Investigation of Quinoa Seeds Fractions and Their Application in Wheat Bread Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quinoa Fractions Preparation
2.3. Sample’s Formulations
2.4. Physico-Chemical Characterization of the Formulated Flours
2.5. Dough and Bread Manufacturing
2.6. Evaluation of Flours Microstructure
2.7. Flours ATR FT-IR Spectra Collection
2.8. Empirical Dough Rheology and Texture Profile Analysis
2.9. Fundamental Dough Rheology
2.10. Physical Properties of Bread
2.11. Bread Texture Parameters Determination
2.12. Statistical Analysis
3. Results and Discussions
3.1. Microstructure of Flours
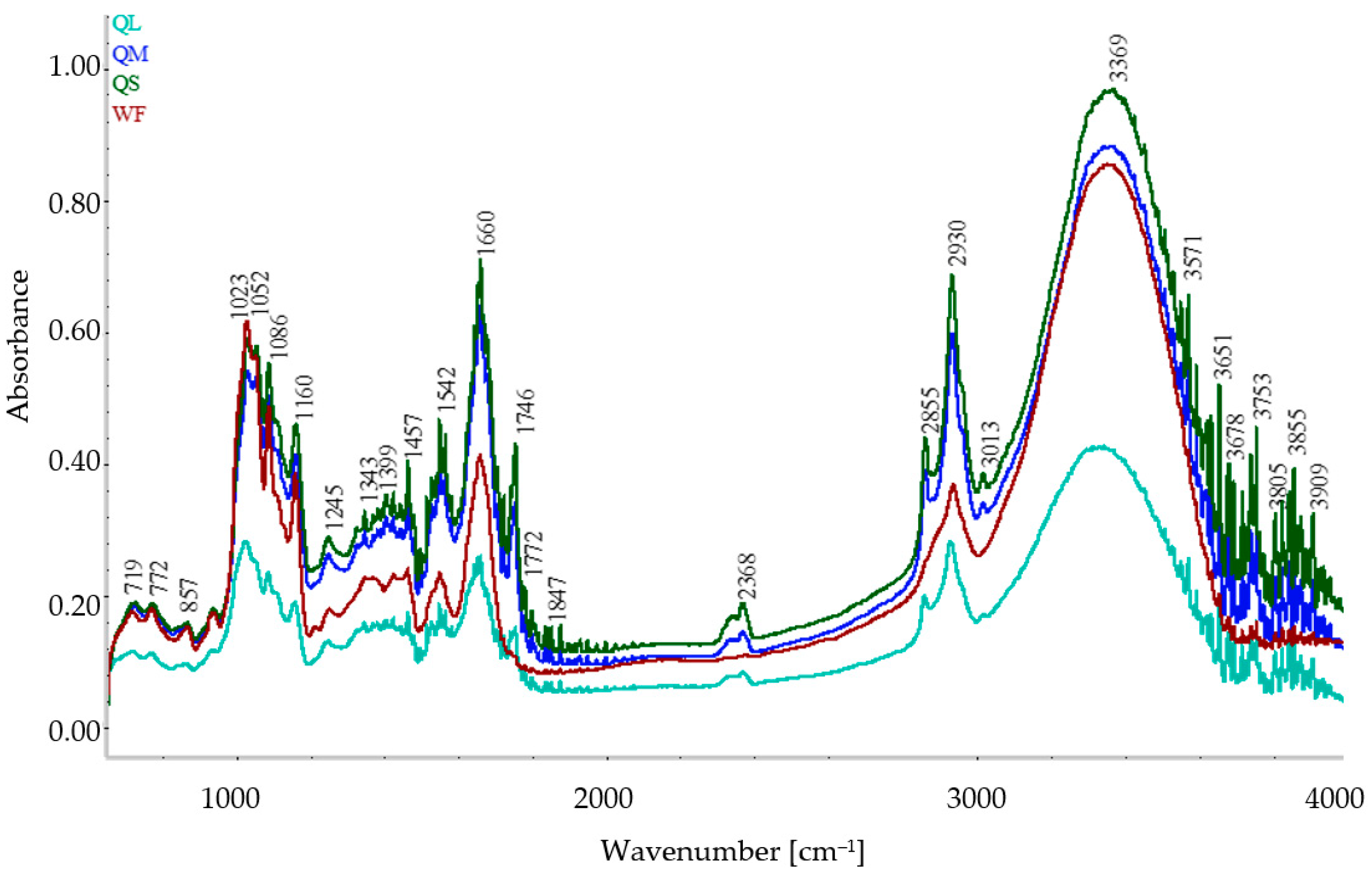
3.2. ATR FT-IR Spectra of Flours
3.3. Physico-Chemical Properties of Composite Flours
3.4. Dough Rheological Properties
3.4.1. Alveographic Parameters
3.4.2. Dynamic Rheological Parameters
3.4.3. Dough Texture Profile Analysis
3.5. Physical Properties of Bread
3.6. Textural Parameters of Bread
3.7. Relations between the Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vignola, M.B.; Moiraghi, M.; Salvucci, E.; Baroni, V.; Pérez, G.T. Whole meal and white flour from Argentine wheat genotypes: Mineral and arabinoxylan differences. J. Cereal Sci. 2016, 71, 217–223. [Google Scholar] [CrossRef]
- Mironeasa, S.; Iuga, M.; Zaharia, D.; Mironeasa, C. Optimization of grape peels particle size and flour substitution in white wheat flour dough. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2019, 20, 29–42. [Google Scholar]
- Saleem Khan, M.; Ali, E.; Ali, S.; Khan, W.M.; Sajjad, M.A.; Hussain, F.; Khan, M.S.; Ali, S.; Hussain, F. Assessment of essential amino acids in wheat proteins: A case study. J. Biodivers. Environ. Sci. 2014, 4, 185–189. [Google Scholar]
- Küster, I.; Vila, N. Health/Nutrition food claims and low-fat food purchase: Projected personality influence in young consumers. J. Funct. Foods 2017, 38, 66–76. [Google Scholar] [CrossRef]
- Encina-Zelada, C.; Cadavez, V.; Pereda, J.; Gómez-Pando, L.; Salvá-Ruíz, B.; Teixeira, J.A.; Ibañez, M.; Liland, K.H.; Gonzales-Barron, U. Estimation of composition of quinoa (Chenopodium quinoa Willd.) grains by Near-Infrared Transmission spectroscopy. LWT 2017, 79, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Nowak, V.; Du, J.; Charrondière, U.R. Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef]
- Valcárcel-Yamani, B.; Lannes, S.C.d.S. Applications of quinoa (Chenopodium quinoa Willd.) and amaranth (Amaranthus spp.) and their influence in the nutritional value of cereal based foods. Food Public Health 2012, 2, 265–275. [Google Scholar]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; Isabel, C.F.R.; Ferreira, I. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: A good alternative to nutritious food. Food Chem. 2019, 280, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lietz, G.; Seal, C.J. Phenolic, apparent antioxidant and nutritional composition of quinoa (Chenopodium quinoa Willd.) seeds. Int. J. Food Sci. Technol. 2021, 56, 3245–3254. [Google Scholar] [CrossRef]
