Alleviation of Chlorpyrifos Toxicity in Maize (Zea mays L.) by Reducing Its Uptake and Oxidative Stress in Response to Soil-Applied Compost and Biochar Amendments
Abstract
:1. Introduction
2. Results and Discussion
2.1. Soil and Amendments Characteristics
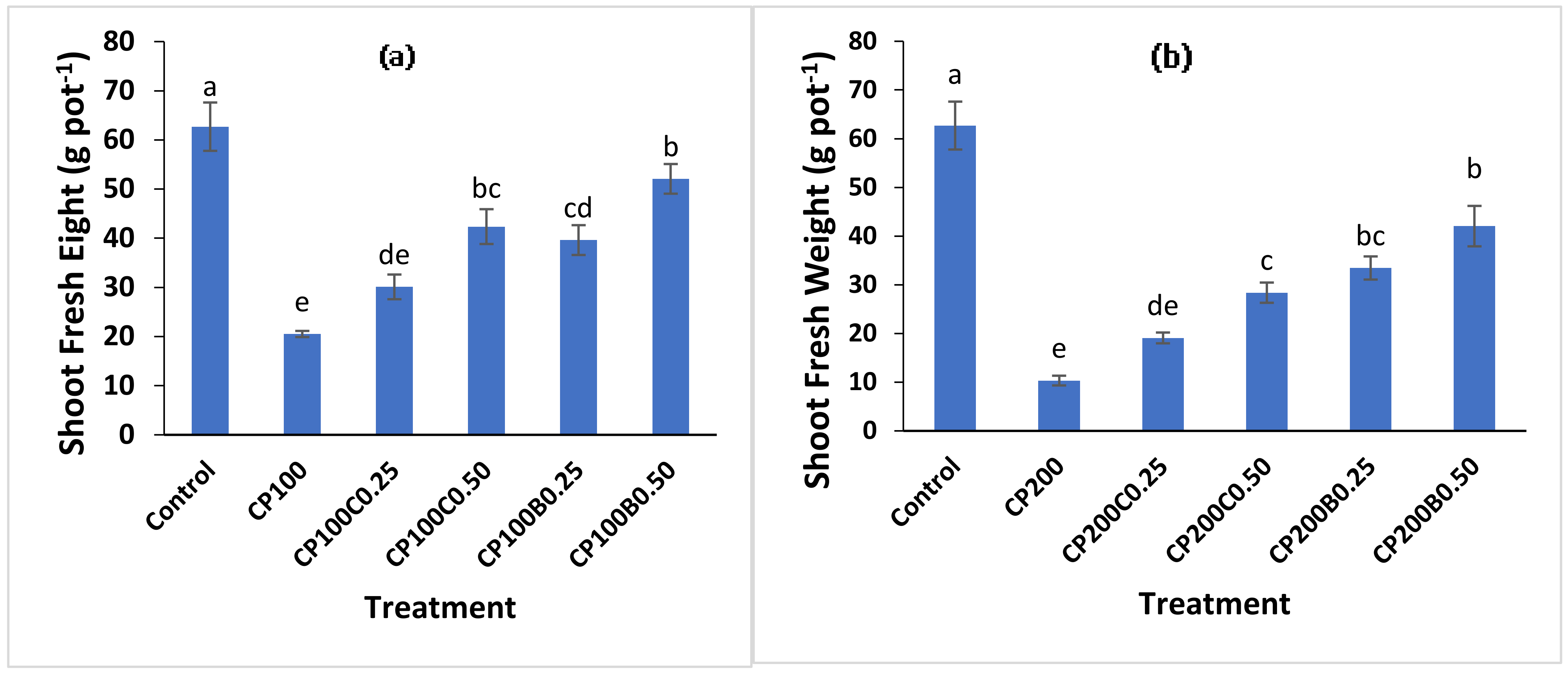
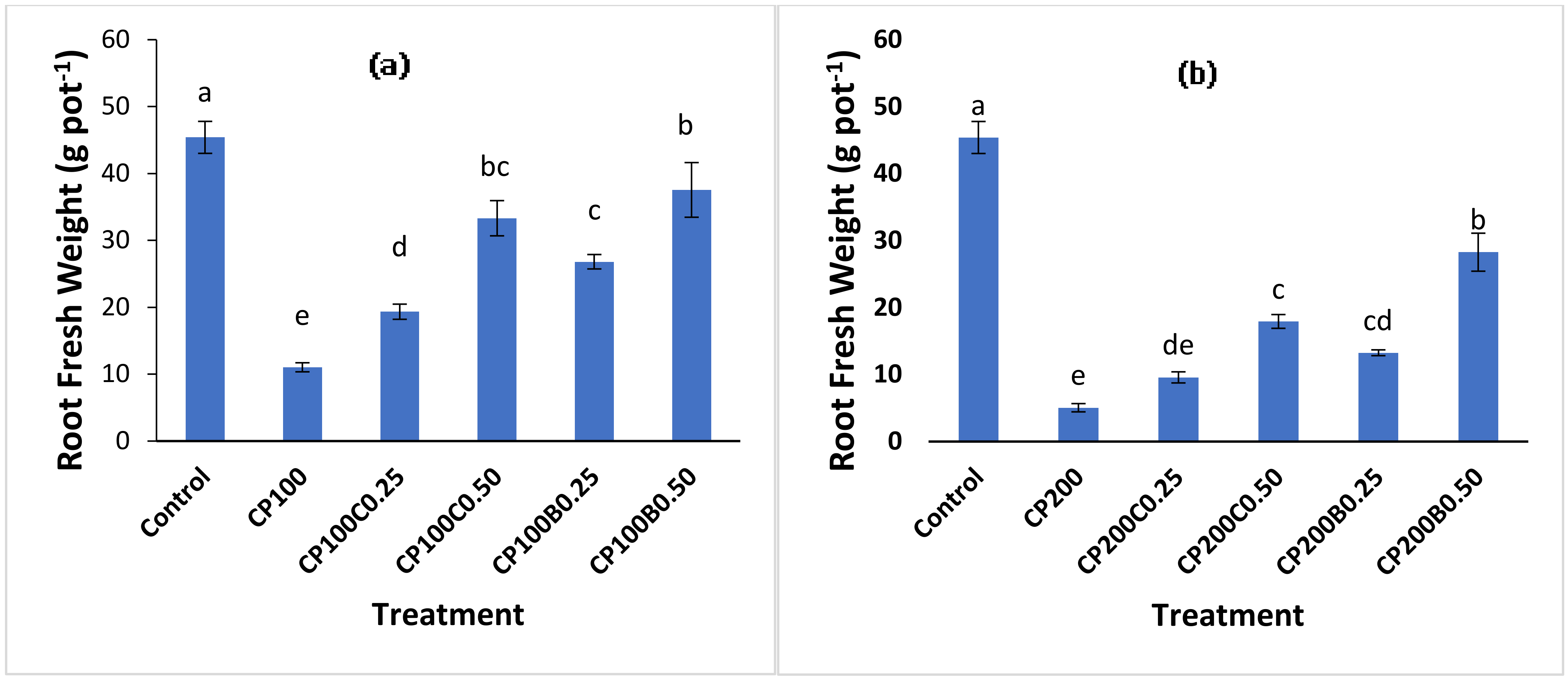
2.2. Plant Growth
2.3. Antioxidant Enzyme Activities of Maize Shoots
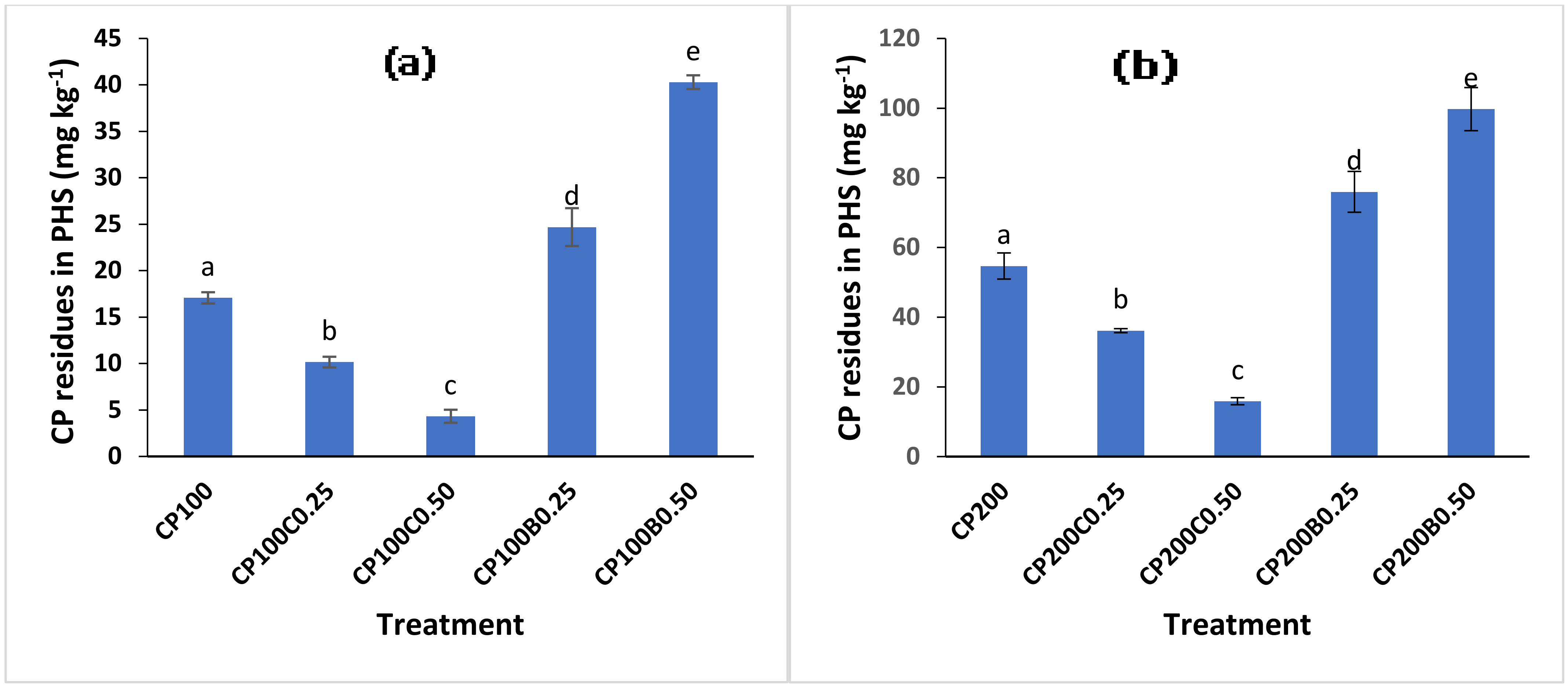
2.4. Chlorpyrifos Residues in Postharvest Soil
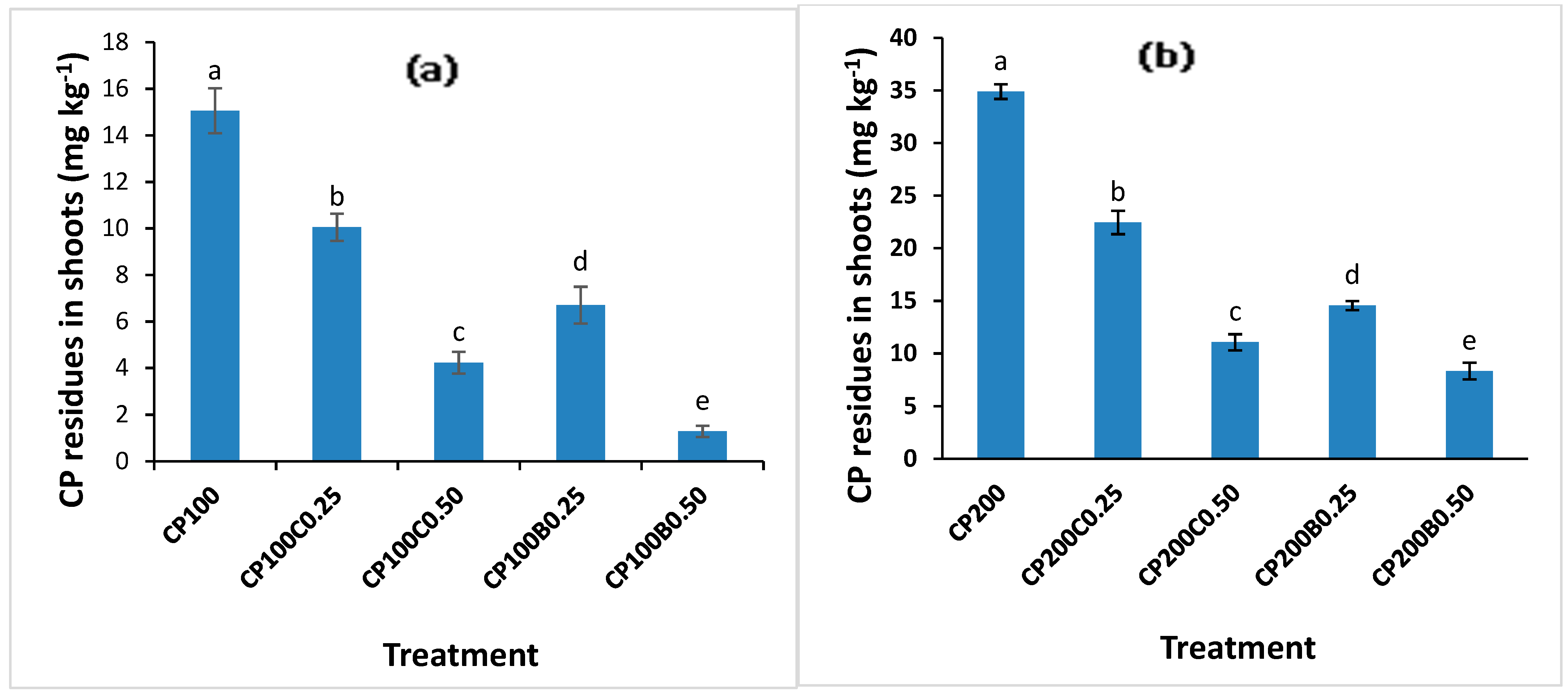
2.5. Chlorpyrifos Concentration in Maize Plants in the Presence and Absence of Organic Amendments
3. Materials and Methods
3.1. Collection and Preparation of Soil and Amendments
3.2. Analytical Methods
3.3. Pesticide and Chemicals
3.4. Plant Growth Experiment
3.5. Residue Extraction and Cleanup
3.6. Residue Analysis
3.7. Extraction and Determination of Enzyme Activities
3.8. Statistical Analysis
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pelosi, C.; Bertrand, C.; Daniele, G.; Coeurdassier, M.; Benoit, P.; Nelieu, S.; Fritsch, C. Residues of currently used pesticides in soils and earthworms: A silent threat? Agric. Ecosyst. Environ. 2021, 305, 107–167. [Google Scholar] [CrossRef]
- Liu, D.B.; Chen, W.W.; Wei, J.H.; Li, X.B.; Wang, Z.; Jiang, X.Y. A highly sensitive, dual-readout assay based on gold nanoparticles for organophosphorus and carbamate pesticides. Anal. Chem. 2012, 84, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Howard, P.H.; Gray, D.A.; Sage, G.W.; Jarvis, W.F. Handbook of Environmental Fate and Exposure 311 Data for Organic Chemicals. Volume II: Solvents; CRC Press: Boca Raton, FL, USA, 1990; Volume IV. [Google Scholar]
- Rajmohan, K.; Chandrasekaran, R.; Varjani, S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Ahad, K.; Anwar, T.; Ahmad, I.; Mohammad, A.; Tahir, S.; Aziz, S.; Baloch, U.K. Determination of insecticides residues in ground water of Mardan Division, NWFP. Pakistan: A case study. Water SA 2000, 26, 409–412. [Google Scholar]
- Parveen, Z.; Khuhro, M.I.; Rafiq, N.; Kausar, N. Evaluation of multiple pesticide residues in apple and citrus fruits, 1999–2001. Bull. Environ. Contam. Toxicol. 2004, 73, 312–318. [Google Scholar] [CrossRef]
