Emerging Roles of γ Aminobutyric Acid (GABA) Gated Channels in Plant Stress Tolerance
Abstract
:1. Introduction
2. GABA Affects Physiological Processes
3. GABA Regulation of Gene Expression
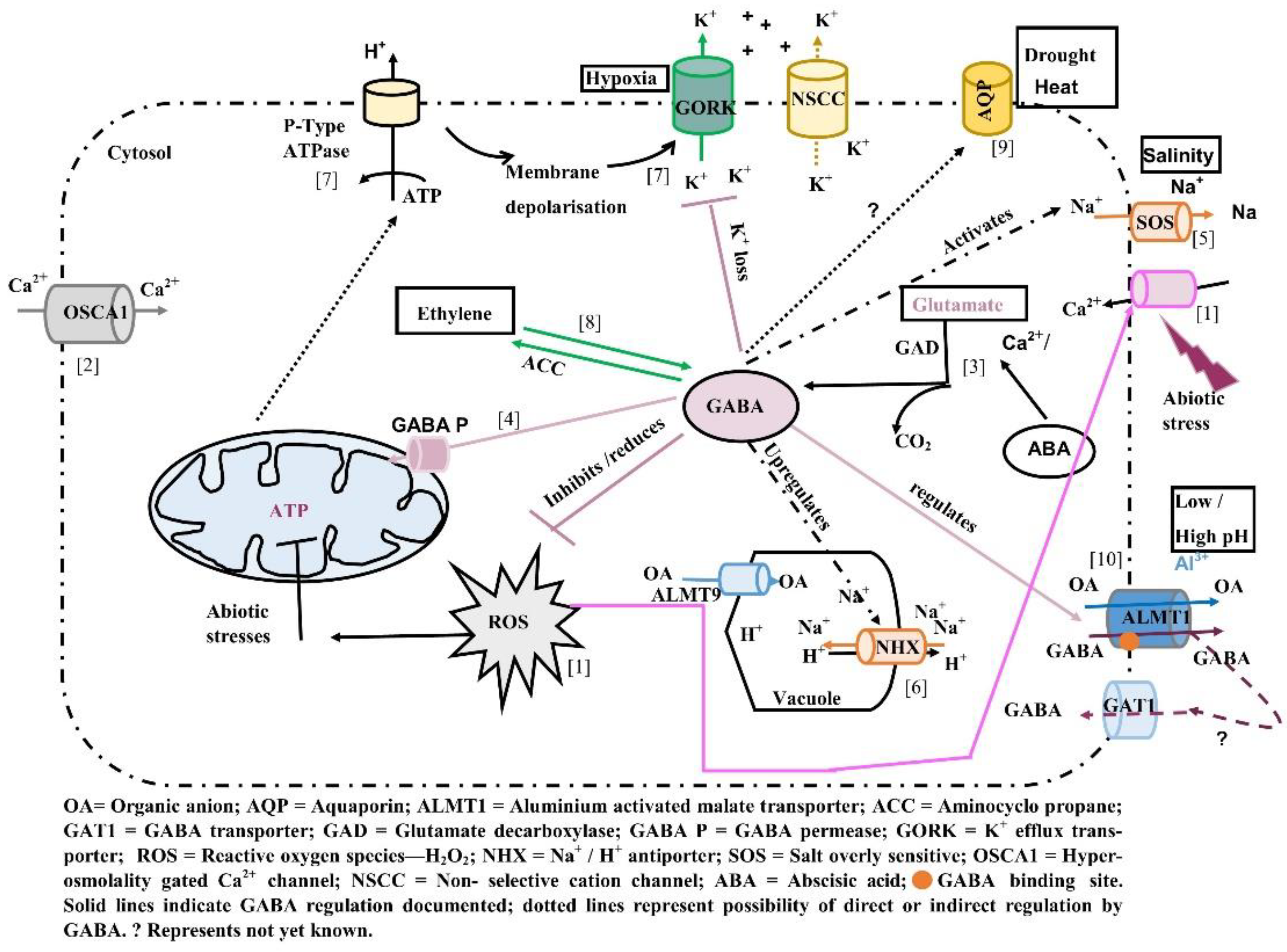
4. GABA Is Involved in Cross Talk with Other Stress Signalling Pathways
5. GABA Gated Channels
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steward, F.C. γ-aminobutyric acid, a constituent of the potato tuber? Science 1949, 110, 439–440. [Google Scholar]
- Roberts, E.; Frankel, S. γ-aminobutyric acid in brain: Its formation from glutamic acid. J. Biol. Chem. 1950, 187, 55–63. [Google Scholar] [CrossRef]
- Awapara, J.; Landua, A.J.; Fuerst, R.; Seale, B. Free γ-aminobutyric acid in brain. J. Biol. Chem. 1950, 187, 35–39. [Google Scholar] [CrossRef]
- Watanabe, M.; Fukuda, A. Development and regulation of chloride homeostasis in the central nervous system. Front. Cell. Neurosci. 2015, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ari, Y.; Gaiarsa, J.-L.; Tyzio, R.; Khazipov, R. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 2007, 87, 1215–1284. [Google Scholar] [CrossRef]
- Li, K.; Xu, E. The role and the mechanism of γ-aminobutyric acid during central nervous system development. Neurosci. Bull. 2008, 24, 195. [Google Scholar] [CrossRef] [Green Version]
- Erdö, S.L.; Wolff, J.R. γ-Aminobutyric Acid Outside the Mammalian Brain. J. Neurochem. 1990, 54, 363–372. [Google Scholar] [CrossRef]
- Barragan, A.; Weidner, J.M.; Jin, Z.; Korpi, E.; Birnir, B. GABAergic signalling in the immune system. Acta Physiol. 2015, 213, 819–827. [Google Scholar] [CrossRef]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef]
- Narayan, V.S.; Nair, P. Metabolism, enzymology and possible roles of 4-aminobutyrate in higher plants. Phytochemistry 1990, 29, 367–375. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef]
- Bouche, N.; Fait, A.; Bouchez, D.; Moller, S.G.; Fromm, H. Mitochondrial succinic-semialdehyde dehydrogenase of the gamma-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 6843–6848. [Google Scholar] [CrossRef] [Green Version]
- Bouche, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. Gamma-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef] [PubMed]
- Bown, A.; Shelp, B. The metabolism and functions of -aminobutyric acid. Plant Physiol. Biochem. 1997, 115, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Gao, X.Q.; Wang, F.; Dong, Y.; Li, X.; Zhang, X.S. Transcriptional evidence for inferred pattern of pollen tube-stigma metabolic coupling during pollination. PLoS ONE 2014, 9, e107046. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Kinnersley, A.M. Physiological evidence for GABA receptors in plants. Plant Biol. 1999, 1999, 153. [Google Scholar]
- Bouche, N.; Lacombe, B.; Fromm, H. GABA signaling: A conserved and ubiquitous mechanism. Trends Cell Biol. 2003, 13, 607–610. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193–194, 130–135. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not just a metabolite. Trends Plant Sci. 2016, 21, 811–813. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.; Huang, X.; Cui, J.; Shabala, L.; Zhou, M.; Yu, M.; Shabala, S. Hypoxia-induced increase in GABA content is essential for restoration of membrane potential and preventing ROS-induced disturbance to ion homeostasis. Plant Commun. 2021, 2, 100188. [Google Scholar] [CrossRef]