- Prego, I.; Maldonado, S.; Otegui, M. Seed structure and localization of reserves in Chenopodium quinoa. Ann. Bot. 1998, 82, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Ando, H.; Chen, Y.C.; Tang, H.; Shimizu, M.; Watanabe, K.; Mitsunaga, T. Food Components in Fractions of Quinoa Seed. Food Sci. Technol. Res. 2002, 8, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Tsatsaragkou, K.; Yiannopoulos, S.; Kontogiorgi, A.; Poulli, E.; Krokida, M.; Mandala, I. Mathematical approach of structural and textural properties of gluten free bread enriched with carob flour. J. Cereal Sci. 2012, 56, 603–609. [Google Scholar] [CrossRef]
- Cappelli, A.; Cini, E.; Guerrini, L.; Masella, P.; Angeloni, G.; Parenti, A. Predictive models of the rheological properties and optimal water content in doughs: An application to ancient grain flours with different degrees of refining. J. Cereal Sci. 2018, 83, 229–235. [Google Scholar] [CrossRef]
- Solaesa, Á.G.; Villanueva, M.; Vela, A.J.; Ronda, F. Protein and lipid enrichment of quinoa (cv.Titicaca) by dry fractionation. Techno-functional, thermal and rheological properties of milling fractions. Food Hydrocoll. 2020, 105, 105770. [Google Scholar] [CrossRef]
- Mironeasa, S.; Zaharia, D.; Codină, G.G.; Ropciuc, S.; Iuga, M. Effects of Grape Peels Addition on Mixing, Pasting and Fermentation Characteristics of Dough from 480 Wheat Flour Type. Bull. UASVM Food Sci. Technol. 2018, 75, 27–35. [Google Scholar] [CrossRef]
- Kurek, M.A.; Sokolova, N. Optimization of bread quality with quinoa flour of different particle size and degree of wheat flour replacement. Food Sci. Technol. 2020, 40, 307–314. [Google Scholar] [CrossRef] [Green Version]
- El-Sohaimy, A.S.; Shehata, G.M.; Djapparovec, T.A.; Mehany, T.; Zeitoun, A.M.; Zeitoun, M.A. Development and characterization of functional pan bread supplemented with quinoa flour. J. Food Process. Preserv. 2021, 45. [Google Scholar] [CrossRef]
- Wang, X.; Lao, X.; Bao, Y.; Guan, X.; Li, C. Effect of whole quinoa flour substitution on the texture and in vitro starch digestibility of wheat bread. Food Hydrocoll. 2021, 119, 106840. [Google Scholar] [CrossRef]
- Calderelli, V.A.S.; de Benassi, M.T.; Visentainer, J.V.; Matioli, G. Quinoa and flaxseed: Potential ingredients in the production of bread with functional quality. Brazilian Arch. Biol. Technol. 2010, 53, 981–986. [Google Scholar] [CrossRef] [Green Version]
- Stikic, R.; Glamoclija, D.; Demin, M.; Vucelic-Radovic, B.; Jovanovic, Z.; Milojkovic-Opsenica, D.; Jacobsen, S.-E.; Milovanovic, M. Agronomical and nutritional evaluation of quinoa seeds (Chenopodium quinoa Willd.) as an ingredient in bread formulations. J. Cereal Sci. 2012, 55, 132–138. [Google Scholar] [CrossRef]