- Muhammad, F.; Akhtar, M.; Rahman, Z.U.; Farooq, H.U.; Khaliq, T.; Anwar, M.I. Multi-residue determination of pesticides in the meat of cattle in Faisalabad Pakistan. Egypt Acad. J. Biol. Sci. 2010, 2, 19–28. [Google Scholar]
- Ismail, M.; Ali, R.; Shahid, M.; Khan, M.A.; Zubair, M.; Ali, T.; Khan, Q.M. Genotoxic and hematological effects of chlorpyrifos exposure on freshwater fish Labeorohita. Drug Chem. Toxicol. 2017, 30, 1–5. [Google Scholar]
- Duntas, L.H.; Stathatos, N. Toxic chemicals and thyroid function: Hard facts and lateral thinking. Rev. Endocr. Metab. Dis. 2015, 16, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Q. Exogenous salicylic acid alleviates the toxicity of chlorpyrifos in wheat plants (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2017, 137, 218–224. [Google Scholar] [CrossRef]
- Yu, X.Y.; Ying, G.; Kookana, R.S. Reduced plant uptake of pesticides with biochar additions to soil. Chemosphere 2009, 76, 665–671. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Shan, W.L.; Song, W.C.; Gong, Y.; Liu, X.J. Phytotoxicity and uptake of chlorpyrifos in cabbage. Environ. Chem. Lett. 2011, 9, 547–552. [Google Scholar] [CrossRef]
- Gvozdenac, S.; Indic, D.; Vukovic, S. Phytotoxicity of Chlorpyrifos to White Mustard (Sinapis alba L.) and Maize (Zea mays L.): Potential Indicators of Insecticide Presence in Water. Pestic. Phytomed. 2013, 28, 265–271. [Google Scholar] [CrossRef]
- Dubey, P.; Mishra, A.K.; Shukla, P.; Singh, A.K. Differential sensitivity of barley (Hordeum vulgare L.) to chlorpyrifos and propiconazole: Morphology, cytogenetic assay and photosynthetic pigments. Pestic. Biochem. Phys. 2015, 124, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Hassan, A.U.; Maqbool, S.; Barber, B.; Koskinen, W.C.; Xinhua, P.E.N.G.; Mulla, D.J. Sorption and leaching potential of isoproturon and atrazine in low organic carbon soil of Pakistan under a wheat-maize rotation. Pedosphere 2016, 26, 687–698. [Google Scholar] [CrossRef]
- Tahir, M.; Hassan, A.U.; Zahir, Z.A.; Ur-Rehman, K. Modeling the water retention capacity and hydraulic properties of a manure-amended loam soil and its effect on wheat and maize yield. Int. J. Agric. Biol. 2012, 14, 96–102. [Google Scholar]
- Saleem, M.; Ali, S.; Rehman, M.; Rana, M.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Hussein, M.; Elkelish, A.; et al. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef]