- Baum, G.; Chen, Y.; Arazi, T.; Takatsuji, H.; Fromm, H. A plant glutamate decarboxylase containing a calmodulin binding domain. Cloning, sequence, and functional analysis. J. Biol. Chem. 1993, 268, 19610–19617. [Google Scholar] [CrossRef]
- Akama, K.; Akihiro, T.; Kitagawa, M.; Takaiwa, F. Rice (Oryza sativa) contains a novel isoform of glutamate decarboxylase that lacks an authentic calmodulin-binding domain at the C-terminus. Biochim. Et Biophys. Acta (BBA)-Gene Struct. Expr. 2001, 1522, 143–150. [Google Scholar] [CrossRef]
- Trobacher, C.P.; Zarei, A.; Liu, J.; Clark, S.M.; Bozzo, G.G.; Shelp, B.J. Calmodulin-dependent and calmodulin-independent glutamate decarboxylases in apple fruit. BMC Plant Biol. 2013, 13, 144. [Google Scholar] [CrossRef] [Green Version]
- Mei, X.; Chen, Y.; Zhang, L.; Fu, X.; Wei, Q.; Grierson, D.; Zhou, Y.; Huang, Y.; Dong, F.; Yang, Z. Dual mechanisms regulating glutamate decarboxylases and accumulation of gamma-aminobutyric acid in tea (Camellia sinensis) leaves exposed to multiple stresses. Sci. Rep. 2016, 6, 23685. [Google Scholar] [CrossRef] [Green Version]
- Baum, G.; Lev-Yadun, S.; Fridmann, Y.; Arazi, T.; Katsnelson, H.; Zik, M.; Fromm, H. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 1996, 15, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Kinnersley, A.M.; Lin, F. Receptor modifiers indicate that 4-aminobutyric acid (GABA) is a potential modulator of ion transport in plants. Plant Growth Regul. 2000, 32, 65–76. [Google Scholar] [CrossRef]
- Palanivelu, R.; Brass, L.; Edlund, A.F.; Preuss, D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 2003, 114, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Beuve, N.; Rispail, N.; Laine, P.; CLIQUET, J.B.; Ourry, A.; Le Deunff, E. Putative role of γ-aminobutyric acid (GABA) as a long-distance signal in up-regulation of nitrate uptake in Brassica napus L. Plant Cell Environ. 2004, 27, 1035–1046. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, J.M.; Singh, N.K.; Cherry, J.H.; Locy, R.D. GABA increases the rate of nitrate uptake and utilization in Arabidopsis roots. Plant Biol. 2000, 2000, 133. [Google Scholar]
- Barbosa, J.M.; Singh, N.K.; Cherry, J.H.; Locy, R.D. Nitrate uptake and utilization is modulated by exogenous gamma-aminobutyric acid in Arabidopsis thaliana seedlings. Plant Physiol. Biochem. 2010, 48, 443–450. [Google Scholar] [CrossRef]
- Jin, H.; Dilworth, M.; Glenn, A. 4-Aminobutyrate is not available to bacteroids of cowpea Rhizobium MNF2030 in snake bean nodules. Arch. Microbiol. 1990, 153, 455–462. [Google Scholar] [CrossRef]
- Miller, R.; McRae, D.; Joy, K. Glutamate and g-aminobutyrate metabolism in isolated Rhizobium meliloti bacteroids. Mol. Plant-Microbe Interact. 1991, 4, 37–45. [Google Scholar] [CrossRef]
- Sulieman, S. Does GABA increase the efficiency of symbiotic N2 fixation in legumes? Plant Signal Behav. 2011, 6, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Diaz, C.; Lemaître, T.; Christ, A.; Azzopardi, M.; Kato, Y.; Sato, F.; Morot-Gaudry, J.-F.; Le Dily, F.; Masclaux-Daubresse, C. Nitrogen recycling and remobilization are differentially controlled by leaf senescence and development stage in Arabidopsis under low nitrogen nutrition. Plant Physiol. 2008, 147, 1437–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, W.L.; Shelp, B.J. A potential role for gamma-hydroxybuty rate production in redox homeostasis. Plant Biol. 2006, 2006, 219. [Google Scholar]