- El-Sohaimy, S.A.; Shehata, M.G.; Mehany, T.; Zeitoun, M.A. Nutritional, Physicochemical, and Sensorial Evaluation of Flat Bread Supplemented with Quinoa Flour. Int. J. Food Sci. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Luo, Z.; Yang, Q.; Xiao, Z.; Lu, X. Effect of quinoa flour on baking performance, antioxidant properties and digestibility of wheat bread. Food Chem. 2019, 294, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Coțovanu, I.; Mironeasa, S. Impact of different amaranth particle size addition level on wheat flour dough rheology and bread features. Foods 2021, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Wang, Z.; Hao, Y.; Wang, Y.; Yang, Z.; Li, W.; Wang, J. Physicochemical and functional properties of soluble dietary fiber from different colored quinoa varieties (Chenopodium quinoa Willd). J. Cereal Sci. 2020, 95, 103045. [Google Scholar] [CrossRef]
- Shotts, M.L.; Plans Pujolras, M.; Rossell, C.; Rodriguez-Saona, L. Authentication of indigenous flours (Quinoa, Amaranth and kañiwa) from the Andean region using a portable ATR-Infrared device in combination with pattern recognition analysis. J. Cereal Sci. 2018, 82, 65–72. [Google Scholar] [CrossRef]
- Czekus, B.; Pećinar, I.; Petrović, I.; Paunović, N.; Savić, S.; Jovanović, Z.; Stikić, R. Raman and Fourier transform infrared spectroscopy application to the Puno and Titicaca cvs. of quinoa seed microstructure and perisperm characterization. J. Cereal Sci. 2019, 87, 25–30. [Google Scholar] [CrossRef]
- Mironeasa, S.; Codină, G.G.; Mironeasa, C. Effect of Composite Flour Made from Tomato Seed and Wheat of 650 Type of a Strong Quality for Bread Making on Bread Quality and Alveograph Rheological Properties. Int. J. Food Eng. 2018, 4, 22–26. [Google Scholar] [CrossRef]
- Mironeasa, S.; Iuga, M.; Zaharia, D.; Mironeasa, C. Rheological Analysis of Wheat Flour Dough as Influenced by Grape Peels of Different Particle Sizes and Addition Levels. Food Bioprocess Technol. 2019, 12, 228–245. [Google Scholar] [CrossRef]
- Iuga, M.; Boestean, O.; Ghendov-Mosanu, A.; Mironeasa, S. Impact of Dairy Ingredients on Wheat Flour Dough Rheology and Bread Properties. Foods 2020, 9, 828–854. [Google Scholar] [CrossRef]
- Mironeasa, S. Mironeasa Costel Dough bread from refined wheat flour partially replaced by grape peels: Optimizing the rheological properties. J. Food Process Eng. 2019, 42, 1–14. [Google Scholar] [CrossRef]
- Bordei, D.; Bahrim, G.; Pâslaru, V.; Gasparotti, C.; Elisei, A.; Banu, I.; Ionescu, L.; Codină, G. Quality Control in the Bakery Industry-Analysis Methods. Galați Acad. 2007, 1, 203–212. [Google Scholar]
- Romano, A.; Masi, P.; Nicolai, M.A.; Falciano, A.; Ferranti, P. QUINOA (Chenopodium quinoa willd.) flour as novel and safe ingredient in bread formulation. Chem. Eng. Trans. 2019, 75, 301–306. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Auty, M.; Arendt, E.K.; Gallagher, E. Baking properties and microstructure of pseudocereal flours in gluten-free bread formulations. Eur. Food Res. Technol. 2010, 230, 437–445. [Google Scholar] [CrossRef]