- Javed, M.T.; Saleem, M.H.; Aslam, S.; Rehman, M.; Iqbal, N.; Begum, R.; Ali, S.; Alsahli, A.A.; Alyemeni, M.N.; Wijaya, L. Elucidating silicon-mediated distinct morpho-physio-biochemical attributes and organic acid exudation patterns of cadmium stressed Ajwain (Trachyspermum ammi L.). Plant Physiology and Biochemistry 2020, 157, 23–37. [Google Scholar] [CrossRef]
- Iqbal, S.; Christian, T.; Haroon, Z.K.; Hafiz, M.R.J.; Muhammad, A.; Muhammad, S. Maximizing maize quality, productivity and profitability through a combined use of compost and nitrogen fertilizer in a semi-arid environment in Pakistan. Nutr. Cycl. Agroecosyst. 2017, 7, 197–213. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef]
- Mahmood, F.; Khan, I.; Ashraf, U.; Shahzad, T.; Hussain, S.; Shahid, M.; Ullah, S. Effects of organic and inorganic manures on maize and their residual impact on soil physico-chemical properties. J. Soil Sci. Plant Nutr. 2017, 17, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Cederlund, H.; Börjesson, E.; Stenström, J. Effects of a wood-based biochar on the leaching of pesticides chlorpyrifos, diuron, glyphosate and MCPA. J. Environ. Manag. 2017, 191, 28–34. [Google Scholar] [CrossRef]
- Burgess, R.M.; Perron, M.M.; Friedman, C.L.; Suuberg, E.M.; Pennell, K.G.; Cantwell, M.G.; Ryba, S.A. Evaluation of the effects of coal fly ash amendments on the toxicity of a contaminated marine sediment. Environ. Toxicol. Chem. 2009, 28, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; He, J.; Qi, H.; Li, H.; Boyd, S.A.; Zhang, W. Impact of biochar amendment on the uptake, fate and bioavailability of pharmaceuticals in soil-radish systems. J. Hazard. Mater. 2020, 398, 122852. [Google Scholar] [CrossRef] [PubMed]
- Hilber, I.; Wyss, G.S.; Mader, P.; Bucheli, T.D.; Meier, I.; Vogt, L.; Schulin, R. Influence of activated charcoal amendment to contaminated soil on dieldrin and nutrient uptake by cucumbers. Environ. Pollut. 2009, 157, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.N.; Sheng, G.Y. Enhanced pesticide sorption by soils containing particulate matter from crop residue burns. Environ. Sci. Technol. 2003, 37, 3635–3639. [Google Scholar] [CrossRef] [PubMed]