- Allan, W.L.; Shelp, B.J. Fluctuations of γ-aminobutyrate, γ-hydroxybutyrate, and related amino acids in Arabidopsis leaves as a function of the light–dark cycle, leaf age, and N stress. Botany 2006, 84, 1339–1346. [Google Scholar]
- Renault, H.; Roussel, V.; El Amrani, A.; Arzel, M.; Renault, D.; Bouchereau, A.; Deleu, C. The Arabidopsis pop2-1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol. 2010, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Renault, H.; El Amrani, A.; Palanivelu, R.; Updegraff, E.P.; Yu, A.; Renou, J.P.; Preuss, D.; Bouchereau, A.; Deleu, C. GABA accumulation causes cell elongation defects and a decrease in expression of genes encoding secreted and cell wall-related proteins in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 894–908. [Google Scholar] [CrossRef] [Green Version]
- Michaeli, S.; Fromm, H. Closing the loop on the GABA shunt in plants: Are GABA metabolism and signaling entwined? Front. Plant Sci. 2015, 6, 419. [Google Scholar] [CrossRef] [Green Version]
- Saikusa, T.; Horino, T.; Mori, Y. Distribution of free amino acids in the rice kernel and kernel fractions and the effect of water soaking on the distribution. J. Agric. Food Chem. 1994, 42, 1122–1125. [Google Scholar] [CrossRef]
- Dluzniewska, P.; Gessler, A.; Kopriva, S.; Strnad, M.; NOVÁK, O.; Dietrich, H.; Rennenberg, H. Exogenous supply of glutamine and active cytokinin to the roots reduces NO3–uptake rates in poplar. Plant Cell Environ. 2006, 29, 1284–1297. [Google Scholar] [CrossRef] [Green Version]
- Mazzucotelli, E.; Tartari, A.; Cattivelli, L.; Forlani, G. Metabolism of gamma-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J. Exp. Bot. 2006, 57, 3755–3766. [Google Scholar] [CrossRef] [Green Version]
- Vannini, C.; Iriti, M.; Bracale, M.; Locatelli, F.; Faoro, F.; Croce, P.; Pirona, R.; Di Maro, A.; Coraggio, I.; Genga, A. The ectopic expression of the rice Osmyb4 gene in Arabidopsis increases tolerance to abiotic, environmental and biotic stresses. Physiol. Mol. Plant Pathol. 2006, 69, 26–42. [Google Scholar] [CrossRef]
- Xing, S.G.; Jun, Y.B.; Hau, Z.W.; Liang, L.Y. Higher accumulation of γ-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol. Biochem. 2007, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Bor, M.; Seckin, B.; Ozgur, R.; Yılmaz, O.; Ozdemir, F.; Turkan, I. Comparative effects of drought, salt, heavy metal and heat stresses on gamma-aminobutryric acid levels of sesame (Sesamum indicum L.). Acta Physiol. Plant. 2009, 31, 655–659. [Google Scholar] [CrossRef]
- Patterson, J.H.; Newbigin, E.; Tester, M.; Bacic, A.; Roessner, U. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar]
- Al-Quraan, N.A.; Locy, R.D.; Singh, N.K. Implications of paraquat and hydrogen peroxide-induced oxidative stress treatments on the GABA shunt pathway in Arabidopsis thaliana calmodulin mutants. Plant Biotechnol. Rep. 2011, 5, 225–234. [Google Scholar] [CrossRef]
- Akçay, N.; Bor, M.; Karabudak, T.; Özdemir, F.; Türkan, İ. Contribution of Gamma amino butyric acid (GABA) to salt stress responses of Nicotiana sylvestris CMSII mutant and wild type plants. J. Plant Physiol. 2012, 169, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, R.; Chen, H.; Song, Y.; Gu, Z. Accumulation of γ-aminobutyric acid in germinated soybean (Glycine max L.) in relation to glutamate decarboxylase and diamine oxidase activity induced by additives under hypoxia. Eur. Food Res. Technol. 2012, 234, 679–687. [Google Scholar] [CrossRef]