- Marti, A.; Bock, J.E.; Pagani, M.A.; Ismail, B.; Seetharaman, K. Structural characterization of proteins in wheat flour doughs enriched with intermediate wheatgrass (Thinopyrum intermedium) flour. Food Chem. 2016, 194, 994–1002. [Google Scholar] [CrossRef]
- Cozzolino Daniel The role of visible and infrared spectroscopy combined with chemometrics to measure phenolic compounds in grape and wine samples. Molecules 2015, 20, 726–737. [CrossRef] [Green Version]
- García-Salcedo, Á.J.; Torres-Vargas, O.L.; Ariza-Calderón, H. Physical-chemical characterization of quinoa (Chenopodium quinoa Willd.), amaranth (Amaranthus caudatus L.), and chia (Salvia hispanica L.) flours and seeds. Agroindustry Food Sci. 2017, 67, 215–222. [Google Scholar]
- Prasopsunwattana, N.; Omary, M.B.; Arndt, E.A.; Cooke, P.H.; Flores, R.A.; Yokoyama, W.; Toma, A.; Chongcham, S.; Lee, S.P. Particle size effects on the quality of flour tortillas enriched with whole grain waxy barley. Cereal Chem. 2009, 86, 439–451. [Google Scholar] [CrossRef]
- Coțovanu, I.; Stoenescu, G.; Mironeasa, S. Amaranth Influence on Wheat Flour Dough Rheology: Optimal Particle Size and Amount of Flour Replacement. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 366–373. [Google Scholar] [CrossRef]
- Chauhan, G.S.; Zillman, R.R.; Eskin, N.A.M. Dough mixing and breadmaking properties of quinoa-wheat flour blends. Int. J. Food Sci. Technol. 1992, 27, 701–705. [Google Scholar] [CrossRef]
- Dhen, N.; Román, L.; Ben Rejeb, I.; Martínez, M.M.; Garogouri, M.; Gómez, M. Particle size distribution of soy flour affecting the quality of enriched gluten-free cakes. LWT 2016, 66, 179–185. [Google Scholar] [CrossRef]
- Ahmed, J.; Thomas, L.; Arfat, Y.A. Functional, rheological, microstructural and antioxidant properties of quinoa flour in dispersions as influenced by particle size. Food Res. Int. 2019, 116, 302–311. [Google Scholar] [CrossRef]
- Demir, M.K. Use of quinoa flour in the production of gluten-free tarhana. Food Sci. Technol. Res. 2014, 20, 1087–1092. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, E.; Gormley, T.R.; Arendt, E.K. Crust and crumb characteristics of gluten free breads. J. Food Eng. 2003, 56, 153–161. [Google Scholar] [CrossRef]
- Sluimer, P.; Sluimer, P. Principles of Breadmaking: Functionality of Raw Materials and Process Steps; American Association of Cereal Chemists (AACC): St. Paul, MN, USA, 2005. [Google Scholar]
- Coțovanu, I.; Mironeasa, S. Buckwheat seeds: Impact of milling fractions and addition level on wheat bread dough rheology. Appl. Sci. 2021, 11, 1731. [Google Scholar] [CrossRef]
- Hoseney, R.C. Principles of Cereal Science and Technology; American Association of Cereal Chemists (AACC): St. Paul, MN, USA, 1994; ISBN 0913250791. [Google Scholar]
- Ratnawati, L.; Desnilasari, D.; Surahman, D.N.; Kumalasari, R. Evaluation of Physicochemical, Functional and Pasting Properties of Soybean, Mung Bean and Red Kidney Bean Flour as Ingredient in Biscuit. IOP Conf. Ser. Earth Environ. Sci. 2019, 251. [Google Scholar] [CrossRef]