- Parween, T.; Jan, S.; Fatma, T. Alteration in nitrogen metabolism and plant growth during different developmental stages of green gram (Vigna radiata L.) in response to chlorpyrifos. Acta Physiol. Plant. 2011, 33, 2321. [Google Scholar] [CrossRef]
- Dubey, K.K.; Fulekar, M.H. Effect of pesticides on the seed germination of Cenchrus setigerus and Pennisetum pedicellatum as monocropping and co-cropping system: Implications for rhizospheric bioremediation. Rom. Biotechnol. Lett. 2011, 16, 5908–5918. [Google Scholar]
- Yang, X.; Guoying, G.; Anpeng, P.; Zhao, L.J.L.; Zhang, L.J.; Yuan, P.; He, H.P. Influence of Biochars on Plant Uptake and Dissipation of Two Pesticides in an Agricultural Soil. J. Agric. Food Chem. 2010, 58, 7915–7921. [Google Scholar] [CrossRef]
- Tang, X.; Huang, W.; Guo, J.; Yang, Y.; Tao, R.; Xu, F. Use of Fe-impregnated Biochar to Efficiently Sorb Chlorpyrifos, reduce uptake by Allium fistulosum L. and Enhance Microbial Community diversity. J. Agric. Food Chem. 2017, 65, 5238–5243. [Google Scholar] [CrossRef]
- Antonious, G.F. Decontamination of Pesticide Residues for Sustainable Agriculture. JSM. Environ. Sci. Ecol. 2015, 3, 1014. [Google Scholar]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strat. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Song, H.; Yin, X.; Chen, G.F.; Yang, H. Biological responses of wheat (Triticum aestivum L.) plants to the herbicide chlorotoluron in soils. Chemosphere 2007, 69, 1779–1787. [Google Scholar] [CrossRef]
- Chen, S.; Chen, M.; Wang, Z.; Qiu, W.; Wang, J.; Shen, Y.; Ge, S. Toxicological effects of chlorpyrifos on growth, enzyme activity and chlorophyll a synthesis of freshwater microalgae. Environ. Toxicol. Pharmacol. 2016, 45, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Parween, T.A.; Jan, S.; Fatma, T. Evaluation of oxidative stress in Vigna radiata L. in response to chlorpyrifos. Int. J. Environ. Sci. Technol. 2012, 9, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Bashir, F.; Siddiqi, T.O.; Iqbal, M. The anti-oxidative response system in Glycine max L.) Merr. exposed to deltamethrin, a synthetic pyrethroid insecticide. Environ. Pollut. 2007, 147, 94–100. [Google Scholar] [CrossRef]
