ± 41 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Pino, A.M.; Regni, L.; D’Amato, R.; Di Michele, A.; Proietti, P.; Palmerini, C.A. Persistence of the Effects of Se-Fertilization in Olive Trees over Time, Monitored with the Cytosolic Ca2+ and with the Germination of Pollen. Plants 2021, 10, 2290. https://doi.org/10.3390/plants10112290
Del Pino AM, Regni L, D’Amato R, Di Michele A, Proietti P, Palmerini CA. Persistence of the Effects of Se-Fertilization in Olive Trees over Time, Monitored with the Cytosolic Ca2+ and with the Germination of Pollen. Plants. 2021; 10(11):2290. https://doi.org/10.3390/plants10112290
Chicago/Turabian StyleDel Pino, Alberto Marco, Luca Regni, Roberto D’Amato, Alessandro Di Michele, Primo Proietti, and Carlo Alberto Palmerini. 2021. "Persistence of the Effects of Se-Fertilization in Olive Trees over Time, Monitored with the Cytosolic Ca2+ and with the Germination of Pollen" Plants 10, no. 11: 2290. https://doi.org/10.3390/plants10112290
APA StyleDel Pino, A. M., Regni, L., D’Amato, R., Di Michele, A., Proietti, P., & Palmerini, C. A. (2021). Persistence of the Effects of Se-Fertilization in Olive Trees over Time, Monitored with the Cytosolic Ca2+ and with the Germination of Pollen. Plants, 10(11), 2290. https://doi.org/10.3390/plants10112290