- Tamba-Berehoiu, R.M.; Turtoi, M.O.; Popa, C.N. Assessment of quinoa flours effect on wheat flour dough rheology and bread quality. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2019, 43, 173–188. [Google Scholar] [CrossRef]
- Föste, M.; Nordlohne, S.D.; Elgeti, D.; Linden, M.H.; Heinz, V.; Jekle, M.; Becker, T. Impact of quinoa bran on gluten-free dough and bread characteristics. Eur. Food Res. Technol. 2014, 239, 767–775. [Google Scholar] [CrossRef]
- Park, S.H.; Maeda, T.; Morita, N. Effect of Whole Quinoa Flours and Lipase on the Chemical, Rheological and Breadmaking Characteristics of Wheat Flour. J. Appl. Glycosci. 2005, 52, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Coțovanu, I.; Batariuc, A.; Mironeasa, S. Characterization of quinoa seeds milling fractions and their effect on the rheological properties of wheat flour dough. Appl. Sci. 2020, 10, 7225. [Google Scholar] [CrossRef]
- Bilgiçli, N.; İbanoğlu, Ş. Effect of pseudo cereal flours on some physical, chemical and sensory properties of bread. J. Food Sci. Technol. 2015, 52, 7525–7529. [Google Scholar] [CrossRef]
- Ruffino, A.M.C.; Rosa, M.; Hilal, M.; González, J.A.; Prado, F.E. The role of cotyledon metabolism in the establishment of quinoa (Chenopodium quinoa) seedlings growing under salinity. Plant Soil 2010, 326, 213–224. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.S.; Zannini, E.; Arendt, E.K. In vitro starch digestibility and predicted glycaemic indexes of buckwheat, oat, quinoa, sorghum, teff and commercial gluten-free bread. J. Cereal Sci. 2013, 58, 431–436. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Miñarro, B.; Albanell, E.; Aguilar, N.; Guamis, B.; Capellas, M. Effect of legume flours on baking characteristics of gluten-free bread. J. Cereal Sci. 2012, 56, 476–481. [Google Scholar] [CrossRef]
- Elgeti, D.; Nordlohne, S.D.; Föste, M.; Besl, M.; Linden, M.H.; Heinz, V.; Jekle, M.; Becker, T. Volume and texture improvement of gluten-free bread using quinoa white flour. J. Cereal Sci. 2014, 59, 41–47. [Google Scholar] [CrossRef]




| Sample | Moisture (%) | Protein (%) | Lipids (%) | Ash (%) | Carbohydrates (%) | Color | |||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||||
| Control | 14.08 ± 0.08 e | 12.45 ± 0.15 a | 1.41 ± 0.01 a | 0.69 ± 0.04 a | 71.36 ± 0.01 e | 91.46 ± 0.15 d | -5.13 ± 0.03 a | 15.09 ± 0.07 b | |
| QL_5 | 13.82 ±0.00 dy | 12.57 ± 0.03 bx | 1.65 ± 0.00 by | 0.70 ± 0.00 bx | 71.25 ± 0.04 dz | 90.87 ± 0.07 cz | −4.89 ± 0.02 bx | 14.98 ± 0.05 abx | |
| QL_10 | 13.64 ± 0.00 cy | 12.54 ± 0.06 cx | 1.90 ± 0.00 cy | 0.76 ± 0.00 cx | 71.15 ± 0.06 cz | 90.43 ± 0.12 bz | −4.83 ± 0.01 cx | 14.51 ± 0.04 ax | |
| QL_15 | 13.47 ± 0.01 by | 12.50 ± 0.09 dx | 2.16 ± 0.00 dy | 0.81 ± 0.00 dx | 71.05 ± 0.11 bz | 90.82 ± 0.25 bz | −4.99 ± 0.07 cx | 14.50 ± 0.11 abx | |
