Vegetal Compounds as Sources of Prophylactic and Therapeutic Agents in Dentistry
Abstract
:1. General Aspects
2. Herbal Compounds in Dentistry
3. Vegetal Sources of Compounds on Dentistry Products
4. Herbs as Therapeutic Agents in Dentistry
5. In Vitro Experimental Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Petersen, P.; Kwan, S. The 7(Th) WHO Global Conference on Health Promotion—towards Integration of Oral Health (Nairobi, Kenya 2009). Community Dent. Health 2021, 26, 1109. [Google Scholar] [CrossRef]
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The Global Burden of Oral Diseases and Risks to Oral Health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar] [PubMed]
- Rodrigues, F.; Lehmann, M.; do Amaral, V.S.; Reguly, M.L.; de Andrade, H.H.R. Genotoxicity of Three Mouthwash Products, Cepacol, Periogard, and Plax, in the Drosophila Wing-Spot Test. Environ. Mol. Mutagen. 2007, 48, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Taheri, J.B.; Azimi, S.; Rafieian, N.; Akhavan Zanjani, H. Herbs in Dentistry. Int. Dent. J. 2011, 61, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Jalaluddin, M.; Rout, P.; Mohanty, R.; Dileep, C.L. Emerging Trends of Herbal Care in Dentistry. J. Clin. Diagn. Res. 2013, 7, 1827–1829. [Google Scholar] [CrossRef]
- Pancu, D.F.; Scurtu, A.; Macasoi, I.G.; Marti, D.; Mioc, M.; Soica, C.; Coricovac, D.; Horhat, D.; Poenaru, M.; Dehelean, C. Antibiotics: Conventional Therapy and Natural Compounds with Antibacterial Activity—A Pharmaco-Toxicological Screening. Antibiotics 2013, 7, 1827–1829. [Google Scholar]
- Laheij, A.M.G.A.; Kistler, J.O.; Belibasakis, G.N.; Välimaa, H.; de Soet, J.J.; EOMW. Healthcare-Associated Viral and Bacterial Infections in Dentistry. J. Oral Microbiol. 2012, 4, 17659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral Effect of Phytochemicals from Medicinal Plants: Applications and Drug Delivery Strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef] [Green Version]
- Petersen, P.E. The World Oral Health Report 2003: Continuous Improvement of Oral Health in the 21st Century--the Approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2003, 31 (Suppl. 1), 3–23. [Google Scholar] [CrossRef]
- Petersen, P.E. The Burden of Oral Disease: Challenges to Improving Oral Health in the 21st Century. Bull. World Health Organ. 2005, 83, 3. [Google Scholar]
- Kim, H.-S. Do Not Put Too Much Value on Conventional Medicines. J. Ethnopharmacol. 2005, 100, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A Status Review on the Medicinal Properties of Essential Oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Adámez, J.D. Agrifood By-Products as a Source of Phytochemical Compounds. Descr. Food Sci. 2018. [Google Scholar] [CrossRef] [Green Version]
- Chioibas, R.; Susan, R.; Susan, M.; Mederle, O.; Vaduva, D.; Radulescu, M.; Berceanu, M.; Danciu, C.; Khaled, Z.; Draghici, G.; et al. Antimicrobial Activity Exerted by Total Extracts of Germander. Rev. Chim. 2019, 70, 3242–3244. [Google Scholar] [CrossRef]
- Gibbons, S. Anti-Staphylococcal Plant Natural Products. Nat. Prod. Rep. 2004, 21, 263–277. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between Natural Products and Antibiotics against Infectious Diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Ambrosio, S.R.; Furtado, N.A.J.C.; de Oliveira, D.C.R.; da Costa, F.B.; Martins, C.H.G.; de Carvalho, T.C.; Porto, T.S.; Veneziani, R.C.S. Antimicrobial Activity of Kaurane Diterpenes against Oral Pathogens. Z. Naturforsch. C 2008, 63, 326–330. [Google Scholar] [CrossRef]
- Porto, T.S.; Rangel, R.; Furtado, N.A.J.C.; de Carvalho, T.C.; Martins, C.H.G.; Veneziani, R.C.S.; Da Costa, F.B.; Vinholis, A.H.C.; Cunha, W.R.; Heleno, V.C.G.; et al. Pimarane-Type Diterpenes: Antimicrobial Activity against Oral Pathogens. Molecules 2009, 14, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Urzúa, A.; Rezende, M.C.; Mascayano, C.; Vásquez, L. A Structure-Activity Study of Antibacterial Diterpenoids. Molecules 2008, 13, 882–891. [Google Scholar] [CrossRef] [Green Version]