- Vergara, R.; Parada, F.; Rubio, S.; Pérez, F.J. Hypoxia induces H2O2 production and activates antioxidant defence system in grapevine buds through mediation of H2O2 and ethylene. J. Exp. Bot. 2012, 63, 4123–4131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayyar, H.; Kaur, R.; Kaur, S.; Singh, R. γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J. Plant Growth Regul. 2014, 33, 408–419. [Google Scholar] [CrossRef]
- Mekonnen, D.W.; Flügge, U.-I.; Ludewig, F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016, 245, 25–34. [Google Scholar] [CrossRef]
- Fougere, F.; Le Rudulier, D.; Streeter, J.G. Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiol. 1991, 96, 1228–1236. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, Y.; Du, Y.; Chen, S.; Tang, H. Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. J. Proteome Res. 2011, 10, 1904–1914. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W.; Meng, Y.; Xie, T.; Li, L.; Li, J.; Wei, S. γ-Aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Hamoud, Y.A.; Shaghaleh, H.; Khan, N.U.; Yang, R.; Tang, B. GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int. J. Mol. Sci. 2019, 20, 5709. [Google Scholar] [CrossRef] [Green Version]
- Salvatierra, A.; Pimentel, P.; Almada, R.; Hinrichsen, P. Exogenous GABA application transiently improves the tolerance to root hypoxia on a sensitive genotype of Prunus rootstock. Environ. Exp. Bot. 2016, 125, 52–66. [Google Scholar] [CrossRef]
- Abdel Razik, E.S.; Alharbi, B.M.; Pirzadah, T.B.; Alnusairi, G.S.; Soliman, M.H.; Hakeem, K.R. γ-Aminobutyric acid (GABA) mitigates drought and heat stress in sunflower (Helianthus annuus L.) by regulating its physiological, biochemical and molecular pathways. Physiol. Plant. 2021, 172, 505–527. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Peng, C.; Shi, H.; Yang, J.; He, M.; Zhang, M.; Zhou, Y.; Duan, L. Exogenous Gamma-aminobutyric Acid Coordinates Active Oxygen and Amino Acid Homeostasis to Enhance Heat Tolerance in Wheat Seedlings. J. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Priya, M.; Sharma, L.; Kaur, R.; Bindumadhava, H.; Nair, R.M.; Siddique, K.; Nayyar, H. GABA (γ-aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci. Rep. 2019, 9, 7788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xiong, F.; Nong, S.; Liao, J.; Xing, A.; Shen, Q.; Ma, Y.; Fang, W.; Zhu, X. Effects of nitric oxide on the GABA, polyamines, and proline in tea (Camellia sinensis) roots under cold stress. Sci. Rep. 2020, 10, 12240. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, X.; Yang, S.; Zhang, Z.; Ye, X.; Li, J.; Qi, H.; Hu, X. Crosstalk between GABA and ALA to improve antioxidation and cell expansion of tomato seedling under cold stress. Environ. Exp. Bot. 2020, 180, 104228. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Wei, B.; Cheng, S.; Zhou, Q.; Ji, S. GABA application improves the mitochondrial antioxidant system and reduces peel browning in ‘Nanguo’pears after removal from cold storage. Food Chem. 2019, 297, 124903. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Huang, X.; Yang, K.; Gao, S.; Du, R. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hortic. 2014, 168, 132–137. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.; Luo, Y.; Sun, X.; Wang, J.; Luo, T.; Zeng, Y.; Xu, J.; Deng, X.; Cheng, Y. Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 2017, 216, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, P.; Khosravi-Nejad, F.; Hatamnia, A.A.; Mehr, R.S. Impact of postharvest exogenous γ-aminobutyric acid treatment on cucumber fruit in response to chilling tolerance. Physiol. Mol. Biol. Plants 2017, 23, 827–836. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Razavi, F.; Karamneghad, F. Maintaining the postharvest nutritional quality of peach fruits by γ-Aminobutyric acid. Iran. J. Plant Physiol. 2016, 5, 1457–1463. [Google Scholar]
- Lancien, M.; Roberts, M.R. Regulation of Arabidopsis thaliana 14-3-3 gene expression by gamma-aminobutyric acid. Plant Cell Environ. 2006, 29, 1430–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Dubey, A.K.; Upadhyay, A.K.; Gautam, A.; Ranjan, R.; Srikishna, S.; Sahu, N.; Behera, S.K.; Mallick, S. GABA accretion reduces Lsi-1 and Lsi-2 gene expressions and modulates physiological responses in Oryza sativa to provide tolerance towards arsenic. Sci. Rep. 2017, 7, 8786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Cheng, B.; Zeng, W.; Zhang, X.; Peng, Y. Proteomic and Metabolomic Profilings Reveal Crucial Functions of γ-Aminobutyric Acid in Regulating Ionic, Water, and Metabolic Homeostasis in Creeping Bentgrass under Salt Stress. J. Proteome Res. 2020, 19, 769–780. [Google Scholar] [CrossRef]
- Li, Z.; Huang, T.; Tang, M.; Cheng, B.; Peng, Y.; Zhang, X. iTRAQ-based proteomics reveals key role of γ-aminobutyric acid (GABA) in regulating drought tolerance in perennial creeping bentgrass (Agrostis stolonifera). Plant Physiol. Biochem. 2019, 145, 216–226. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kobayashi, Y.; Sugimoto, M.; Lakshmanan, V.; Iuchi, S.; Kobayashi, M.; Bais, H.P.; Koyama, H. Characterization of the complex regulation of AtALMT1 expression in response to phytohormones and other inducers. Plant Physiol. 2013, 162, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.R. Does GABA Act as a Signal in Plants? Hints from Molecular Studies: Hints from Molecular Studies. Plant Signal. Behav. 2007, 2, 408–409. [Google Scholar] [CrossRef] [Green Version]
- Pavlíková, D.; Zemanová, V.; Procházková, D.; Pavlík, M.; Száková, J.; Wilhelmová, N. The long-term effect of zinc soil contamination on selected free amino acids playing an important role in plant adaptation to stress and senescence. Ecotoxicol. Environ. Saf. 2014, 100, 166–170. [Google Scholar] [CrossRef]
- Tuteja, N. Abscisic acid and abiotic stress signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Dittrich, M.; Mueller, H.M.; Bauer, H.; Peirats-Llobet, M.; Rodriguez, P.L.; Geilfus, C.-M.; Carpentier, S.C.; Al Rasheid, K.A.; Kollist, H.; Merilo, E. The role of Arabidopsis ABA receptors from the PYR/PYL/RCAR family in stomatal acclimation and closure signal integration. Nat. Plants 2019, 5, 1002–1011. [Google Scholar]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E., Jr. Ethylene in Plant Biology; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Morgan, P.W.; Drew, M.C. Ethylene and plant responses to stress. Physiol. Plant. 1997, 100, 620–630. [Google Scholar] [CrossRef]
