Grapevine Shoot Tip Cryopreservation and Cryotherapy: Secure Storage of Disease-Free Plants
Abstract
1. Introduction
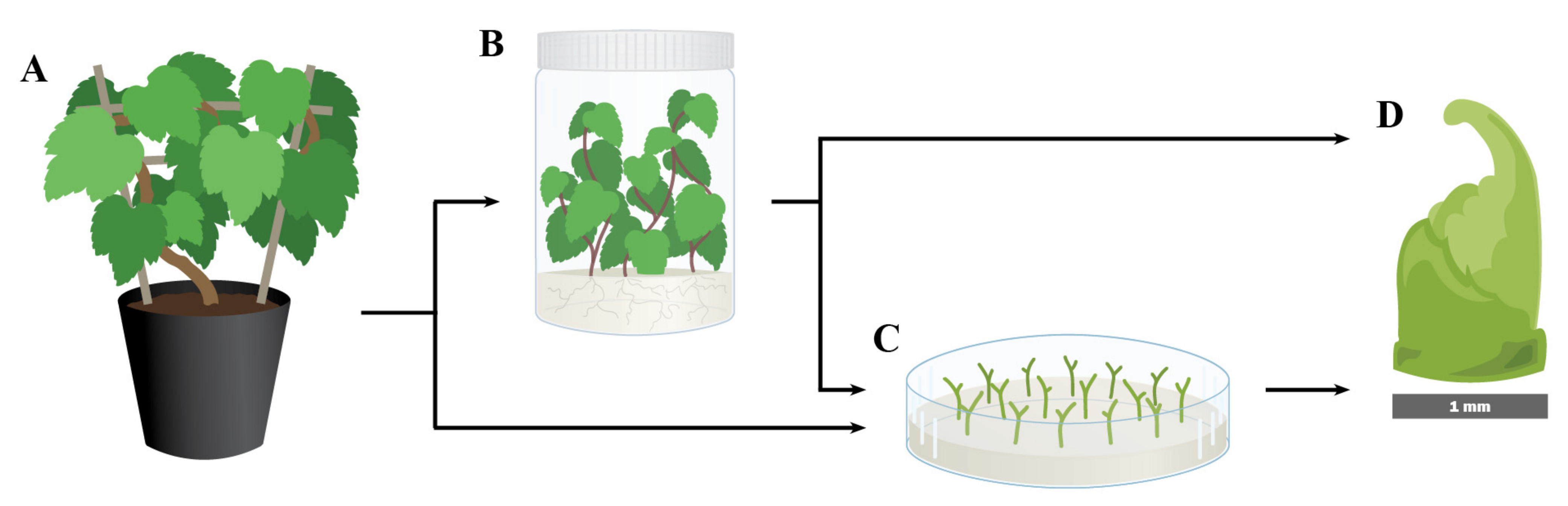
2. Explant Sources
| Species, No. Genotypes Tested | Explant Source | Cryo Method | Pretreatment | Preculture | Best Cryoprotectant/ Dehydration Treatment | Regrowth (%) | Year Ref. |
|---|---|---|---|---|---|---|---|
| V. labrusca, 3 | ST (1–2 mm; type n/s) harvested from greenhouse plants | TEC | None | None | 10% DMSO + 60 g L−1 sucrose (2 h at 20 °C) → cooled (0.5 °C min−1) to −20 °C/−30 °C/−40 °C → LN | 87–100% survival | 1989 [84] |
| V. vinifera, 1 | AxST (size n/s) harvested from in vitro cultures that are 7 weeks old | ED + TEC | None | Liquid MS with increased sucrose concentrations every 2 d of 0.3, 0.5 and 0.75 M every 2 d, and then every 1 day of 1, 1.25 and 1.5 M (temperature n/s) | Bead desiccation to 20% + cooled (0.5 °C min−1) from +20 °C to −80 °C → LN | 24 | 1991 [85] |
| ED | Bead desiccation to 20% → LN | No shoot regrowth | |||||
| V. vinifera, 1 | AxST (size n/s) harvested from in vitro cultures that are 7 to 8 weeks old | ED + TEC | None | Liquid MS with increased sucrose concentrations every 2 days of 0.3, 0.5 and 0.75 M and 1 M (temperature n/s) | Bead desiccation to 22% + cooled (0.5 °C min−1) from + 20 °C to −100 °C → LN | 30 | 1993 [86] |
| ED | Bead desiccation to 22% → LN | 30% survival | |||||
| V. vinifera, 3 | AxST (size n/s) harvested from in vitro cultures (age n/s) | ED + TEC | None | Liquid MS with increased sucrose concentrations every 24 h of 0.3, 0.6 and 1 M (25 °C) | Bead desiccation to 30% + beads cooled from 0.5 °C min−1 to −80 °C → LN | No shoot regrowth | 2000 [87] |
| LN33 (Vitis L.), 1; V. vinifera, 1 | ST (1 mm; type n/s) harvested from in vitro cultures that are 4 weeks old | ED | None | 1/2 MS with increased sucrose concentrations every 24 h of 0.25, 0.5, 0.75 and 1 M + 2.6 g L−1 gellan gum (24 °C) | Bead desiccation to 16% → LN | 40–60% survival | 2000 [88] |
| V. vinifera, 4 | AxST (2 mm) harvested from in vitro cultures that are 5 months old | ED + TEC | Cold-hardening for 4 weeks at 5 °C | B5 medium with increased sucrose concentrations every 24 h of 0.1, 0.3, 0.7 and 1 M + 5 g L−1 agar (5 °C) | Bead desiccation to 21% + cooled (0.2 °C min−1) to −40 °C → LN | 15–40 | 2001 [89] |
| Table 1 (continued) | |||||||
| Species, No. Genotypes Tested | Explant Source | Cryo Method | Pretreatment | Preculture | Best Cryoprotectant/ Dehydration Treatment | Regrowth (%) | Year Ref. |
| LN33 (Vitis L.), 1 | ST (1 mm; type n/s) harvested from in vitro cultures that are 4 weeks old | VI | None | 1/2 MS with increased sucrose concentrations every 24 h of 0.25, 0.5 and 0.75 M + 2.6 g L−1 Gelrite (24 °C) | 2 M glycerol + 0.75 M sucrose (60 min at 25 °C) → 1/2 PVS2 (30 min at 0 °C) → PVS2 (50 min at 0 °C) → LN | 45% survival | 2003 [33] |
| ED | Preculture described above + additional 1 d on 1 M sucrose + 2.6 g L−1 Gelrite | Bead desiccation by air-drying for 8 h (beads moisture content n/s) → LN | 63% survival | ||||
| V. vinifera, 1 | ST (1 mm; type n/s) harvested from in vitro cultures that are 4 weeks old | VI | None | 1/2 MS with increased sucrose concentrations every 24 h of 0.25, 0.5 and 0.75 M + 2.6 g L−1 Gelrite (24 °C) | 2 M glycerol + 0.75 M sucrose (60 min at 25 °C) → 1/2 PVS2 (30 min at 0 °C) → PVS2 (50 min at 0 °C) → LN | 50% survival | 2003 [64] |
| ED | Preculture described above + additional 1 day on 1 M sucrose + 2.6 g L−1 Gelrite | Bead desiccation by air-drying for 7 h (beads moisture content n/s) → LN | 62% survival | ||||
| V. berlandieri x V. riparia, 1 | AST and AxST (1–2 mm) harvested from in vitro cultures (age n/s) | EN-VI | Cold-hardening for 3 weeks at 4 °C | None | PVS2 (30 and 90 min at 0 °C) → LN | Low (n/s) | 2003 [90] |
| V. vinifera, 7; V. berlandieri x riparia, 2; V. mourvedre × V. rupestris, 1; V. coignetiae, 1 | AxST (1 mm) harvested from in vitro cultures that are 4 to 5 months old | VI | None | 1/2 MS + 0.3 M sucrose + 2 g L−1 gellan gum for 3 days at 25 °C | 2 M glycerol + 0.4 M sucrose (20 min at 25 °C) → 1/2 PVS2 (30 min at 0 °C) → PVS2 (50 min at 0 °C) → LN | 30–87 | 2000 [31] 2003 [32] |
| V. vinifera, 4 | AST (2 mm) harvested from in vitro cultures that are 50 days old | ED + TEC | None | Culture medium with increased sucrose concentrations every 24 h of 0.5, 0.70 and 1 M (5 °C) | Bead desiccation to 26% + cooled (0.2 °C min−1) from 0 °C to −40 °C → LN | 36 (average) | 2003 [91] |
| V. berlandieri × riparia, 1 | AxST (size n/s) harvested from in vitro cultures (age n/s) | VI | None | 0.2, 0.3 or 0.4 M sucrose (duration and conditions n/s) | 2 M glycerol + 0.4 M sucrose (30 min) → PVS2 (30, 60 or 90 min) (conditions n/s) → LN | No shoot regrowth | 2007 [92] |
| Table 1 (continued) | |||||||
| Species, No. Genotypes Tested | Explant Source | Cryo Method | Pretreatment | Preculture | Best Cryoprotectant/ Dehydration Treatment | Regrowth (%) | Year Ref. |
| V. vinifera, 1 | ST (1 mm; type n/s) harvested from shoots of greenhouse plants | ED | None | 3/4 MS with increased sucrose concentrations every 24 h of 0.25, 0.5, 0.75 and 1 M (conditions n/s) | Bead desiccation by air-drying for 12 h (beads moisture content n/s) → LN | 59 | 2011 [63] |
| V. vinifera, 1 | ST (2–3 mm; type n/s) harvested from in vitro cultures (age n/s) | VI | None | MS + 0.3 M sucrose + 8 g L−1 agar for 1 day at 24 °C | 5% (w/v) DMSO + 5% (w/v) glycerol + 5% (w/v) sucrose (50 min at 0 °C) → PVS2 (40 min at 0 °C) or MPVS2 **** (40 min at 0 °C) → LN | 47 and 55 | 2011 [93] |
| V. berlandieri × V. riparia, 1 | AxST (2 ± 1 mm) harvested from in vitro cultures (age n/s) | VI | Cold-hardening for 2 weeks at 4 °C | MS + 0.2, 0.3 or 0.4 M sucrose + 8 g L−1 agar for 2 days at 4 °C | 2 M glycerol + 0.4 M sucrose (30 min at 4 °C) → PVS2 (30, 60 or 90 min at 0 °C) → LN | No shoot regrowth | 2012 [94] |