| QL_20 | 13.29 ± 0.01 ay | 12.47 ± 0.12 ex | 2.41 ± 0.00 ey | 0.87 ± 0.00 ex | 70.95 ± 0.13 az | 89.38 ± 0.04 az | −4.60 ± 0.00 dx | 14.76 ± 0.02 abx | |
| QM_5 | 13.80 ± 0.00 dxy | 12.91 ± 0.00 bz | 1.65 ± 0.00 bxy | 0.78 ± 0.00 bz | 70.85 ± 0.00 dx | 89.35 ± 0.08 cx | −4.85 ± 0.07 bx | 14.81 ± 0.13 aby | |
| QM_10 | 13.60 ± 0.00 cxy | 13.22 ± 0.00 cz | 1.90 ± 0.00 cxy | 0.90 ± 0.00 cz | 70.36 ± 0.00 cx | 88.64 ± 0.11 bx | −4.72 ± 0.06 cx | 14.82 ± 0.12 ay | |
| QM_15 | 13.47 ± 0.01 bxy | 13.54 ± 0.01 dz | 2.15 ± 0.00 dxy | 1.03 ± 0.00 dz | 69.87 ± 0.01 bx | 88.10 ± 0.12 bx | −4.69 ± 0.03 cx | 15.43 ± 0.52 aby | |
| QM_20 | 13.21 ± 0.00 axy | 13.85 ± 0.03 ez | 2.40 ± 0.00 exy | 1.16 ± 0.01 ez | 69.38 ± 0.02 ax | 87.59 ± 0.23 ax | −4.53 ± 0.02 dx | 15.33 ± 0.19 aby | |
| QS_5 | 13.79 ± 0.00 dx | 12.75 ± 0.00 by | 1.65 ± 0.00 bx | 0.74 ± 0.00 by | 71.06 ± 0.02 dy | 89.80 ± 0.23 cy | −4.93 ± 0.10 by | 14.91 ± 0.07 aby | |
| QS_10 | 13.57 ± 0.03 cx | 12.91 ± 0.01 cy | 1.89 ± 0.00 cx | 0.84 ± 0.00 cy | 70.78 ± 0.04 cy | 89.05 ± 0.19 by | −4.80 ± 0.02 cy | 14.98 ± 0.04 ay | |
| QS_15 | 13.36 ± 0.05 bx | 13.06 ± 0.02 dy | 2.14 ± 0.00 dx | 0.93 ± 0.00 dy | 70.50 ± 0.07 by | 88.71 ± 0.13 by | −4.69 ± 0.03 cy | 15.22 ± 0.08 aby | |
| QS_20 | 13.15 ± 0.07 ax | 13.22 ± 0.03 ey | 2.39 ± 0.00 ex | 1.03 ± 0.00 ey | 70.20 ± 0.09 ay | 88.63 ± 0.05 ay | −4.59 ± 0.05 dy | 15.01 ± 0.04 aby | |
| Two-way ANOVA p value | |||||||||
| F1: | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p = 0.01 | |
| F2 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | |
| F1 × F2 | p = 0.35 | p < 0.01 | p = 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | |
| Sample | P (mm H2O) | L (mm) | G | W (×10−4 J) | P/L |
|---|---|---|---|---|---|
| Control | 86.33 ± 0.57 a | 94.00 ± 3.00 d | 21.55 ± 0.35 d | 253.00 ± 4.00 d | 0.92 ± 0.03 a |
| QL_5 | 88.50 ± 0.50 by | 46.50 ± 0.50 cy | 15.25 ± 0.05 cy | 167.50 ± 2.50 cx | 1.80 ± 0.06 by |
| QL_10 | 103.50 ± 0.50 cy | 39.50 ± 0.50 by | 13.80 ± 0.10 by | 166.00 ± 0.00 cx | 2.65 ± 0.01 cy |
| QL_15 | 104.00 ± 1.00 cy | 35.50 ± 0.50 ay | 12.75 ± 0.05 ay | 158.00 ± 2.00 bx | 3.39 ± 0.00 ey |
| QL_20 | 113.00 ± 1.00 dy | 32.00 ± 3.00 ay | 12.20 ± 0.60 ay | 142.00 ± 5.00 ax | 3.21 ± 0.01 dy |
| QM_5 | 102.50 ± 1.50 bz | 42.00 ± 1.00 cx | 14.75 ± 0.15 cx | 173.50 ± 0.50 cx | 2.44 ± 0.09 bz |
| QM_10 | 101.00 ± 0.00 cz | 38.00 ± 1.00 bx | 14.60 ± 0.00 bx | 172.00 ± 1.73 cx | 2.35 ± 0.00 cz |
| QM_15 | 113.00 ± 3.00 cz | 29.00 ± 0.00 ax | 12.00 ± 0.00 ax | 138.50 ± 4.50 bx | 4.53 ± 0.11 ez |
| QM_20 | 117.00 ±3.00 dz | 26.00 ± 2.00 ax | 11.40 ± 0.40 ax | 141.50 ± 3.50 ax | 3.92 ± 0.37 dz |
| QS_5 | 98.50 ± 0.50 bx | 58.50 ± 1.50 cz | 17.00 ± 0.20 cz | 211.50 ± 8.31 cx | 1.66 ± 0.01 bx |
| QS_10 | 96.00 ± 1.00 cx | 53.00 ± 3.00 bz | 16.05 ± 0.35 bz | 179.50 ± 0.50 cx | 1.87 ± 0.03 cx |
| QS_15 | 92.00 ± 1.00 cx | 43.50 ± 1.50 az | 14.60 ± 0.20 az | 158.00 ± 2.00 bx | 2.22 ± 0.01 ex |