- Jianga, L.; Maa, L.; Suia, Y.; Hna, S.Q.; Wua, Z.Y.; Fenga, Y.X.; Yanga, H. Effect of manure compost on the herbicide prometryne bioavailability to wheat plants. J. Hazard. Mater. 2010, 184, 337–344. [Google Scholar] [CrossRef]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Lead-induced stress, which triggers the production of nitric oxide (NO) and superoxide anion (O2−) in Arabidopsis peroxisomes, affects catalase activity. Nitric Oxide 2017, 68, 103–110. [Google Scholar] [CrossRef]
- Azcon, R.; del, M.; Peralvarez, C.; Biro, B.; Roldan, A.; Ruiz-Lozano, J.M. Antioxidant activities and metal acquisition in mycorrhizal plants growing in a heavy-metal multicontaminated soil amended with treated ligno cellulosic agrowaste. Appl. Soil Ecol. 2009, 41, 168–177. [Google Scholar] [CrossRef]
- Fang, H.; Yun-long, Y.; Xiao Wang, M.S.; Xiao-mao, W.; Jing-quan, Y. Dissipation of chlorpyrifos in pakchoi-vegetated soil in a greenhouse. J. Environ. Sci. 2006, 18, 760–764. [Google Scholar]
- Mutua, G.K.; Ngigi, A.N.; Getenga, Z.M. Chlorpyrifos Degradation in Soils with Different Treatment Regimes within Nzoia River Drainage Basin, Kenya. Bull. Environ. Contam. Toxicol. 2015, 94, 387–392. [Google Scholar] [CrossRef]
- Garcia-Jaramillo, M.; Redondo-Gómez, S.; Barcia-Piedras, J.M.; Aguilar, M.; Jurado, V.; Hermosín, M.C.; Cox, L. Dissipation and effects of tricyclazole on soil microbial communities and rice growth as affected by amendment with alperujo compost. Sci. Total Environ. 2016, 550, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Kravvariti, K.; Tsiropoulos, N.G.; Karpouzas, D.G. Degradation and adsorption of terbuthylazine and chlorpyrifos in biobedbiomixtures from composted cotton crop residues. Pest Manag. Sci. 2010, 66, 1122–1128. [Google Scholar] [CrossRef]
- Lopez-Pin eiro, A.; Cabrera, D.; Albarran, A.; Pena, D. Influence of two-phase olive mill waste application to soil on terbuthylazine behavior and persistence under controlled and field conditions. J. Soils Sediments 2011, 11, 771–782. [Google Scholar] [CrossRef]
- Copaja, S.V.; Vergara, R.; Bravo, H.R. Bioavailability of Chlorpyrifos in Wheat Plants (Triticum aestivun L.). Agric. Sci. 2014, 5, 660. [Google Scholar]
- Hamaker, J.W.; Thompson, J.M. Adsorption of organic chemicals in the soil environment. In Organic Chemicals in the Soil Environment; Hamaker, J.W., Goring, C.A.I., Eds.; Marcel Decker Inc.: New York, NY, USA, 1972; Volume 1, pp. 49–144. [Google Scholar]