- Armaka, M.; Papanikolaou, E.; Sivropoulou, A.; Arsenakis, M. Antiviral Properties of Isoborneol, a Potent Inhibitor of Herpes Simplex Virus Type 1. Antivir. Res. 1999, 43, 79–92. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative Study on the Antiviral Activity of Selected Monoterpenes Derived from Essential Oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Kharouf, N.; Haikel, Y.; Ball, V. Polyphenols in Dental Applications. Bioengineering 2020, 7, 72. [Google Scholar] [CrossRef]
- Leme-Kraus, A.A.; Aydin, B.; Vidal, C.M.P.; Phansalkar, R.M.; Nam, J.W.; McAlpine, J.; Pauli, G.F.; Chen, S.; Bedran-Russo, A.K. Biostability of the Proanthocyanidins-Dentin Complex and Adhesion Studies. J. Dent. Res. 2017, 96, 406–412. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Li, K.; Yan, H.; Liu, S.; Wang, Y.; Huang, C. High-Performance Therapeutic Quercetin-Doped Adhesive for Adhesive-Dentin Interfaces. Sci. Rep. 2017, 7, 8189. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhou, X.D.; Wu, C.D. The Tea Catechin Epigallocatechin Gallate Suppresses Cariogenic Virulence Factors of Streptococcus Mutans. Antimicrob. Agents Chemother. 2011, 55, 1229–1236. [Google Scholar] [CrossRef] [Green Version]
- Hara, K.; Ohara, M.; Hayashi, I.; Hino, T.; Nishimura, R.; Iwasaki, Y.; Ogawa, T.; Ohyama, Y.; Sugiyama, M.; Amano, H. The Green Tea Polyphenol (-)-Epigallocatechin Gallate Precipitates Salivary Proteins Including Alpha-Amylase: Biochemical Implications for Oral Health. Eur. J. Oral Sci. 2012, 120, 132–139. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Mucsi, I.; Gyulai, Z.; Beladi, I. Combined Effects of Flavonoids and Acyclovir against Herpesviruses in Cell Cultures. Acta Microbiol. Hung. 1992, 39, 137–147. [Google Scholar]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Cirkovic Velickovic, T.D.; Stanic-Vucinic, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef] [Green Version]
- Bennick, A. Interaction of Plant Polyphenols with Salivary Proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef]
- Hu, J.P.; Takahashi, N.; Yamada, T. Coptidis Rhizoma Inhibits Growth and Proteases of Oral Bacteria. Oral Dis. 2000, 6, 297–302. [Google Scholar] [CrossRef]
- Sayhan, H.; Beyaz, S.G.; Çeliktaş, A. The Local Anesthetic and Pain Relief Activity of Alkaloids. In Alkaloids—Alternatives in Synthesis, Modification and Application; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Ghildiyal, R.; Prakash, V.; Chaudhary, V.K.; Gupta, V.; Gabrani, R. Phytochemicals as Antiviral Agents: Recent Updates BT—Plant-Derived Bioactives: Production, Properties and Therapeutic Applications; Swamy, M.K., Ed.; Springer: Singapore, 2020; pp. 279–295. ISBN 978-981-15-1761-7. [Google Scholar]
- Narayanan, N.; Lakshmi, D. Salvia Officinalis in Dentistry. Dent. Hypotheses 2015, 6, 27–30. [Google Scholar] [CrossRef]
- Bachrach, G.; Jamil, A.; Naor, R.; Tal, G.; Ludmer, Z.; Steinberg, D. Garlic Allicin as a Potential Agent for Controlling Oral Pathogens. J. Med. Food 2011, 14, 1338–1343. [Google Scholar] [CrossRef]
- Sakanaka, S.; Kim, M.; Taniguchi, M.; Yamamoto, T. Antibacterial Substances in Japanese Green Tea Extract against Streptococcus Mutans, a Cariogenic Bacterium. Agric. Biol. Chem. 2014, 53, 2307–2311. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Cervino, G. Chitosan Use in Dentistry: A Systematic Review of Recent Clinical Studies. Mar. Drugs 2019, 17, 417. [Google Scholar] [CrossRef] [Green Version]
- Karatas, O.; Balci Yuce, H.; Taskan, M.M.; Gevrek, F.; Alkan, C.; Isiker Kara, G.; Temiz, C. Cinnamic Acid Decreases Periodontal Inflammation and Alveolar Bone Loss in Experimental Periodontitis. J. Periodontal Res. 2020, 55, 676–685. [Google Scholar] [CrossRef]
- Yanakiev, S. Effects of Cinnamon (Cinnamomum spp.) in Dentistry: A Review. Molecules 2020, 25, 4184. [Google Scholar] [CrossRef]