| V. vinifera, 1 | AST (1 mm) harvested from the lateral shoots of in vitro nodal sections cultured for 2 weeks | ED | Nodal sections on 1/2 MS + Morel’s vitamins * + 20 g L−1 sucrose + 1 μmol ZR + 7 g L−1 agar for 2 weeks at 24 °C | Liquid 1/2 MS with increased sucrose concentrations every 12 h of 0.25, 0.5, 0.75 and 1 M (24 °C) | Bead desiccation to 22.3% → LN | 37 | 2013 [95] |
| DV | MS + 1 M sucrose + 7 g L−1 agar for 24 h at 24 °C | 2 M glycerol + 0.4 M sucrose (20 min at RT) → PVS2 (50 min at 0 °C) → LN | 50 | ||||
| V. vinifera, 2 | AxST (1 mm) harvested from greenhouse-grown plants | DV | None | 1/2 MS + 0.3 M sucrose + 0.16 mM GSH reduced + 0.14 mM AsA + 2.5 g L−1 gellan gum for 3 days at 25 °C | 2 M glycerol + 0.4 M sucrose (20 min at 22 °C) → 1/2 PVS2 (10–15 min at 0 °C) → PVS2 (10–20 min at 0 °C) → LN | 40–46 | 2013 [96] |
| Table 1 (continued) | |||||||
| Species, No. Genotypes Tested | Explant Source | Cryo Method | Pretreatment | Preculture | Best Cryoprotectant/ Dehydration Treatment | Regrowth (%) | Year Ref. |
| V. vinifera, 1 | AST (1 mm) harvested from the lateral shoots of in vitro nodal sections cultured for 2 weeks | DV | Nodal sections on 1/2 MS + Morel’s vitamins * + 20 g L−1 sucrose + 1 μmol BA or ZR + 7 g L−1 agar for 2 weeks at 24 °C | 1/2 MS + 0.1 M sucrose + 7 g L−1 agar for 24 h at 25 °C | 2 M glycerol + 0.4 M sucrose (20 min at RT) → 1/2 PVS2 (30 min at RT) → PVS2 (50 min at 0 °C) → LN | 44 | 2014 [97] |
| AxST (1 mm) harvested from in vitro cultures that are 2 months old | None | 2 M glycerol + 0.4 M sucrose (20 min at RT) → 1/2 PVS2 (30 min at RT) → PVS2 (75 min at 0 °C) → LN | 41.6 | ||||
| V. vinifera, 1 | AxST (size n/s) harvested from vineyard | VI | None | None | PVS2 (180 min at 25 °C) → LN | n/s | 2015 [98] |
| V. vinifera, 9 | AST (1 mm) harvested from the lateral shoots of in vitro nodal sections cultured for 2 weeks | DV | Nodal sections on 1/2 MS + Morel’s vitamins * + 20 g L−1 sucrose + 1 μmol BA + 7 g L−1 agar for 2 weeks at 24 °C | 1/2 MS + 0.1 M sucrose + 7 g L−1 agar for 24 h at 25 °C | 2 M glycerol + 0.4 M sucrose (20 min at RT) → 1/2 PVS2 (30 min at RT) → PVS2 (50 min at 0 °C) → LN | 0–70 | 2015 [34] |
| V. vinifera, 7; V. labrusca, 1; V. riparia, 1; V. berlandieri × V. rupestris, 2; V. berlandieri × V. riparia, 1 | AST harvest from in vitro cultures (size and age of cultures n/s) | ED | None | Following Wang et al. [32] | 0–9% survival | 2015 [99] | |
| VI | None | Following Shatnawi et al. [83] | 0–1% survival | ||||
| V. vinifera, 5 | AST and AxST harvested from in vitro cultures that are 2 weeks old (size n/s) | DV | Cultures on 1/2 MS + B5 vitamins ** + 20 g L−1 sucrose + 0.5 mg L−1 BA + 0.1 mM SA + 3 g L−1 gellan gum for 2 weeks at 24 °C | 1/2 MS + B5 vitamins ** with increased sucrose concentrations of 0.25 M, 0.5 M, 0.75 M and 1 M (every 24 h) + 3 g L−1 Gelrite (24 °C) | 2 M glycerol + 0.4 M sucrose (20 min at RT) → PVS2 (36–41.5 min at 0 °C) → LN | 13–30 | 2015 [62] |
| Table 1 (continued) | |||||||
| Species, No. Genotypes Tested | Explant Source | Cryo Method | Pretreatment | Preculture | Best Cryoprotectant/ Dehydration Treatment | Regrowth (%) | Year Ref. |
| V. vinifera, 3 | AST (1–2 mm) harvested from the lateral shoots of in vitro nodal sections cultured for 10 days | VI | None | Medium with increased sucrose concentrations of 0.3, 0.5 and 0.75 M every 24 h (conditions n/s) | 2 M glycerol + 0.4 M sucrose (30 min at RT) → 2 M glycerol + 0.75 M sucrose (30 min at RT) → 1/2 PVS3 (30 min at RT) → 80 % PVS3 (60–90 min) | n/s after LN exposure | 2015 [100] |
| V. vinifera, 4; V. riparia × V. rupestris, 1; V. vinifera Chasselas × V. berlandieri, 1 | AST and AxST (1–1.5 mm) harvested from the lateral shoots of in vitro nodal sections cultured for 2 weeks | DV | Nodal sections on 1/2 MS + B5 vitamins ** + 20 g L−1 sucrose + 0.5 mg L−1 BA + 0.1 Mm SA + 3 g L−1 gellan gum for 2 weeks at 24 °C | 1/2 MS + B5 vitamins ** with increased sucrose concentrations every 24 h of 0.25 M, 0.5 M, 0.75 M and 1 M + 3 g L−1 Gelrite (24 °C) | 2 M glycerol + 0.4 M sucrose (20 min at RT) → PVS2 (36–43 min at 0 °C) → LN | 7–45 | 2016 [16] |
| V. vinifera, 6; V. pseudoreticulata, 2 | AST (1 mm) harvested from the lateral shoots of in vitro nodal sections cultured for 2 weeks | DV | None | 1/2 MS + 0.3 M sucrose + 0.16 mM GSH + 0.14 mM AsA + 7 g L−1 agar for 3 days at 24 °C | 2 M glycerol + 0.4 M sucrose (20 min at 24 °C) → 1/2 PVS2 (30 min at 0 °C) → PVS2 (50 min at 0 °C) → LN | 24–72 | 2018 [35] |
| V. vinifera, 2; V. vinifera × V. labrusca, 1; V. pseudoreticulata, 1 | 43–59 | 2018 [51] | |||||
| V. vinifera, 1; V. aestivalis, 1; V. afghanistan, 1; V. flexuosa, 1; V. palmate, 1; V. riparia, 1; V. rupestris, 1; V. sylvestris, 1; V. treleasii, 1 | AST (1–1.5 mm) harvested from the lateral shoots of in vitro nodal sections cultured for 2 to 3 weeks | DV | Nodal sections on MS + 30 g L−1 sucrose + 0.2 mg L−1 BA + 0.1 mM SA + 1 mM GSH*** + 1 mM AsA + 3 g L−1 gellan gum for 2–3 weeks at 25 °C | 1/2 MS + 0.3 M sucrose + 0.1 mM SA + 1 mM GSH *** + 1 mM AsA + 3 g L−1 gellan gum for 3 days at 25 °C | 2 M glycerol + 0.4 M sucrose (20 min at 22 °C) → 1/2 PVS2 (30 min at 22 °C) → PVS2 (90 min at 0 °C) → LN | 25–43 | 2018 [36] |
| Table 1 (continued) | |||||||
| Species, No. Genotypes Tested | Explant Source | Cryo Method | Pretreatment | Preculture | Best Cryoprotectant/ Dehydration Treatment | Regrowth (%) | Year Ref. |
| V. champinii × 1613 Couderc, 1; V. berlandieri × V. riparia, 1; V. shampinii, 1 | ST (3 mm; type n/s) harvested from shoots of greenhouse plants | DV | None | 1/2 MS + 0.3 M sucrose for 3 days at 25 °C | 2 M glycerol + 0.4 M sucrose (20 min at 25 °C) → PVS2 (0–50 min at 0 °C) → LN | No shoot regrowth | 2019 [101] |