| QS_20 | 80.50 ± 1.50 dx | 37.00 ± 1.00 az | 13.55 ± 0.15 az | 117.50 ± 6.50 ax | 2.18 ± 0.01 dx |
| Two-way ANOVA p value | |||||
| F1 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| F2 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| F1 × F2 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| Type of Sample | G′ at 1 Hz (Pa) | G″ at 1 Hz (Pa) | Tan δ at 1 Hz (adim.) | Tmax (°C) |
|---|---|---|---|---|
| Control | 26,370 ± 70.15 a | 9488 ± 60.00 a | 0.3598 ± 0.00 d | 82.74 ± 0.49 c |
| QL_5 | 44,600 ± 270.00 by | 17,125 ± 145.00 cy | 0.3839 ± 0.00 cy | 78.32 ± 0.05 abx |
| QL_10 | 47,150 ± 190.00 cy | 15,615 ± 25.00 by | 0.3311 ± 0.01 by | 78.87 ± 0.02 bx |
| QL_15 | 52,790 ± 285.00 dy | 19,970 ± 100.00 dy | 0.3782 ± 0.00 dy | 78.68 ± 0.19 abx |
| QL_20 | 55,420 ± 40.00 ey | 20,525 ± 535.00 dy | 0.3703 ± 0.00 dy | 78.97 ± 1.01 ax |
| QM_5 | 34,865 ± 525.00 bz | 11,240 ± 60.00 cz | 0.3223 ± 0.00 cy | 78.47 ± 0.05 abxy |
| QM_10 | 47,905 ± 615.00 cz | 16,935 ± 145.00 bz | 0.3535 ± 0.00 by | 79.06 ± 0.14 bxy |
| QM_15 | 57,440 ± 310.00 dz | 18,640 ± 170.00 dz | 0.3245 ± 0.00 dy | 79.45 ± 0.08 abxy |
| QM_20 | 65,245 ± 205.00 ez | 19,745 ± 95.00 dz | 0.3026 ± 0.00 dy | 79.41 ± 0.03 axy |
| QS_5 | 31,320 ± 280.00 bx | 10,175 ± 335.00 cx | 0.3248 ± 0.00 cx | 80.28 ± 0.15 aby |
| QS_10 | 32,360 ± 200.00 cx | 10,853 ± 116.50 bx | 0.3353 ± 0.00 bx | 80.45 ± 0.18 by |
| QS_15 | 39,260 ± 585.00 dx | 14,360 ± 420.00 dx | 0.3657 ± 0.00 dx | 78.74 ± 0.07 aby |
| QS_20 | 48,305 ± 240.00 ex | 15,725 ± 45.00 dx | 0.3255 ± 0.00 dx | 78.97 ± 0.11 ay |
| Two-way ANOVA p value | ||||
| F1 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| F2 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| F1 × F2 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| Sample | Loaf Volume (cm 3) | Specific Volume (g/cm 3) | Porosity (%) | Elasticity (%) |
|---|---|---|---|---|
| Control | 378.70 ± 1.12 e | 2.45 ± 0.00 e | 64.33 ± 0.11 b | 91.72 ± 0.07 b |
| QL_5 | 372.60 ± 0.52 dx | 2.25 ± 0.02 dxy | 72.38 ± 0.16 ex | 97.92 ± 0.37 ez |
| QL_10 | 358.87 ± 1.02 cx | 2.20 ± 0.00 cxy | 67.93 ± 0.05 dx | 94.11 ± 0.84 dz |
| QL_15 | 335.27 ± 0.37 bx | 2.00 ± 0.06 bxy | 66.35 ± 0.34 cx | 93.17 ± 0.45 cz |
| QL_20 | 317.01 ± 1.24 ax | 1.93 ± 0.01 axy | 57.27 ± 0.52 ax | 89.99 ± 1.66 az |
| QM_5 | 371.30 ± 1.21 dx | 2.24 ± 0.01 dy | 72.47 ± 0.07 ez | 96.36 ± 0.29 eyz |
| QM_10 | 363.53 ± 1.27 cx | 2.22 ± 0.01 cy | 70.87 ± 0.46 dz | 94.51 ± 0.31 dyz |
| QM_15 | 338.86 ± 0.15 bx | 2.05 ± 0.00 by | 67.63 ± 0.81 cz | 93.48 ± 0.15 cyz |
| QM_20 | 318.63 ± 0.81 ax | 1.93 ± 0.00 ay | 66.32 ± 0.58 az | 89.74 ± 0.50 ayz |
| QS_5 | 356.66 ± 1.52 dy | 2.21 ± 0.02 dx | 71.97 ± 0.52 ey | 96.17 ± 0.10 exy |
| QS_10 | 347.33 ± 2.08 cy | 2.18 ± 0.00 cx | 70.51 ± 0.09 dy | 94.86 ± 0.93 dxy |
| QS_15 | 303.66 ± 3.51 by | 2.04 ± 0.03 bx | 66.63 ± 0.80 cy | 92.00 ± 0.63 cxy |