- Moyo, F.; Tandlich, R.; Wilhelmi, B.S.; Balaz, S. Sorption of hydrophobic organic compounds on natural sorbents and organoclays from aqueous and non-aqueous solutions: A mini-review. Int. J. Environ. Res. Public Health 2014, 11, 5020–5048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Feng, D.; He, J.X.; Li, F.Z.; Yu, H.M.; Ge, C.J. Influence of biochar amendments to soil on the mobility of atrazine using sorption-desorption and soil thin-layer chromatography. Ecol. Eng. 2017, 99, 381–390. [Google Scholar] [CrossRef]
- Zbytniewski, R.; Buszewski, B. Sorption of pesticides in soil and compost. Pol. J. Environ. Stud. 2002, 11, 179–184. [Google Scholar]
- Medina, J.; Monreal, C.; Chabot, D.; Meier, S.; Gonzalez, M.E.; Morales, E.; Cornejo, P. Microscopic and spectroscopic characterization of humic substances from a compost amended copper contaminated soil: Main features and their potential effects on Cu immobilization. Environ. Sci. Pollut. Res. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Yu, X.; Liu, X. Dissipation of chlorpyrifos and residue analysis in rice, soil and water under paddy field conditions. Ecotoxicol. Environ. Saf. 2012, 78, 276–280. [Google Scholar] [CrossRef]
- Khorram, M.S.; Zheng, Y.; Lin, D.; Zhang, Q.; Fang, H.; Yu, Y. Dissipation of fomesafen in biochar-amended soil and its availability to corn (Zea mays L.) and earthworm (Eisenia fetida L.). J. Soils Sediments 2016, 16, 2439–2448. [Google Scholar] [CrossRef]
- Murtaza, B.; Murtaza, G.; Saqib, M.; Khaliq, A. Efficiency of nitrogen use in rice-wheat cropping system in salt-affected soils with contrasting texture. Pak. J. Agric. Sci. 2014, 51, 431–441. [Google Scholar]
- Sanchez, M.E.; Lindao, E.; Margaleff, D.; Martınez, O.; Moran, A. Pyrolysis of agricultural residues from rape and sunflowers: Production and characterization of bio-fuels and biochar soil management. J. Anal. Appl. Pyrol. 2009, 85, 142–144. [Google Scholar] [CrossRef]
- Ahmad, R.; Shahzad, S.M.; Khalid, A.; Arshad, M.; Mahmood, M.H. Growth and yield response of wheat (Triticum aestivum L.) and maize (Zea mays L.) to nitrogen and L-tryptophan enriched compost. Pak. J. Bot. 2007, 39, 541. [Google Scholar]
- Salinity Lab. Staff US. Diagnosis and Improvement of Saline and Alkali Soils; USDA Handbook No. 60; USDA: Washington, DC, USA, 1954.