| 1/2 MS with increased sucrose concentrations every 24 h of 0.25, 0.5, 0.75 and 1 M (25 °C) | 2 M glycerol + 0.4 M sucrose (20 min at 25 °C) → PVS2 (50 min at 0 °C) → LN | 27–47 | |||||
| V. vinifera, 2; V. berlandieri × V. riparia, 1 | AST (1 mm) harvested from the lateral shoots of nodal sections cultured for 2 weeks from growth chamber stock plants | DV | Nodal sections on MS + 30 g L−1 sucrose + 0.2 mg L−1 BA + 0.1 mM SA + 1 mM GSH *** + 1 mM AsA + 1.5 % (v/v) PPM + 3 g L−1 gellan gum for 2 weeks at 25 °C | 1/2 MS + 0.3 M sucrose + 0.1 mM SA + 1 mM GSH *** + 1 mM AsA + 1.5 % (v/v) PPM + 3 g L−1 gellan gum for 3 days at 25 °C | 2 M glycerol + 0.4 M sucrose (20 min at 22 °C) → 1/2 PVS2 (30 min at 22 °C) → PVS2 (30–40 min at 0 °C) → LN | 43–64 | 2019 [102] |
| V. vinifera, 2; V. actinifolia, 1; V. aestivalis, 1; V. jacquemontii, 1; V. flexuosa, 1; V. palmate, 1; V. riparia, 1; V. rupestris, 1; V. sylvestris, 1; V. ficifolia, 1; V. treleasi, 1; V. xnovae angeliae, 1 | AST (1 mm) harvested from the lateral shoots of in vitro nodal sections cultured for 2 weeks | DV | Nodal sections on MS + 30 g L−1 sucrose + 0.2 mg L−1 BA + 0.1 mM SA + 1 mM GSH *** + 1 mM AsA + 3 g L−1 gellan gum for 2 weeks at 25 °C | 1/2 MS + 0.3 M sucrose + 0.1 Mm SA + 1 mM GSH *** + 1 mM AsA + 3 g L−1 gellan gum for 3 days at 25 °C | 2 M glycerol + 0.4 M sucrose (20 min at 22 °C) → 1/2 PVS2 (30 min at 22 °C) → PVS2 (90 min at 0 °C) → LN | 35–72 | 2019 [37] |
| Pretreatment described above + cold-hardening for 2 weeks at 5 °C | 2 M glycerol + 0.4 M sucrose (20 min at 22 °C) → 1/2 PVS2 (30 min at 22 °C) → PVS2 (75 min at 0 °C) → LN | 43–70 | |||||
| V. aestivalis, 1; V. jacquemontii, 1 | AST (1 mm) harvested from the lateral shoots of in vitro nodal sections cultured for 2 weeks | DV | Nodal sections on MS + 30 g L−1 sucrose + 0.2 mg L−1 BA + 0.1 mM SA + 1 mM GSH *** + 1 mM AsA + 3 g L−1 gellan gum for 2 weeks at 25 °C | 1/2 MS + 0.3 M sucrose + 0.1 mM SA + 1 mM GSH *** + 1 mM AsA + 3 g L−1 gellan gum for 3 days at 25 °C | 2 M glycerol + 0.4 M sucrose (20 min at 22 °C) → 1/2 PVS2 (30 min at 22 °C) → PVS2 (90 min at 0 °C) → LN | 53–70 | 2019 [76] |
| V-CP | 2 M glycerol + 0.4 M sucrose (30 min at 22 °C) → PVS2 (40 min at 22 °C) → LN | 68–70 | |||||
| Table 1 (continued) | |||||||
| Species, No. Genotypes Tested | Explant Source | Cryo Method | Pretreatment | Preculture | Best Cryoprotectant/ Dehydration Treatment | Regrowth (%) | Year Ref. |
| V. vinifera, 1 | AST (1 mm) harvested from the lateral shoots of in vitro nodal sections cultured for 2 to 3 weeks | DV | Nodal sections on MS + 30 g L−1 sucrose + 0.2 mg L−1 BA + 0.1 mM SA + 1 mM GSH *** + 1 mM AsA + 3 g L−1 gellan gum for 2–3 weeks at 25 °C | 1/2 MS + 0.3 M sucrose + 0.1 mM SA + 1 mM GSH *** + 1 mM AsA + 3 g L−1 gellan gum for 3 days at 25 °C | 2 M glycerol + 0.4 M sucrose (30 min at 22 °C) → 1/2 PVS2 (30 min at 22 °C) → PVS2 (75 min at 0 °C) → LN | 68 | 2019 [103] |
| AST (1 mm) harvested from growth chamber nodal sections cultured in vitro for 2 weeks before ST excision | Pretreatment described above + additional + 1.5% (v/v) PPM for 2 weeks at 25 °C | Preculture described above + additional + 1.5% (v/v) PPM for 3 days at 25 °C | 2 M glycerol + 0.4 M sucrose (30 min at 22 °C) → 1/2 PVS2 (30 min at 22 °C) → PVS2 (40 min at 0 °C) → LN | 43 | |||
| V. vinifera, 1 | ST (size and type n/s) harvested from in vitro stock cultures that are 4 weeks old | ED | Cold-hardening for 4 weeks at 5 °C | 1/2 MS with increased sucrose concentrations every 24 h of 0.25, 0.5, 0.75 and 1 M + 2.5 g L−1 Phytagel (5 °C) | Bead desiccation to 18.4% → LN | 33% survival | 2021 [104] |
3. Pretreatment, Excision and Preculture of Shoot Tips
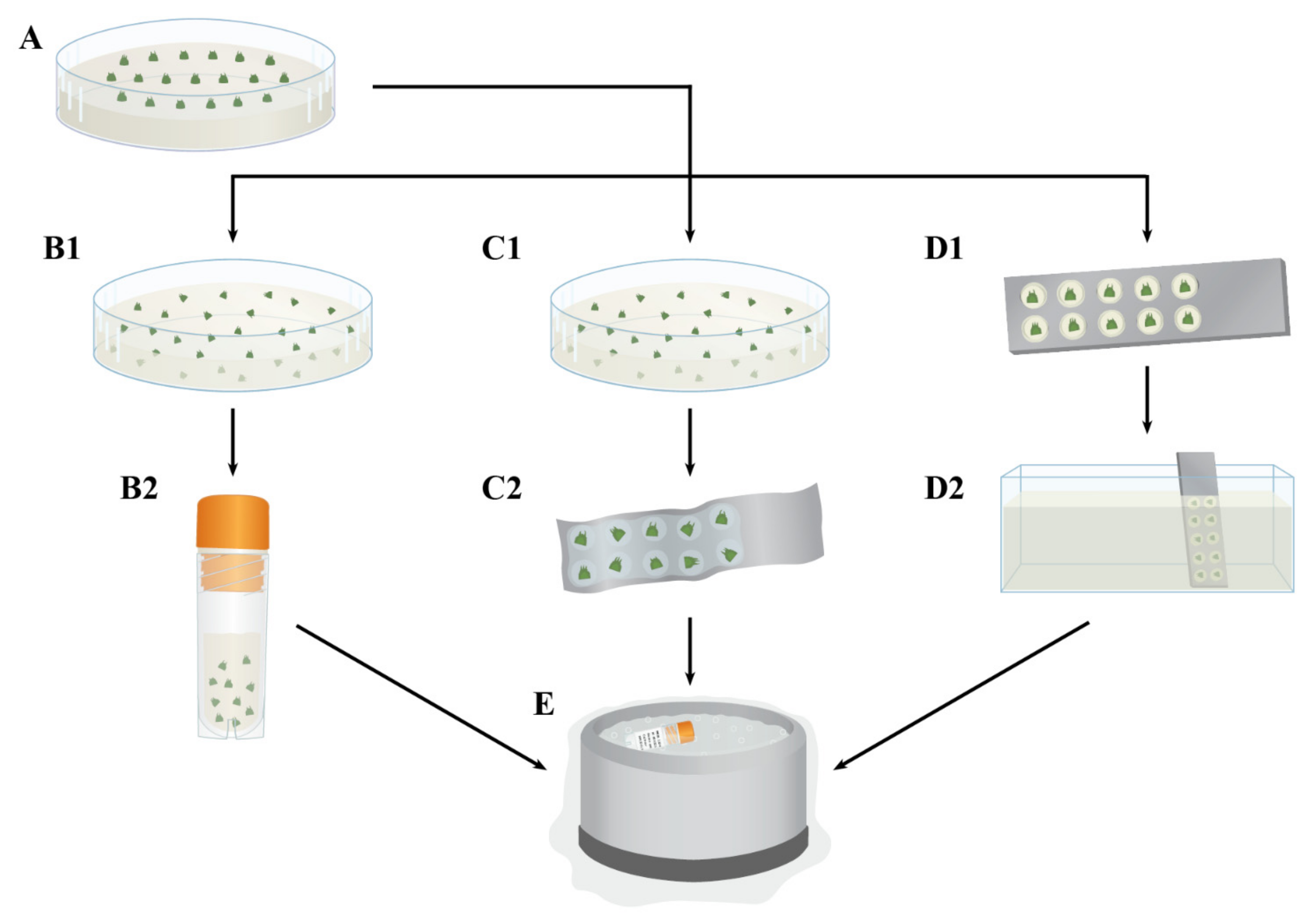
4. Methods for Shoot Tip Cryopreservation
4.1. Two-Step Cooling
4.2. Encapsulation-Dehydration
4.3. Vitrification
4.4. Encapsulation-Vitrification
4.5. Droplet-Vitrification
4.6. V Cryo-Plate
5. Shoot Tip Cryotherapy
5.1. Methods for Shoot Tip Cryotherapy
5.2. Mechanism Involved in Shoot Tip Cryotherapy for Eradication of Phloem-Limited Grapevine Viruses
5.3. Comparison of Virus Eradication Efficiency between the Traditional Methods and Shoot Tip Cryotherapy
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Apical dome |
| AsA | Ascorbic acid |
| AST | Apical shoot tips |
| AxST | Axillary shoot tips |
| BA | Benzylaminopurine |
| DAS-ELISA | Double-antibody sandwich enzyme-linked immunosorbent assay |
| DMSO | Dimethyl sulfoxide |
| ELISA | Enzyme-linked immunosorbent assay |
| FWB | Fresh weight basis |
| IHC | Immunohistochemical |
| LN | Liquid nitrogen |
| LNV | Liquid nitrogen vapor |
| LS | Loading solution |
| ULS | Unloading solution |
| SA | Salicylic acid |
| GVA | Grapevine virus A |
| GFKV | Grapevine fleck virus |
| GFLV | Grapevine fanleaf virus |
| GLRaV-1 | Grapevine leafroll-associated virus-1 |
| GLRaV-2 | Grapevine leafroll-associated virus-2 |
| GLRaV-3 | Grapevine leafroll-associated virus-3 |
| GSH | Glutathione |
| LP | Leaf primordia |
| MD RT-PCR | Microtissue direct reverse transcription polymerase chain reaction |
| MS | Murashige and Skoog (1962) medium |
| NAA | Naphthaleneacetic acid |
| PPV | Plum pox virus |
| PVS | Plant vitrification solution |
| PVS2 | Plant vitrification solution 2 |
| PVS3 | Plant vitrification solution 3 |
| RT-PCR | Reverse transcription polymerase chain reaction |
| ROS | Reactive oxygen species |
| ST | Shoot tips |
| V cryo-plate | Vitrification cryo-plate |
| ZR | Zeatin riboside |
References
- OIV (International Organisation of Vine and Wine). State of the World Vitivinicultural Sector in 2019; International Organisation of Vine and Wine: Paris, France, 2020; Available online: https://www.oiv.int/public/medias/7298/oiv-state-of-the-vitivinicultural-sector-in-2019.pdf (accessed on 5 July 2021).