| QS_20 | 260.00 ± 3.00 ay | 1.81 ± 0.08 ax | 61.60 ± 1.01 ay | 87.72 ± 0.96 axy |
| Two-way ANOVA p value | ||||
| F1 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| F2 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| F1 × F2 | p < 0.01 | p = 0.01 | p < 0.01 | p = 0.01 |
| Sample | Crust Color | Crumb Color | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| Control | 67.36 ± 0.19 d | 0.78 ± 0.22 a | 32.27 ± 0.28 cy | 72.30 ± 0.27 e | −4.48 ± 0.03 a | 19.02 ± 0.23 a |
| QL_5 | 64.99 ± 0.74 dx | 4.09 ± 0.30 by | 29.65 ± 0.17 az | 75.21 ± 0.19 dy | −4.24 ± 0.09 bx | 19.75 ± 0.13 bx |
| QL_10 | 61.79 ± 0.07 cx | 5.29 ± 0.10 cy | 32.74 ± 0.20 bz | 64.64 ± 1.07 cy | −3.95 ± 0.24 cx | 20.09 ± 0.59 cx |
| QL_15 | 60.71 ± 0.40 bx | 6.64 ± 0.24 dy | 34.00 ± 0.78 cz | 64.00 ± 0.50 by | −3.86 ± 0.03 dx | 21.30 ± 0.56 dx |
| QL_20 | 59.88 ± 0.97 ax | 6.79 ± 0.53 dy | 34.28 ± 0.44 dz | 63.37 ± 0.47 ay | −3.67 ± 0.04 ex | 21.26 ± 0.10 ex |
| QM_5 | 63.36 ± 0.56 by | 3.59 ± 0.25 bx | 24.56 ± 0.22 ax | 69.88 ± 0.73 dy | −4.17 ± 0.14 by | 19.54 ± 0.60 by |
| QM_10 | 65.48 ± 0.43 cy | 4.74 ± 0.38 cx | 30.66 ± 0.59 bx | 66.96 ± 0.85 cy | −3.78 ± 0.02 cy | 21.54 ± 0.22 cy |
| QM_15 | 63.62 ± 0.26 by | 4.90 ± 0.18 dx | 31.32 ± 0.87 cx | 65.64 ± 0.38 by | −3.33 ± 0.02 dy | 21.89 ± 0.07 dy |
| QM_20 | 62.23 ± 0.51 ay | 5.11 ± 0.32 dx | 32.76 ± 0.69 dx | 63.40 ± 0.67 ay | −3.17 ± 0.09 ey | 23.50 ± 0.22 ey |
| QS_5 | 64.25 ± 0.31 dx | 3.54 ± 0.09 bx | 29.03 ± 1.14 ay | 65.45 ± 1.27 dx | −3.74 ± 0.08 bz | 20.42 ± 0.49 by |
| QS_10 | 62.01 ± 0.61 cx | 4.42 ± 0.20 cx | 30.51 ± 0.36 by | 65.45 ± 0.33 cx | −3.66 ± 0.04 cz | 22.12 ± 0.70 cy |
| QS_15 | 60.01 ± 0.74 bx | 4.78 ± 0.32 dx | 31.71 ± 0.43 cy | 65.35 ± 0.51 bx | −3.03 ±0.09 dz | 22.78 ± 0.49 dy |
| QS_20 | 57.79 ± 0.88 ax | 5.11 ± 0.14 dx | 34.57 ± 0.41 dy | 64.37 ± 1.69 ax | −2.23 ± 0.10 ez | 22.19 ± 1.25 ey |
| Two-way ANOVA p value | ||||||
| F1 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| F2 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| F1 × F2 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coţovanu, I.; Ungureanu-Iuga, M.; Mironeasa, S. Investigation of Quinoa Seeds Fractions and Their Application in Wheat Bread Production. Plants 2021, 10, 2150. https://doi.org/10.3390/plants10102150
Coţovanu I, Ungureanu-Iuga M, Mironeasa S. Investigation of Quinoa Seeds Fractions and Their Application in Wheat Bread Production. Plants. 2021; 10(10):2150. https://doi.org/10.3390/plants10102150
Chicago/Turabian StyleCoţovanu, Ionica, Mădălina Ungureanu-Iuga, and Silvia Mironeasa. 2021. "Investigation of Quinoa Seeds Fractions and Their Application in Wheat Bread Production" Plants 10, no. 10: 2150. https://doi.org/10.3390/plants10102150
APA StyleCoţovanu, I., Ungureanu-Iuga, M., & Mironeasa, S. (2021). Investigation of Quinoa Seeds Fractions and Their Application in Wheat Bread Production. Plants, 10(10), 2150. https://doi.org/10.3390/plants10102150