- Kathiresan, A.; Tung, P.; Chinnappa, C.C.; Reid, D.M. gamma-Aminobutyric acid stimulates ethylene biosynthesis in sunflower. Plant Physiol. 1997, 115, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Tian, Q.; Zhang, X.; Ramesh, S.; Gilliham, M.; Tyerman, S.D.; Zhang, W.-H. Ethylene negatively regulates aluminium-induced malate efflux from wheat roots and tobacco cells transformed with TaALMT1. J. Exp. Bot. 2014, 65, 2415–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevrot, R.; Rosen, R.; Haudecoeur, E.; Cirou, A.; Shelp, B.J.; Ron, E.; Faure, D. GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 2006, 103, 7460–7464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouche, N.; Fait, A.; Zik, M.; Fromm, H. The root-specific glutamate decarboxylase (GAD1) is essential for sustaining GABA levels in Arabidopsis. Plant Mol. Biol. 2004, 55, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.-M.; Chiu, J.; Hsieh, M.-H.; Meisel, L.; Oliveira, I.C.; Shin, M.; Coruzzi, G. Glutamate-receptor genes in plants. Nature 1998, 396, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shabala, S. Mechanisms of cytosolic calcium elevation in plants: The role of ion channels, calcium extrusion systems and NADPH oxidase-mediated ‘ROS-Ca2+ Hub’. Funct. Plant Biol. 2017, 45, 9–27. [Google Scholar] [CrossRef]
- Gordeeva, A.; Zvyagilskaya, R.; Labas, Y.A. Cross-talk between reactive oxygen species and calcium in living cells. Biochemistry 2003, 68, 1077–1080. [Google Scholar]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B. OSCA1 mediates osmotic-stress-evoked Ca 2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef]
- Shelp, B.J.; Mullen, R.T.; Waller, J.C. Compartmentation of GABA metabolism raises intriguing questions. Trends Plant Sci. 2012, 17, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Wu, Q.; Chen, J.; Shabala, L.; Mithöfer, A.; Wang, H.; Qu, M.; Yu, M.; Cui, J.; Shabala, S. GABA operates upstream of H+-ATPase and improves salinity tolerance in Arabidopsis by enabling cytosolic K+ retention and Na+ exclusion. J. Exp. Bot. 2019, 70, 6349–6361. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Q.; Shi, Z.; Jiang, Z.P.; Qi, L.W.; Sun, X.M.; Li, C.X.; Liu, J.F.; Xiao, W.F.; Zhang, S.G. Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: Regulatory roles for H2O2 and ethylene production. Plant Cell Env. 2010, 33, 149–162. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004, 37, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Žárský, V. Signal transduction: GABA receptor found in plants. Nat. Plants 2015, 1, 15115. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Ryan, P.R.; Sasaki, T.; Yamamoto, Y.; Sullivan, W.; Tyerman, S.D. Characterization of the TaALMT1 protein as an Al3+-activated anion channel in transformed tobacco (Nicotiana tabacum L.) cells. Plant Cell Physiol. 2008, 49, 1316–1330. [Google Scholar] [CrossRef] [Green Version]
- Pineros, M.A.; Cançado, G.M.; Kochian, L.V. Novel properties of the wheat aluminum tolerance organic acid transporter (TaALMT1) revealed by electrophysiological characterization in Xenopus oocytes: Functional and structural implications. Plant Physiol. 2008, 147, 2131–2146. [Google Scholar] [CrossRef] [Green Version]
- Meyer, S.; Mumm, P.; Imes, D.; Endler, A.; Weder, B.; Al-Rasheid, K.A.; Geiger, D.; Marten, I.; Martinoia, E.; Hedrich, R. AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 2010, 63, 1054–1062. [Google Scholar] [CrossRef] [Green Version]
- Palmer, A.J.; Baker, A.; Muench, S.P. The varied functions of aluminium-activated malate transporters–much more than aluminium resistance. Biochem. Soc. Trans. 2016, 44, 856–862. [Google Scholar]