- This, P.; Lacombe, T.; Thomas, M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef]
- Gardiman, M.; Bavaresco, L. The Vitis germplasm repository at the CRA-VIT, Conegliano (Italy): Conservation, characterization and valorisation of grapevine genetic resources. Acta Hortic. 2015, 1082, 239–244. [Google Scholar] [CrossRef]
- OIV (International Organisation of Vine and Wine). Focus OIV 2017: Distribution of the of the World’s Grapevine Varieties; International Organisation of Vine and Wine: Paris, France, 2018; Available online: https://www.oiv.int/public/medias/5888/en-distribution-of-the-worlds-grapevine-varieties.pdf (accessed on 5 July 2021).
- Li, B.; Jiang, J.; Fan, X.; Zhang, Y.; Sun, H.; Zhang, G.; Liu, C. Molecular characterization of Chinese grape landraces (Vitis L.) using microsatellite DNA markers. HortScience 2017, 52, 533–540. [Google Scholar] [CrossRef]
- Riaz, S.; Pap, D.; Uretsky, J.; Laucou, V.; Boursiquot, J.M.; Kocsis, L.; Walker, M.A. Genetic diversity and parentage analysis of grape rootstocks. App Genet. 2019, 132, 1847–1860. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.P.; Morales, N.B.; Thomas, M.R.; Smith, H.M.; Clingeleffer, P.R. Grapevine rootstocks resistant to the root-knot nematode Meloidogyne javanica. Aust. J. Grape Wine Res. 2017, 23, 125–131. [Google Scholar] [CrossRef]
- Hou, H.; Li, H.; Wang, H.; Wang, X. Potential novel bZIP-like gene for resistance to Erysiphe necator identified in Chinese wild Vitis pseudoreticulata. Afr. J. Biotechnol. 2012, 11, 10926–10933. [Google Scholar] [CrossRef]
- Sapkota, S.; Chen, L.L.; Yang, S.; Hyma, K.E.; Cadle-Davidson, L.; Hwang, C.F. Construction of a high-density linkage map and QTL detection of downy mildew resistance in Vitis aestivalis-derived ‘Norton’. Appl. Genet. 2019, 132, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Blanc, S.; Wiedemann-Merdinoglu, S.; Dumas, V.; Mestre, P.; Merdinoglu, D. A reference genetic map of Muscadinia rotundifolia and identification of Ren5, a new major locus for resistance to grapevine powdery mildew. Appl. Genet. 2012, 125, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Myles, S. Improving fruit and wine: What does genomics have to offer? Trends Gen. 2013, 29, 190–196. [Google Scholar] [CrossRef]
- Maia, J.D.G.; Carmargo, U.A.; Tonietto, J.; Zanus, M.C.; Quecini, V.; Ferreira, M.E.; Ritschel, P. Grapevine breeding programs in Brazil. In Grapevine Breeding Programs for the Wine Industry—Traditional and Molecular Techniques; Reynolds, A., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 247–271. [Google Scholar] [CrossRef]
- Postman, J.; Hummer, K.; Stover, E.; Krueger, R.; Forsline, P.; Grauke, L.J.; Zee, F.; Ayala-Silva, T.; Irish, B. Fruit and nut genebanks in the U.S. National Plant Germplasm System. HortScience 2006, 41, 1188–1194. [Google Scholar] [CrossRef]
- Prins, B.; Volk, G.M.; Preece, J.E. Grape collection. In Field Tour of the USDA National Clonal Germplasm Repository for Tree Fruit, Nut Crops, and Grapes in Davis, California; Volk, G.M., Preece, J.E., Eds.; Colorado State University: Fort Collins, CO, USA, 2021; Available online: https://colostate.pressbooks.pub/davisrepositoryfieldtour/chapter/grapes/ (accessed on 7 August 2021).
- Elgelmann, F. In vitro conservation methods. In Biotechnology and Plant Genetic Resources; Callow, J.A., Ford-Lloye, B.V., Newbury, H.J., Eds.; CAB International: Wallingford, UK, 1997; pp. 119–161. [Google Scholar]
- Pathirana, R.; McLachlan, A.; Hedderley, D.; Panis, B.; Carimi, F. Pre-treatment with salicylic acid improves plant regeneration after cryopreservation of grapevine (Vitis spp.) by droplet vitrification. Acta Physiol. Plant. 2016, 38, 12. [Google Scholar] [CrossRef]
- Gisbert, C.; Peiró, R.; San Pedro, T.; Olmos, A.; Jiménez, C.; García, J. Recovering ancient grapevine varieties: From genetic variability to in vitro conservation, a case study. In Grapes and Wines—Advances in Production, Processing, Analysis and Valorization; Jordão, A.M., Cosme, F., Eds.; Intech: London, UK, 2018; pp. 3–21. [Google Scholar]
- Silva, R.C.; Luis, Z.G.; Scherwinski-Pereira, J.E. Short-term storage in vitro and large-scale propagation of grapevine genotypes. Pesquisa Agropecuária Brasileira 2012, 47, 344–350. [Google Scholar] [CrossRef]
- Hassanen, S.A.; Abido, A.I.A.; Aly, M.A.M.; Rayan, G.A. In vitro preservation of grapevine (Vitis vinifera L.) Muscat of Alexandria and Black Monukka cultivars as genetic resource. Afr. J. Basic Appl. Sci. 2013, 5, 55–63. [Google Scholar]
- Hassan, N.A.; Stino, R.G.; Gomaa, A.H.; Al-Mousa, R.N. In vitro medium-term germplasm conservation and genetic stability of rape (Vitis vinifera L.). J. Hortic. Sci. Ornam. Plants 2014, 6, 9–17. [Google Scholar]
- Barlass, M.; Skene, K.G.M. Long-term storage of grape in vitro. Plant. Genet. Resour. News 1983, 53, 19–21. [Google Scholar]
- Panis, B.; Nagel, M.; Van den Houwe, I. Challenges and prospects for the conservation of crop genetic resources in field genebanks, in in vitro collection and/or in liquid nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef]
- Rodrigues, P.H.V.; Tulmann Neto, A.; Cassieri Neto, P.; Mendes, B.M.J. Influence of the number of subcultures on somaclonal variation in micropropagated Nanico (Musa spp., AAA group). Acta Hortic. 1998, 490, 469–473. [Google Scholar] [CrossRef]
- Kulus, D.; Zalewska, M. Cryopreservation as a tool used in long-term storage of ornamental species—A review. Sci. Hortic. 2014, 168, 88–107. [Google Scholar] [CrossRef]
- Höfer, M.; Hanke, M.V. Cryopreservation of fruit germplasm. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 372–381. [Google Scholar] [CrossRef]
- Dalla Costa, L.; Malnoy, M.; Lecourieux, D.; Deluc, L.; Ouaked-Lecourieux, F.; Thomas, M.R.; Torregrosa, L. The state-of-the-art of grapevine biotechnology and new breeding technologies (NBTS). OENO One 2019, 53, 205–228. [Google Scholar] [CrossRef]
- Benson, E.E. Cryopreservation of phytodiversity: A critical appraisal of theory & practice. Crit. Rev. Plant Sci. 2008, 27, 141–219. [Google Scholar] [CrossRef]
- Harding, K. Genetic integrity of cryopreserved plant cells: A review. Cryo Lett. 2004, 25, 3–22. [Google Scholar]
- Wang, M.R.; Chen, L.; Teixeira da Silva, J.A.; Volk, G.M.; Wang, Q.C. Cryobiotechnology of apple (Malus spp.): Development, progress and future prospects. Plant Cell Rep. 2018, 37, 689–709. [Google Scholar] [CrossRef]
- Esensee, V.; Stushnoff, C. Cryoconservation of dormant grape (Vitis sp.) buds. In Proceedings of the 87th Annual Meeting of the American Society for Horticultural Science, Tucson, AZ, USA, 4–8 November 1990; Volume 25. Contributed Papers (Oral and Poster) Abstract #190. [Google Scholar]
- Matsumoto, T.; Sakai, A. Cryopreservation of in vitro-cultured axillary shoot tips of Vitis by vitrification. Acta Hortc. 2000, 538, 177–181. [Google Scholar] [CrossRef]
- Matsumoto, T.; Sakai, A. Cryopreservation of axillary shoot tips of in vitro-grown grape (Vitis) by a two-step vitrification protocol. Euphytica 2003, 131, 299–304. [Google Scholar] [CrossRef]
- Wang, Q.; Li, P.; Batuman, Ö.; Gafny, R.; Mawassi, M. Effect of benzyladenine on recovery of cryopreserved shoot tips of grapevine and citrus cultured in vitro. Cryo Lett. 2003, 24, 293–302. [Google Scholar] [PubMed]
- Marković, Z.; Preiner, D.; Stupić, D.; Andabaka, Ž.; Šimon, S.; Vončina, D.; Maletić, E.; Karoglan Kontić, J.; Chatelet, P.; Engelmann, F. Cryopreservation and cryotherapy of grapevine (Vitis vinifera L.). Vitis 2015, 54, 247–251. [Google Scholar] [CrossRef]
- Bi, W.L.; Hao, X.Y.; Cui, Z.H.; Volk, G.M.; Wang, Q. Droplet-vitrification cryopreservation of in vitro-grown shoot tips of grapevine (Vitis spp.). In Vitro Cell. Dev. Biol. -Plant 2018, 54, 590–599. [Google Scholar] [CrossRef]
- Volk, G.M.; Shepherd, A.N.; Bonnart, R. Successful cryopreservation of Vitis shoot tips: Novel pre-treatment combinations applied to nine species. Cryo Lett. 2018, 39, 322–330. [Google Scholar] [PubMed]
- Bettoni, J.; Kretzschmar, A.A.; Bonnart, R.; Shepherd, A.; Volk, G.M. Cryopreservation of 12 Vitis species using apical shoot tips derived from plants grown in vitro. HortScience 2019, 54, 976–981. [Google Scholar] [CrossRef]
- Parfitt, D.E.; Almehdi, A.A. Cryogenic storage of grape pollen. Am. J. Enol. Vitic. 1983, 34, 227–228. [Google Scholar]
- Ganeshan, S. Cryogenic preservation of grape, Vitis vinifera L., pollen. Vitis 1985, 173, 169–173. [Google Scholar]
- Hassan, N.A.; Gomma, A.H.; Shahin, M.A.; El Homosany, A.A. In vitro storage and cryopreservation of some grape varieties. J. Hortc. Sci. Ornam. Plants 2013, 5, 183–193. [Google Scholar]
- Wang, Q.; Mawassi, M.; Sahar, N.; Li, P.; Violeta, C.T.; Gafny, R.; Sela, I.; Tanne, E.; Perl, A. Cryopreservation of grapevine (Vitis spp.) embryogenic cell suspensions by encapsulation-vitrification. Plant Cell Tissue Organ. Cult. 2004, 77, 267–275. [Google Scholar] [CrossRef]
- Wang, Q.C.; Gafny, R.; Sahar, N.; Sela, I.; Mawassi, M.; Tanne, E.; Perl, A. Cryopreservation of grapevine (Vitis vinifera L.) embryogenic cell suspensions and subsequent plant regeneration by encapsulation-dehydration. Plant Sci. 2002, 162, 551–558. [Google Scholar] [CrossRef]
- Dussert, S.; Mauro, M.C.; Deloire, A.; Hamon, A.; Engelmann, F. Cryopreservation of grape embryogenic cell suspensions 1: Influence of pretreatment, freezing and thawing conditions. Cryo Lett. 1991, 12, 287–298. [Google Scholar]