- Haynes, R.J.; Murtaza, G.; Naidu, R. Inorganic and organic constituents and contaminants of biosolids: Implications for land application. Adv. Agron. 2009, 104, 165–267. [Google Scholar]
- Wolf, B. The comprehensive system of leaf analysis and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1983, 13, 1035–1059. [Google Scholar] [CrossRef]
- Barbano, D.M.; Clark, J.L.; Dunham, C.E.; Flemin, R.J. Kjeldahl method for determination of total nitrogen content of milk: Collaborative study. J. Assoc. Off. Anal. Chem. 1990, 73, 849–859. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Usman, A.R.; El-Naggar, A.H.; Aly, A.A.; Ibrahim, H.M.; Elmaghraby, S.; Al-Omran, A. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J. Biol. Sci. 2015, 22, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Aziz, H.; Sabir, M.; Ahmad, H.R.; Aziz, T.; Zia-ur-Rehman, M.; Hakeem, K.R.; Ozturk, M. Alleviating effect of calcium on nickel toxicity in rice. CLEAN-Soil Air Water 2015, 43, 901–909. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Reis, S.K. Superoxide dismutase occurrence in higher plants. Plant Physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef]
- Teranishi, Y.; Tanaka, A.; Osumi, M.; Fukui, S. Catalase activity of hydrocarbon utilizing candida yeast. Agric. Biol. Chem. 1974, 38, 1213–1216. [Google Scholar] [CrossRef]
- Castillo, F.I.; Penel, I.; Greppin, H. Peroxidase release induced by ozone in Sedum album leaves. Plant Physiol. 1984, 74, 846–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics; McGraw Hill Book Co. Inc.: New York, NY, USA, 1997. [Google Scholar]




| Characteristic | Soil | Compost | Biochar |
|---|---|---|---|
| Texture | Sandy clay loam | -- | -- |
| Sand (%) | 56.4 ± 1.04 | -- | -- |
| Silt (%) | 18.9 ± 0.98 | -- | -- |
| Clay (%) | 24.7 ± 1.01 | -- | -- |
| pHw(1:10) | 7.44 ± 0.10 | 6.25 ± 0.09 | 7.89 ± 0.08 |
| ECw(1:10) (dS m−1) | 3.21 ± 0.08 | 3.10 ± 0.15 | 4.01 ± 0.08 |
| TSS (mmolc L−1) | 32 ± 0.20 | -- | -- |
| CaCO3 | 4.91 | -- | -- |
| Total organic carbon (%) | 0.87± 0.03 | 35.36 ± 1.32 | 43.80 ± 1.65 |
| Specific surface area (m2 g−1) | -- | 31.37 ± 0.04 | 94.83± 0.09 |
| Pore width (nm) | -- | 21 ± 1.32 | 15.0 ± 0.91 |
| Pore volume (cm3 g−1) | -- | 0.0035 ± 0.0001 | 0.09 ± 0.0001 |
| CEC cmolckg−1 | 5.2 ± 0.87 | 107.5 ± 4.34 | 85 ± 3.94 |