- Piñeros, M.A.; Cançado, G.M.; Maron, L.G.; Lyi, S.M.; Menossi, M.; Kochian, L.V. Not all ALMT1-type transporters mediate aluminum-activated organic acid responses: The case of ZmALMT1–an anion-selective transporter. Plant J. 2008, 53, 352–367. [Google Scholar] [CrossRef]
- Kovermann, P.; Meyer, S.; Hörtensteiner, S.; Picco, C.; Scholz-Starke, J.; Ravera, S.; Lee, Y.; Martinoia, E. The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J. 2007, 52, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Delhaize, E.; Ryan, P.R. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995, 107, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, P.; Delhaize, E.; Jones, D. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef]
- Barbier-Brygoo, H.; De Angeli, A.; Filleur, S.; Frachisse, J.-M.; Gambale, F.; Thomine, S.; Wege, S. Anion channels/transporters in plants: From molecular bases to regulatory networks. Annu. Rev. Plant Biol. 2011, 62, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Kamran, M.; Sullivan, W.; Chirkova, L.; Okamoto, M.; Degryse, F.; McLaughlin, M.; Gilliham, M.; Tyerman, S.D. Aluminum-Activated Malate Transporters Can Facilitate GABA Transport. Plant Cell 2018, 30, 1147–1164. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Tyerman, S.D.; Gilliham, M. Cytosolic GABA inhibits anion transport by wheat ALMT1. New Phytol. 2020, 225, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Gilliham, M.; Tyerman, S.D. Linking metabolism to membrane signaling: The GABA–malate connection. Trends Plant Sci. 2016, 21, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabala, L.; Zhang, J.; Pottosin, I.; Bose, J.; Zhu, M.; Fuglsang, A.T.; Velarde-Buendia, A.; Massart, A.; Hill, C.B.; Roessner, U. Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol. 2016, 172, 2445–2458. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, I.; Blatt, M.R. What makes a gate? The ins and outs of Kv-like K+ channels in plants. Trends Plant Sci. 2009, 14, 383–390. [Google Scholar] [CrossRef]
- Eisenach, C.; Papanatsiou, M.; Hillert, E.K.; Blatt, M.R. Clustering of the K+ channel GORK of A rabidopsis parallels its gating by extracellular K+. Plant J. 2014, 78, 203–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adem, G.D.; Chen, G.; Shabala, L.; Chen, Z.-H.; Shabala, S. GORK Channel: A Master Switch of Plant Metabolism? Trends Plant Sci. 2020, 25, 434–445. [Google Scholar] [CrossRef]
- Felle, H.H. pH regulation in anoxic plants. Ann. Bot. 2005, 96, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabala, S.; Bose, J.; Fuglsang, A.T.; Pottosin, I. On a quest for stress tolerance genes: Membrane transporters in sensing and adapting to hostile soils. J. Exp. Bot. 2016, 67, 1015–1031. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaspal, M.; Kanapaddalagamage, M.H.; Ramesh, S.A. Emerging Roles of γ Aminobutyric Acid (GABA) Gated Channels in Plant Stress Tolerance. Plants 2021, 10, 2178. https://doi.org/10.3390/plants10102178
Kaspal M, Kanapaddalagamage MH, Ramesh SA. Emerging Roles of γ Aminobutyric Acid (GABA) Gated Channels in Plant Stress Tolerance. Plants. 2021; 10(10):2178. https://doi.org/10.3390/plants10102178
Chicago/Turabian StyleKaspal, Mona, Madhuka H. Kanapaddalagamage, and Sunita A. Ramesh. 2021. "Emerging Roles of γ Aminobutyric Acid (GABA) Gated Channels in Plant Stress Tolerance" Plants 10, no. 10: 2178. https://doi.org/10.3390/plants10102178