- Dussert, S.; Mauro, M.C.; Engelmann, F. Cryopreservation of grape embryogenic cell suspensions 2: Influence of post-culture conditions and application to different strains. Cryo Lett. 1992, 13, 15–22. [Google Scholar]
- Carra, A.; Carimi, F.; Bettoni, J.C.; Pathirana, R. Progress and Challenges in the Application of Synthetic Seed Technology for Ex Situ Germplasm Conservation in Grapevine (Vitis spp.). In Synthetic Seeds; Faisal, A., Alatar, A.A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 439–467. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ. Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Walters, C.; Richards, C.R.; Volk, G.M. Genebank conservation of germplasm collected from wild species. In North American Crop Wild Relatives, Volume 1; Greene, S.L., Williams, K.A., Khoury, C.K., Kantar, M.B., Marek, L.F., Eds.; Springer Nature: New York, NY, USA, 2018; pp. 245–280. [Google Scholar] [CrossRef]
- Chofong, G.N.; Minarovits, J.; Richert-Pöggeler, K.R. Virus latency: Heterogeneity of host-virus interaction in shaping the virosphere. In Plant Virus-Host Interaction (Second Edition), Molecular Approaches and Viral Evolution; Gaur, R.K., Paul Khurana, S.M., Sharma, P., Hohn, T., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 111–137. [Google Scholar] [CrossRef]
- Hu, R.; Dias, N.P.; Soltani, N.; Vargas-Asencio, J.A.; Hensley, D.; Perry, K.L.; Domier, L.L.; Hajimorad, M.R. Cultivated and wild grapevines in Tennessee possess overlapping but distinct virus populations. Plant Dis. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Mannini, F.; Digiaro, M. The effects of viruses and viral diseases on grapes and wine. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G., Golino, D., Fuchs, M., Eds.; Springer Nature: New York, NY, USA, 2017; pp. 453–482. [Google Scholar] [CrossRef]
- Bi, W.L.; Hao, X.Y.; Cui, Z.H.; Pathirana, R.; Volk, G.M.; Wang, Q.C. Shoot tip cryotherapy for efficient eradication of grapevine leafroll-associated virus-3 from diseased grapevine in vitro plants. Ann. Appl. Biol. 2018, 173, 261–270. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Costa, M.D.; Gardin, J.P.P.; Kretzschmar, A.A.; Pathirana, R. Cryotherapy: A new technique to obtain grapevine plants free of viruses. Rev. Bras. Frutic. 2016, 38, e-833. [Google Scholar] [CrossRef]
- Martelli, G.P.; Walter, B. Virus certification of grapevines. In Plant Virus Disease Control; Hadid, A., Khetarpal, R.K., Koganezawa, H., Eds.; APS Press: St Paul, MN, USA, 1998; pp. 150–166. [Google Scholar]
- Brison, M.; Boucaud, M.T.; Pierronnet, A.; Dosba, F. Effect of cryopreservation on the sanitary state of a cv. Prunus rootstock experimentally contaminated with Plum Pox Potyvirus. Plant Sci. 1997, 123, 189–196. [Google Scholar] [CrossRef]
- Wang, Q.C.; Valkonen, J.P.T. Cryotherapy of shoot tips: Novel pathogen eradication method. Trend Plant Sci. 2009, 14, 119–122. [Google Scholar] [CrossRef]
- Helliot, B.; Panis, B.; Poumay, Y.; Swenen, R.; Lepoivre, P.; Frison, E. Cryopreservation for the elimination of cucumber mosaic and banana streak viruses from banana (Musa spp.). Plant Cell Rep. 2002, 20, 1117–1122. [Google Scholar] [CrossRef]
- Yi, J.Y.; Lee, G.A.; Jeong, J.W.; Lee, S.Y.; Lee, Y.G. Eliminating Potato Virus Y (PVY) and Potato Leaf Roll Virus (PLRV) using cryotherapy of in vitro-grown potato shoot tips. Korean J. Crop. Sci. 2004, 59, 498–504. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Xie, Y.; You, M. Cryotherapy of potato shoot tips for efficient elimination of Potato leaf roll virus (PLRV) and Potato virus Y (PVY). Potato Res. 2006, 49, 119–129. [Google Scholar] [CrossRef]
- Wang, Q.C.; Valkonen, J.P.T. Elimination of two viruses which interact synergistically from sweetpotato by shoot tip culture and cryotherapy. J. Virol. Methods 2008, 154, 135–145. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Souza, J.A.; Volk, G.M.; Dalla Costa, M.; da Silva, F.N.; Kretzschmar, A.A. Eradication of latent viruses from apple cultivar ’Monalisa’ shoot tips using droplet-vitrification cryotherapy. Sci. Hortic. 2019, 250, 12–18. [Google Scholar] [CrossRef]
- Souza, J.A.; Bogo, A.; Bettoni, J.C.; Dalla Costa, M.; da Silva, F.N.; Casa, R.T.; Rufato, L. Droplet-vitrification cryotherapy for eradication of apple stem grooving virus and apple stem pitting virus from “Marubakaido” apple rootstock. Trop. Plant Pathol. 2020, 45, 148–152. [Google Scholar] [CrossRef]
- Pathirana, R.; McLachlan, A.; Hedderley, D.; Carra, A.; Carimi, F.; Panis, B. Removal of leafroll viruses from infected grapevine plants by droplet-vitrification. Acta Hort. 2015, 1083, 491–498. [Google Scholar] [CrossRef]
- Bayati, S.; Shams-Bakhsh, M.; Moieni, A. Elimination of Grapevine virus A (GVA) by cryotherapy and electrotherapy. J. Agric. Sci. Tech. 2011, 13, 443–450. [Google Scholar]
- Wang, Q.; Mawassi, M.; Li, P.; Gafny, R.; Sela, I.; Tanne, E. Elimination of Grapevine virus A (GVA) by cryopreservation of in vitro-grown shoot tips of Vitis vinifera L. Plant Sci. 2003, 165, 321–327. [Google Scholar] [CrossRef]
- Bi, W.L. Cryopreservation of Shoot Tips of Grapevine (Vitis spp.) and Cryotherapy for Eradication of grapevine Leafroll-Associated Virus 3. PhD Thesis, Northwest A&F University, Yangling, Shaanxi, China, 2018. [Google Scholar]
- Marković, Z. Cryopreservation and Cryotherapy of Grapevine (Vitis vinifera L.). PhD Thesis, University of Zagreb, Faculty of Agriculture Croatia, Zagreb, Croatia, 9 December 2013. [Google Scholar]
- Bi, W.L.; Pan, C.; Hao, X.Y.; Cui, Z.H.; Kher, M.M.; Marković, Z.; Wang, Q.C.; Silva, J.A.T. Cryopreservation of grapevine (Vitis spp.)—A review. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 449–460. [Google Scholar] [CrossRef]
- Panis, B. Sixty years of plant cryopreservation: From freezing hardy mulberry twigs to establishing reference crop collections for future generations. Acta Hortic. 2019, 1234, 1–7. [Google Scholar] [CrossRef]
- Agrawal, A.; Singh, S.; Malhotra, E.V.; Meena, D.P.S.; Tyagi, R.K. In vitro conservation and cryopreservation of clonally propagated horticultural species. In Conservation and Utilization of Horticultural Genetic Resources; Rajasekharan, P., Rao, V., Eds.; Springer Nature: New York, NY, USA, 2019; pp. 529–578. [Google Scholar] [CrossRef]
- Malik, S.K.; Chaudhury, R. Cryopreservation techniques for conservation of tropical horticultural species using various explants. In Conservation and Utilization of Horticultural Genetic Resources; Rajasekharan, P., Rao, V., Eds.; Springer Nature: New York, NY, USA, 2019; pp. 579–594. [Google Scholar] [CrossRef]
- Jenderek, M.M.; Reed, B.M. Cryopreserved storage of clonal germplasm in the USDA National plant germplasm system. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 299–308. [Google Scholar] [CrossRef]
- Keller, E.R.; Grübe, M.; Hajirezaei, M.R.; Melzer, M.; Mock, H.P.; Rolletschek, H.; Senula, A.; Subbarayan, K. Experience in large-scale cryopreservation and links to applied research for safe storage of plant germplasm. Acta Hortic. 2016, 1113, 239–249. [Google Scholar] [CrossRef]
- Kim, H.H.; Popova, E.; Shin, D.J.; Yi, J.Y.; Kim, C.H.; Lee, J.S.; Yoon, M.K.; Engelmann, F. Cryobanking of Korean Allium germplasm collections: Results from a 10 year experience. Cryo Lett. 2012, 33, 45–57. [Google Scholar]
- Vollmer, R.; Villagaray, R.; Cárdenas, J.; Castro, M.; Chávez, O.; Anglin, N.L.; Ellis, D. A large-scale viability assessment of the potato cryobank at the International Potato Centre (CIP). In Vitro Cell. Dev. Biol.-Plant 2017, 53, 309–317. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Development of practical and successful Vitis shoot tip cryopreservation protocols. Cryobiology 2019, 91, 194–195. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Shepherd, A.; Kretzschmar, A.A.; Volk, G.M. Modifications to a Vitis shoot tip cryopreservation procedure: Effect of shoot tip size and use of cryoplates. Cryo Lett. 2019, 40, 103–112. [Google Scholar] [PubMed]
- Engelmann., F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Wang, M.R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tissue Organ. Cult. 2021, 144, 7–20. [Google Scholar] [CrossRef]
- Marković, Z.; Preiner, D.; Stupić, D.; Andabaka, Z.; Šikuten, I.; Kontić, J.K.; Maletić, E.; Štambuk, P. Cryopreservation Protocols for Grapevine Shoot Tips Cryopreservation. In Biotechnology in Biomedical and Biological Sciences; Bozkurt, Y., Ed.; IntechOpen Limited: London, UK, 2018; pp. 131–142. [Google Scholar] [CrossRef]
- Takagi, H. Recent developments in cryopreservation of shoot tips of tropical species. In Cryopreservation of Tropical Plant Germplasm; Engelmann, F., Takagi, H., Eds.; International Plant Genetics Research Institute: Rome, Italy, 2000; pp. 178–193. [Google Scholar]
- Bettoni, J.C. Criopreservação: Uma ferramenta para conservação de recursos genéticos de videira. Agropecu. Catarin. 2019, 32, 92–97. [Google Scholar] [CrossRef]
- Benelli, C.; De Carlo, A.; Engelmann, F. Recent advances in the cryopreservation of shoot-derived germplasm of economically important fruit trees of Actinidia, Diospyros, Malus, Olea, Prunus, Pyrus and Vitis. Biotechnol. Adv. 2013, 31, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.M. Cryopreservation—Practical considerations. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 3–11. [Google Scholar] [CrossRef]