| Fe (mg kg−1) | 5.5 ± 0.91 | 755.6 ± 87 | 154.6 ± 11 |
| Mn (mg kg−1) | 0.51 ± 0.001 | 103.35 ± 9 | 395.62 ± 13 |
| Zn (mg kg−1) | 0.91 ± 0.01 | 130.3 ± 11 | 78.3 ± 8 |
| Total N (%) | 0.03 ± 0.001 | 1.59 ± 0.02 | 0.83 ± 0.01 |
| Available P (%) | 0.0007 ± 0.0001 | 1.30 ± 0.01 | 0.20 ± 0.001 |
| Extractable K (%) | 0.014 ± 0.001 | 2.59 ± 0.03 | 1.06 ± 0.01 |
| Treatment | Abbreviations |
|---|---|
| Control | CP0B0C0 |
| CP 100 mg kg−1 | CP100 |
| CP 200 mg kg−1 | CP200 |
| CP100 mg kg−1 + compost 0.25% | CP100C0.25 |
| CP200 mg kg−1 + compost 0.25% | CP200C0.25 |
| CP100 mg kg−1 + compost 0.50% | CP100C0.50 |
| CP200 mg kg−1 + compost 0.50% | CP200C0.50 |
| CP100 mg kg−1 + biochar 0.25% | CP100B0.25 |
| CP200 mg kg−1 + biochar 0.25% | CP200B0.25 |
| CP100 mg kg−1 + biochar 0.50% | CP100B0.50 |
| CP200 mg kg−1 + biochar 0.50% | CP200B0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, H.; Murtaza, G.; Saleem, M.H.; Ali, S.; Rizwan, M.; Riaz, U.; Niaz, A.; Abualreesh, M.H.; Alatawi, A. Alleviation of Chlorpyrifos Toxicity in Maize (Zea mays L.) by Reducing Its Uptake and Oxidative Stress in Response to Soil-Applied Compost and Biochar Amendments. Plants 2021, 10, 2170. https://doi.org/10.3390/plants10102170
Aziz H, Murtaza G, Saleem MH, Ali S, Rizwan M, Riaz U, Niaz A, Abualreesh MH, Alatawi A. Alleviation of Chlorpyrifos Toxicity in Maize (Zea mays L.) by Reducing Its Uptake and Oxidative Stress in Response to Soil-Applied Compost and Biochar Amendments. Plants. 2021; 10(10):2170. https://doi.org/10.3390/plants10102170
Chicago/Turabian StyleAziz, Humera, Ghulam Murtaza, Muhammad Hamzah Saleem, Shafaqat Ali, Muhammad Rizwan, Umair Riaz, Abdullah Niaz, Muyassar H. Abualreesh, and Aishah Alatawi. 2021. "Alleviation of Chlorpyrifos Toxicity in Maize (Zea mays L.) by Reducing Its Uptake and Oxidative Stress in Response to Soil-Applied Compost and Biochar Amendments" Plants 10, no. 10: 2170. https://doi.org/10.3390/plants10102170
APA StyleAziz, H., Murtaza, G., Saleem, M. H., Ali, S., Rizwan, M., Riaz, U., Niaz, A., Abualreesh, M. H., & Alatawi, A. (2021). Alleviation of Chlorpyrifos Toxicity in Maize (Zea mays L.) by Reducing Its Uptake and Oxidative Stress in Response to Soil-Applied Compost and Biochar Amendments. Plants, 10(10), 2170. https://doi.org/10.3390/plants10102170