- Ezawa, T.; Harada, T.; Yakuwa, T. Studies on freeze-preservation of fruit tree germplasm. III Freeze-preservation of grape shoot tips. J. Fac. Agric. Hokkaido Univ. 1989, 64, 51–55. [Google Scholar]
- Plessis, P.; Leddet, C.; Dereuddre, J. Resistance to dehydration and to freezing in liquid nitrogen of alginate coated shoot tips of grapevine (Vitis vinifera L. cv. Chardonnay). Comptes Rendus l’Acad. Sci. Ser. 3 Sci. Vie 1991, 313, 373–380. [Google Scholar]
- Plessis, P.; Leddet, C.; Collas, A.; Dereuddre, J. Cryopreservation of Vitis vinifera L. cv. Chardonnay shoot tips by encapsulation dehydration: Effects of pretreatment, cooling and postculture conditions. Cryo Lett. 1993, 14, 309–320. [Google Scholar]
- Miaja, M.L.; Gribaudo, I.; Vallania, R.; Fernandez, L.F. Low temperature storage and cryopreservation of a Vitis vinifera L. germplasm collection: First results. Acta Hortic. 2000, 538, 177–181. [Google Scholar] [CrossRef]
- Wang, Q.; Tanne, E.; Arav, A.; Gafny, R. Cryopreservation of in vitro-grow shoot tips of grapevine by encapsulation-dehydration. Plant Cell Tissue Organ. Cult. 2000, 63, 41–46. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, Y.; Engelmann, F.; Zhou, M. Cryopreservation of axillary buds of grape (Vitis vinifera) in vitro plantlets. Cryo Lett. 2001, 22, 321–328. [Google Scholar] [PubMed]
- Benelli, C.; Lambardi, M.; Fabbri, A. Low Temperature Storage and Cryopreservation of the Grape Rootstock “Kober 5BB”. Acta Hortic. 2003, 623, 249–253. [Google Scholar] [CrossRef]
- Zhai, Z.; Wu, Y.; Engelmann, F.; Chen, R.; Zhao, Y. Genetic stability assessments of plantlets regenerated from cryopreserved in vitro cultured grape and kiwi shoot-tips using RAPD. Cryo Lett. 2003, 24, 315–322. [Google Scholar] [PubMed]
- Fabbri, A.; Ganino, T.; Lombardi, M.; Nisi, R. Crioconservazione di gemme di portinnesto Kober 5BB (Vitis berlandieri x Vitis riparia): Aspetti anatomici. Italus Hortus 2007, 3, 82–86. [Google Scholar]
- Shatnawi, M.; Anfoka, G.; Shibli, R.; Al-Mazra‘Awi, M.; Shahrour, W.; Arebiat, A. Clonal propagation and cryogenic storage of virus-free grapevine (Vitis vinifera L.) via meristem culture. Turk. Agric. For. 2011, 35, 173–184. [Google Scholar] [CrossRef]
- Ganino, T.; Silvanini, A.; Beghé, D.; Benelli, C.; Lambardi, M.; Fabbri, A. Anatomy and osmotic potential of the Vitis rootstock shoot tips recalcitrant to cryopreservation. Biol. Plant 2012, 56, 78–82. [Google Scholar] [CrossRef]
- Marković, Z.; Chatelet, P.; Sylvestre, I.; Karoglan Kontić, J.; Engelmann, F. Cryopreservation of grapevine (Vitis vinifera L.) in vitro shoot tips. Cent. Eur. J. Biol. 2013, 8, 993–1000. [Google Scholar] [CrossRef]
- Hassan, N.A.; Haggag, A.M. Cryopreservation of two Egyptian grape (Vitis vinifera) cultivars using two steps vitrification protocol. World Appl. Sci. J. 2013, 28, 254–258. [Google Scholar] [CrossRef]
- Marković, Z.; Chatelet, P.; Preiner, D.; Sylvestre, I.; Karoglan Kontić, J.; Engelmann, F. Effect of shooting medium and source of material on grapevine (Vitis vinifera L.) shoot tip recovery after cryopreservation. Cryo Lett. 2014, 35, 40–47. [Google Scholar] [PubMed]
- Lazo-Javelera, M.F.; Tiznado-Hernández, M.E.; Vargas-Arispuro, I.; Valenzuela-Soto, E.; Rocha-Granados, M.C.; Martínez-Montero, M.E.; Rivera-Domínguez, M. Data on antioxidant activity in grapevine (Vitis vinifera L.) following cryopreservation by vitrification. Data Brief 2015, 5, 549–555. [Google Scholar] [CrossRef][Green Version]
- Dal Bosco, D.; Sinski, I.; Comachio, V.; Maia, J.D.G.; Ritschel, P.S.; Quecini, V. In vitro techniques for grapevine germplasm conservation. Acta Hortic. 2015, 1082, 201–205. [Google Scholar] [CrossRef]
- Faltus, M.; Bilavčík, A.; Zámečník, J. Thermal analysis of grapevine shoot tips during dehydration and vitrification. Vitis 2015, 54, 243–245. [Google Scholar]
- El-Homosany, A.; El-Wagab, A.; Samaan, M. Regeneration of some grape rootstock shoot tips after cryopreservation by droplet-vitrification. Middle East. J. Agric. Res. 2019, 8, 1025–1030. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Shepherd, A.; Kretzschmar, A.A.; Volk, G.M. Cryopreservation of grapevine (Vitis spp.) shoot tips from growth chamber-sourced plants and histological observations. Vitis 2019, 58, 71–78. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Shepherd, A.; Kretzschmar, A.A.; Volk, G.M. Successful cryopreservation of Vitis vinifera ‘Chardonnay’ from both in vitro and growth chamber source plants. Acta Hortc. 2019, 1234, 211–218. [Google Scholar] [CrossRef]
- AlMousa, R.N.; Hassan, N.A.F. Cryopreservation of grape (Vitis vinifera L.) using encapsulation-dehydration technique. J. Genet. Environ. Res. Conserv. 2021, 9, 153–156. [Google Scholar]
- Morel, G. Recherches sur la culture associée de parasites obligatoires et de tissus végétaux. Ann. Epiphyt. 1948, 14, 123–134. [Google Scholar]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Volk, G.M.; Shepherd, A.; Bonnart, R. Strategies for improved efficiency when implementing plant vitrification techniques. Acta Hortic. 2014, 1039, 85–89. [Google Scholar] [CrossRef]
- Mathew, L.; McLachlan, A.; Jibran, R.; Burritt, D.J.; Pathirana, R. Cold, antioxidant and osmotic pre-treatments maintain the structural integrity of meristematic cells and improve plant regeneration in cryopreserved kiwifruit shoot tips. Protoplasma 2018, 255, 1065–1077. [Google Scholar] [CrossRef]
- Panta, A.; Panis, B.; Ynouye, C.; Swennen, R.; Roca, W.; Tay, D.; Ellis, D. Improved cryopreservation method for the long-term conservation of the world potato germplasm collection. Plant Cell Tissue Organ. Cult. 2015, 120, 117–125. [Google Scholar] [CrossRef]
- Kushnarenko, S.V.; Romadanova, N.V.; Reed, B.M. Cold acclimation improves regrowth of cryopreserved apple shoot tips. Cryo Lett. 2009, 30, 47–54. [Google Scholar] [PubMed]
- Chang, Y.; Reed, B.M. Extended alternating-temperature cold acclimation and culture duration improve pear shoot cryopreservation. Cryobiology 2000, 40, 311–322. [Google Scholar] [CrossRef]
- Reed, B.M. Antioxidants and cryopreservation, the new normal? Acta Hortic. 2014, 1039, 41–48. [Google Scholar] [CrossRef]
- Wang, Q.; Laamanen, J.; Uosukainen, M.; Valkonen, J.P.T. Cryopreservation of in vitro-grown shoot tips of raspberry (Rubus idaeus L.) by encapsulation-vitrification and encapsulation-dehydration. Plant Cell Rep. 2005, 24, 280–288. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Charoensub, R.; Hirai, D.; Sakai, A. Cryopreservation of in vitro-grown shoot tips of cassava by encapsulation-vitrification method. Cryo Lett. 2004, 25, 51–58. [Google Scholar] [PubMed]
- Halmagyi, A.; Deliu, C.; Coste, A. Plant regrowth from potato shoot tips cryopreserved by a combined vitrification-droplet method. Cryo Lett. 2005, 26, 313–322. [Google Scholar] [PubMed]
- Bettoni, J.C. Cryopreservation for the Formation of Backup in Grapevine and Cryotherapy for Eradication of Virus from Apple Tree. PhD Thesis, Santa Catarina State University, Lages, Santa Catarina, Brazil, 6 September 2018. [Google Scholar] [CrossRef]
- Uragami, A.; Sakai, A.; Nagai, M.; Takahashi, T. Survival of cultured cells and somatic embryos of Asparagus officinalis cryopreserved by vitrification. Plant Cell Rep. 1989, 8, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef]
- Fabre, L.; Dereuddre, J. Encapsulation-dehydration: A new approach to cryopreservation of Solanum shoot-tips. Cryo Lett. 1990, 11, 413–426. [Google Scholar]
- Dereuddre, J.; Scottez, C.; Arnald, Y.; Doron, M. Resistance of alginate-coated axillary shoot tips of pear tree (Pyrus communis L. cv. Beurre Hardy) in vitro plantlets to dehydration and subsequent freezing in liquid nitrogen: Effects of previous cold hardening. Comptes Rendus l’Acad. Sci. Sér. III Sci. Vie 1990, 310, 317–323. [Google Scholar]
- Engelmann, F. Plant cryopreservation: Progress and prospects. In Vitro Cell. Dev. Biol.-Plant 2004, 40, 427–433. [Google Scholar] [CrossRef]
- Panis, B.; Lambardi, M. Status of cryopreservation technologies in plants (crops and forest trees). In Proceedings of the The Role of Biotechnology for the Characterization and Conservation of Crop, Forestry, Animal and Fishery Genetic Resources, Turin, Italy, 5–7 March 2005; pp. 43–54. [Google Scholar]
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Sakai, A.; Hirai, D.; Niino, T. Development of PVS-Based Vitrification and Encapsulation–Vitrification Protocols. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 33–57. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Pathirana, R.; Bonnart, R.; Shepherd, A.; Volk, G. Cryopreservation of grapevine shoot tips from in vitro plants using droplet vitrification and v cryo-plate techniques. In Synthetic Seeds; Faisal, A., Alatar, A.A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 469–482. [Google Scholar] [CrossRef]
- Bettoni, J.; Bonnart, R.M.; Volk, G.M. Vitis shoot tip cryopreservation (droplet vitrification and V-cryoplate). In Training in Plant. Genetic Resources: Cryopreservation of Clonal Propagules; Volk, G.M., Ed.; Colorado State University: Fort Collins, CO, USA, 2021; Available online: https://colostate.pressbooks.pub/clonalcryopreservation/chapter/grapevine-shoot-tip-cryopreservation-droplet-vitrification-and-cryoplate/ (accessed on 7 August 2021).
- Matsumoto, T.; Sakai, A. An approach to enhance dehydration tolerance of alginate-coated dried meristems cooled to −196 °C. Cryo Lett. 1995, 16, 299–306. [Google Scholar]
- Engelmann, F.; Arnao, M.T.G.; Wu, Y.; Escobar, R. Development of Encapsulation Dehydration. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 59–75. [Google Scholar] [CrossRef]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. Cryo Lett. 2007, 28, 151–172. [Google Scholar] [PubMed]
- Volk, G.M.; Harris, J.L.; Rotindo, K.E. Survival of mint shoot tips after exposure to cryoprotectant solution components. Cryobiology 2006, 52, 305–308. [Google Scholar] [CrossRef]
- Volk, G.M.; Maness, N.; Rotindo, K. Cryopreservation of garlic (Allium sativum L.) using plant vitrification solution 2. Cryo Lett. 2004, 25, 219–226. [Google Scholar] [PubMed]
- Matsumoto, T.; Sakai, A.; Yamada, K. Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep. 1994, 13, 442–446. [Google Scholar] [CrossRef]
- Engelmann, F. Importance of cryopreservation for the conservation of plant genetic resources. In Cryopreservation of Tropical Plant Germplasm; Engelmann, F., Takagi, H., Eds.; International Plant Genetics Research Institute: Rome, Italy, 2000; pp. 8–20. [Google Scholar]
- Matsumoto, T.; Niino, T. The development of plant vitrification solution 2 and recent PVS2-based vitrification protocols. Acta Hortic. 2014, 1039, 21–28. [Google Scholar] [CrossRef]
- Sakai, A. Plant cryopreservation. In Life in the Frozen State; Fuller, B., Lane, N., Benson, E.E., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 329–346. [Google Scholar]
- Matsumoto, T.; Sakai, A.; Takahashi, C.; Yamada, K. Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by encapsulation-vitrification method. Cryo Lett. 1995, 16, 189–196. [Google Scholar]
- Kartha, K.K.; Leung, N.L.; Mroginski, L.A. In vitro growth-responses and plant-regeneration from cryopreserved meristems of cassava (Manihot esculenta Crantz). Z. Pflanzenphysiol. 1982, 107, 133–140. [Google Scholar] [CrossRef]
- Schäfer-Menuhr, A.; Schumacher, H.M.; Mix-Wagner, G. Langzeitlagerung alter Kartoffelsorten durch Kryokonservierung der Meristeme. Landbauforsch. Völkenrode 1994, 44, 301–313. [Google Scholar] [CrossRef]
- Schäfer-Menuhr, A.; Müller, E.; Mix-Wagner, G. Cryopreservation: An alternative for the long-term storage of old potato cultivars. Potato Res. 1996, 39, 507–513. [Google Scholar] [CrossRef]
- Schäfer-Menuhr, A.; Schumacher, H.M.; Mix-Wagner, G. Cryopreservation of potato cultivars-design of a method for routine application in genebanks. Acta Hortic. 1997, 447, 447–482. [Google Scholar] [CrossRef]
- Panis, B.; Piette, B.; Swennen, R. Droplet vitrification of apical meristems: A cryopreservation protocol applicable to all Musaceae. Plant Sci. 2005, 168, 45–55. [Google Scholar] [CrossRef]
- Souza, F.V.; Kaya, E.; Vieira, L.J.; de Souza, E.H.; Amorim, V.B.O.; Skogerboe, D.; Matsumoto, T.; Alves, A.A.C.; Ledo, C.A.S.; Jenderek, M.M. Droplet-vitrification and morphohistological studies of cryopreserved shoot tips of cultivated and wild pineapple genotypes. Plant Cell Tissue Organ. Cult. 2016, 124, 351–360. [Google Scholar] [CrossRef]
- Volk, G.M.; Walters, C. Preservation of Genetic Resources in the National Plant Germplasm Clonal Collections. Plant Breed. Rev. 2003, 23, 291–344. [Google Scholar] [CrossRef]
- Niino, T.; Arizaga, M. Cryopreservation for preservation of potato genetic resources. Breed. Sci. 2015, 65, 41–52. [Google Scholar] [CrossRef][Green Version]
- Panis, B.; Piette, B.; André, E.; Van den Houwe, I.; Swennen, R. Droplet vitrification: The first generic cryopreservation protocol for organized plant tissues? Acta Hortic. 2011, 908, 157–163. [Google Scholar] [CrossRef]
- Yamamoto, S.; Rafique, T.; Priyantha, W.S.; Fukui, K.; Matsumoto, T.; Niino, T. Development of a cryopreservation procedure using aluminium cryo-plates. Cryo Lett. 2011, 32, 256–265. [Google Scholar] [PubMed]
- Matsumoto, T.; Niino, T. Manual of Cryopreservation Methods Using Cryo-Plate: V and D Cryo-Plate Procedure as an Effective Protocol for Cryobanks; Niino, T., Matsumoto, T., Yamamoto, S.-I., Maki, S., Tanata, D., Engelmann, F., Eds.; Plant Tissue Culture and Cryopreservation Group (PTCCcryoG): Tsukuba, Japan, 2017; pp. 8–15. [Google Scholar]
- Matsumoto, T. Cryopreservation of plant genetic resources: Conventional and new methods. Rev. Agric. Sci. 2017, 5, 13–20. [Google Scholar] [CrossRef]
- Niino, T.; Yamamoto, S.; Matsumoto, T.; Elgelmann, F.; Valle Arizaga, M.; Tanaka, D. Development of V and D cryo-plate methods as effective protocols for cryobanking. Acta Hortic. 2019, 1234, 249–262. [Google Scholar] [CrossRef]
- Yamamoto, S.; Rafique, T.; Fukui, K.; Sekizawa, K.; Niino, T. V-cryoplate procedure as an effective protocol for cryobanks: Case study of mint cryopreservation. Cryo Lett. 2012, 33, 12–23. [Google Scholar] [PubMed]
- Wang, Q.C.; Panis, B.; Engelmann, F.; Lambardi, M.; Valkonen, J.P.T. Cryotherapy of shoot tips: A technique for pathogen eradication to produce healthy planting materials and prepare healthy plant genetic resources for cryopreservation. Ann. Appl. Biol. 2009, 154, 351–363. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, M.R.; Li, J.W.; Cui, Z.H.; Volk, G.M.; Wang, Q.C. Cryobiotechnology: A double-edged sword for obligate plant pathogens. Plant Dis. 2019, 103, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Jiroutová, P.; Sedlák, J. Cryobiotechnology of plants: A hot topic not only for gene banks. Appl. Sci. 2020, 10, 4677. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Souza, J.A. Crioterapia: Uma potencial ferramenta para erradicação de vírus em plantas. Rev. Agron. Bras. 2018, 2, 1–3. [Google Scholar] [CrossRef]
- Liu, X.X.; Mou, S.W.; Cheng, Z.H. Effect of cryopreservation on plant growth, bulb characteristics, and virus reduction of garlic (Allium Sativum L.). Cryo Lett. 2019, 40, 322–332. [Google Scholar] [PubMed]
- Luo, Y.; Qiu, J.; Ling, Y.J.; Mo, Q.; Ran, X.R.F.; Tang, H.R. The application of cryotherapy in virus elimination of strawberry. Mol. Plant Breed. 2016, 14, 2488–2494. [Google Scholar]
- Sheng, H.Y.; Wan, J.H.; Xu, C.; Wang, H.Q. Preliminary study on elimination of strawberry mottle virus (SMoV) from shoot tips by vitrification-cryopreservation treatment. J. China Agric. Univ. 2016, 21, 53–57. [Google Scholar]
- Zeng, J.W.; Niu, W.C.; Huang, Y.H.; Sun, Z.H.; Huang, B.Z.; Yi, G.J.; Zhou, B.R. 2009. Elimination of Banana Bunchy Top Virus (BBTV) by cryopreservation of in vitro-grown shoot tips from Banana (Musa spp.). J. Plant Gene Res. 2009, 10, 457–460. [Google Scholar]
- Li, B.Q.; Feng, C.H.; Hu, L.Y.; Wang, M.R.; Wang, Q.C. Shoot tip culture and cryopreservation for eradication of Apple stem pitting virus (ASPV) and Apple stem grooving virus (ASGV) from apple rootstocks ‘M9’ and ‘M26’. Ann. Appl. Biol. 2016, 168, 142–150. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, M.R.; Cui, Z.H.; Chen, L.; Volk, G.M.; Wang, Q.C. Combining thermotherapy with cryotherapy for efficient eradication of Apple stem grooving virus from infected in-vitro-cultured apple shoots. Plant Dis. 2018, 102, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Farhadi-Tooli, S.; Ghanbari, A.; Kermani, M.J.; Zeinalabedini, M.; Bettoni, J.C.; Naji, A.M.; Kazemi, N. Droplet-vitrification cryotherapy and thermotherapy as efficient tools for the eradication of apple chlorotic leaf spot virus and apple stem grooving virus from virus-infected quince in vitro cultures. Eur. J. Plant Pathol. 2021. [Google Scholar] [CrossRef]
- Jeon, S.M.; Naing, A.H.; Kim, H.H.; Chung, M.Y.; Lim, K.B.; Kim, C.K. Elimination of chrysanthemum stunt viroid and chrysanthemum chlorotic mottle viroid from infected chrysanthemum by cryopreservation. Protoplasma 2016, 253, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.R.; Mou, H.Q.; Gao, X.X.; Chen, L.; Li, M.F.; Wang, Q.C. Cryopreservation for eradication of Jujube witches’ broom phytoplasma from Chinese jujube (Ziziphus jujuba). Ann. Appl. Biol. 2015, 166, 218–228. [Google Scholar] [CrossRef]
- Wang, Q.C.; Valkonen, J.P.T. Efficient elimination of Sweetpotato little leaf phytoplasma from sweetpotato by cryotherapy of shoot tips. Plant Pathol. 2008, 57, 338–347. [Google Scholar] [CrossRef]
- Ding, F.; Jin, S.X.; Hong, N.; Zhong, Y.; Cao, Q.; Yi, G.J.; Wang, G.P. Vitrification-cryopreservation, an efficient method for eliminating Candidatus Libero bacterasiaticus, the citrus Huanglongbing pathogen, from in vitro adult shoot tips. Plant Cell Rep. 2008, 27, 241–250. [Google Scholar] [CrossRef]
- Gribaudo, I.; Cuozzo, D.; Ganbino, G.; Vallania, R. Applicazione della tecnica di incapsulazione-vitrificazione per la crioconservazione e la crioterapia in vite. Convegno Nazionale di Viticoltura 2012, 3, 372–374. [Google Scholar]
- Kaya, E.; Galatali, S.; Güldağ, S.; Celik, O. A new perspective on cryotherapy: Pathogen elimination using plant shoot apical meristem via cryogenic techniques. In Plant Stem Cells, Methods in Molecular Biology; Naseem, M., Dandekar, T., Eds.; Humana: New York, NY, USA, 2000; pp. 137–148. [Google Scholar] [CrossRef]
- Barlass, M.; Skene, K.G.M.; Woodham, R.C.; Krake, L.R. Regeneration of virus-free grapevines using in vitro apical culture. Ann. Appl. Biol. 1982, 101, 291–295. [Google Scholar] [CrossRef]
- Youssef, S.A.; Al-Dhaher, M.M.A.; Shalaby, A.A. Elimination of grapevine fanleaf virus (GFLV) and grapevine leaf roll-associated virus-1 (GLRaV-1) from infected grapevine plants using meristem tip culture. Int. J. Virol. 2009, 5, 89–99. [Google Scholar] [CrossRef]
- Fayek, M.A.; Jomaa, A.H.; Shalaby, A.B.A. Meristem tip culture for in vitro eradication of grapevine leaf roll-associated virus-1 (GLRaV-1) and grapevine fan leaf virus (GFLV) from infected flame seedless grapevine plantlets. Iniciación A La Investig. 2009, 4, 1–11. [Google Scholar]
- Dĩaz-Barrita, A.J.; Norton, M.; Martĩnez-Peniche, R.A.; Uchanski, M.; Mulwa, R.; Skirvin, R.M. The use of thermotherapy and in vitro meristem culture to produce virus-free ‘chancellor’ grapevines. Int. J. Fruit Sci. 2008, 7, 15–25. [Google Scholar] [CrossRef]
- Salami, S.A.; Ebadi, A.; Zamani, Z.; Habibi, M.K. Incidence of grapevine fanleaf virus in Iran: A survey study and production of virus-free material using meristem culture and thermotherapy. Eur. J. Hortic. Sci. 2009, 74, 42–46. [Google Scholar]
- Celik, H.; Söylemezoglu, G.; Ertunc, F.; Cakir, A.; Dursunoglu, S.; Akbas, B. Clonal micropropagation of main grape and rootstock varieties of Turkish viticulture for obtaining virus-free basic nursery stocks. In Proceedings of the IX International Conference on Grape Genetics and Breeding, Verona, Italy, 13–18 June 2006; Volume 827, pp. 421–424. [Google Scholar]
- Wang, M.R.; Cui, Z.H.; Li, J.W.; Hao, X.Y.; Zhao, L.; Wang, Q.C. In vitro thermotherapy-based methods for plant virus eradication. Plant Methods 2018, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Skiada, F.G.; Grigoriadou, K.; Maliogka, V.I.; Katis, N.I.; Eleftheriou, E.P. Elimination of grapevine leafroll-associated virus 1 and grapevine rupestris pitting-associated virus from grapevine cv. Agiorgitiko and a micropropagation of protocol for mass production of virus-free plantlets. J. Plant Pathol. 2009, 91, 177–184. [Google Scholar] [CrossRef]
- Panattoni, A.; Triolo, E. Susceptibility of grapevine viruses to thermotherapy on in vitro collection of Kober 5BB. Sci. Hortic. 2010, 125, 63–67. [Google Scholar] [CrossRef]
- Wang, M.R.; Chen, L.; Zhang, Z.; Blystad, D.R.; Wang, Q.C. Cryotherapy: A novel method for virus eradication in economically important plant species. In Plant Cell Culture Protocols, Methods in Molecular Biology; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 257–268. [Google Scholar] [CrossRef]

| Specie and Genotype | Virus | Cryo Method | Explant | Regrowth (%) | Plant Conditions during Virus Detection | Virus-Free Frequency (%) | Virus Confirmation Method | Year Ref. |
|---|---|---|---|---|---|---|---|---|
| V. vinifera ‘Bruti’ | GVA | VI | ST (1 mm; type n/s) | 50 | Plants grown in the greenhouse for 4 months | 97 | Western blotting/ELISA | 2003 [64] |
| ED | 62 | |||||||
| V. vinifera ‘Black’ | GVA | ED | ST (1 mm; type n/s) | 59 | In vitro plants that are 2 to 4 months old | 42.2 | RT-PCR | 2011 [63] |
| V. vinifera Nebbiolo | GVA GLRaV-3 | EV | AST (2 mm) | 15 | n/s | 100 | Multiplex RT-PCR | 2012 [167] |
| V. vinifera Chardonnay | GFLV | DV | AST (1 mm) | 30.7 | In vitro plants that are 2 months old | 77.8 | ELISA | 2015 [34] |
| V. vinifera Cabernet Sauvignon | GLRaV-3 | 41.6 | 100 | |||||
| V. vinifera Pinot gris | GLRaV-2 | DV | AST and AxST (size n/s) | 13.6 | In vitro plants that are 3 months old/plants grown in the greenhouse for 3 and 6 months | 100 | DAS-ELISA | 2015 [62] |
| V. vinifera Sauvignon blanc 316 | GLRaV-2 | 15.7 | 100 and 100 | |||||
| V. vinifera Sauvignon blanc | GLRaV-1 and GLRaV-3 | 30 | 100 | |||||
| V. vinifera ‘Lakemont Seedless’ | GLRaV-3 | 16.2 | 100 | |||||
| V. vinifera Chardonnay | GLRaV-3 | 13 | 100 | |||||
| V. vinifera Cabernet Sauvignon | GLRaV-3 | DV | AST (1 mm) | 59 | Plants grown in the screen-house for 12 months | 100 | RT-PCR and MD RT-PCR | 2018 [51] |
| V. vinifera Chardonnay | 47 | |||||||
| V. vinifera × V. labrusca ‘Kyoho’ | 51 | |||||||
| V. pseudoreticulata ‘Hunan-1’ | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettoni, J.C.; Marković, Z.; Bi, W.; Volk, G.M.; Matsumoto, T.; Wang, Q.-C. Grapevine Shoot Tip Cryopreservation and Cryotherapy: Secure Storage of Disease-Free Plants. Plants 2021, 10, 2190. https://doi.org/10.3390/plants10102190
Bettoni JC, Marković Z, Bi W, Volk GM, Matsumoto T, Wang Q-C. Grapevine Shoot Tip Cryopreservation and Cryotherapy: Secure Storage of Disease-Free Plants. Plants. 2021; 10(10):2190. https://doi.org/10.3390/plants10102190
Chicago/Turabian StyleBettoni, Jean Carlos, Zvjezdana Marković, Wenlu Bi, Gayle M. Volk, Toshikazu Matsumoto, and Qiao-Chun Wang. 2021. "Grapevine Shoot Tip Cryopreservation and Cryotherapy: Secure Storage of Disease-Free Plants" Plants 10, no. 10: 2190. https://doi.org/10.3390/plants10102190
APA StyleBettoni, J. C., Marković, Z., Bi, W., Volk, G. M., Matsumoto, T., & Wang, Q.-C. (2021). Grapevine Shoot Tip Cryopreservation and Cryotherapy: Secure Storage of Disease-Free Plants. Plants, 10(10), 2190. https://doi.org/10.3390/plants10102190