- Chaturvedi, T. Uses of Turmeric in Dentistry: An Update. Indian J. Dent. Res. 2009, 20, 107–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhu, X.; Cao, P.; Wei, S.; Lu, Y. Antibacterial and Antibiofilm Activities of Eugenol from Essential Oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (Clove) Leaf against Periodontal Pathogen Porphyromonas Gingivalis. Microb. Pathog. 2017, 113, 396–402. [Google Scholar] [CrossRef]
- Tiwari, R. Pharmacotherapeutic Properties of Ginger and Its Use in Diseases of the Oral Cavity: A Narrative Review. J. Adv. Oral Res. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Ali, N.; Abbas, M.; Al-Bayaty, F. Evaluation of Potential Effect of Menthol Solution on Oral Hygiene Status of Dental Students in a University in Iraq. Trop. J. Pharm. Res. 2015, 14, 687–692. [Google Scholar] [CrossRef] [Green Version]
- Da Silveira Moretti, A.B.; Abdo, R.C.C.; Carvalho, J.C.T.; Moreira Machado, M.A.d.A.; da Silva, S.M.B. Effect of Sanguinaria Canadensis Tincture Associated to a Chewing Gum on the Bacterial Biofilm. Open Complementary Med. J. 2009, 1, 97–101. [Google Scholar] [CrossRef]
- Koo, H.; Pearson, S.K.; Scott-Anne, K.; Abranches, J.; Cury, J.A.; Rosalen, P.L.; Park, Y.K.; Marquis, R.E.; Bowen, W.H. Effects of Apigenin and Tt-Farnesol on Glucosyltransferase Activity, Biofilm Viability and Caries Development in Rats. Oral Microbiol. Immunol. 2002, 17, 337–343. [Google Scholar] [CrossRef]
- Galvão, L.C.d.C.; Furletti, V.F.; Bersan, S.M.F.; da Cunha, M.G.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Sartoratto, A.; Rehder, V.L.G.; Figueira, G.M.; Teixeira Duarte, M.C.; et al. Antimicrobial Activity of Essential Oils against Streptococcus Mutans and Their Antiproliferative Effects. Evidence-Based Complement. Altern. Med. 2012, 2012, 751435. [Google Scholar] [CrossRef] [Green Version]
- Corega, C.; Vaida, L.; Festila, D.G.; Rigoni, G.; Albanese, M.; D’Agostino, A.; De Santis, D.; Pardo, A.; Nocini, P.F.; Bertossi, D. The Benefits of Quercitin for Dentistry and Maxillofacial Surgery: A Systematic Review. Minerva Stomatol. 2014, 62. in press. [Google Scholar]
- Lakshmi, T.; Roy, A. Yarrow (achillea millefolium linn.) a herbal medicinal plant with broad therapeutic use—A review. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 136–141. [Google Scholar]
- Chou, S.-T.; Peng, H.-Y.; Hsu, J.-C.; Lin, C.-C.; Shih, Y. Achillea Millefolium L. Essential Oil Inhibits LPS-Induced Oxidative Stress and Nitric Oxide Production in RAW 264.7 Macrophages. Int. J. Mol. Sci. 2013, 14, 12978–12993. [Google Scholar] [CrossRef] [Green Version]
- Jaimand, K.; Rezaee, M.B.; Mozaffarian, V. Chemical Constituents of the Leaf and Flower Oils from Achillea Millefolium Ssp. Elbursensis Hub.-Mor. from Iran Rich in Chamazulene. J. Essent. Oil Res. 2006, 18, 293–295. [Google Scholar] [CrossRef]
- Miranzadeh, S.; Adib-Hajbaghery, M.; Soleymanpoor, L.; Ehsani, M. Effect of Adding the Herb Achillea Millefolium on Mouthwash on Chemotherapy Induced Oral Mucositis in Cancer Patients: A Double-Blind Randomized Controlled Trial. Eur. J. Oncol. Nurs. Off. J. Eur. Oncol. Nurs. Soc. 2015, 19, 207–213. [Google Scholar] [CrossRef]
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Iorizzi, M.; Fico, G. Phenolic Compounds from Achillea Millefolium L. and Their Bioactivity. Acta Biochim. Pol. 2011, 58, 203–209. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines: A Guide for Healthcare Professionals; Pharmaceutical Press: London, UK, 2003; ISBN 0853694745. [Google Scholar]
- Karic, V.; Jaiswal, A.; Abrahamse, H.; Thakur, A.; Ganeshpurkar, A. Effectiveness of Allium sativum on Bacterial Oral Infection. In Natural Oral Care in Dental Therapy; Chauhan, D.N., Singh, P.R., Shah, K., Chauhan, N.S., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 345–369. ISBN 9781119614227. [Google Scholar]
- Groppo, F.C.; Ramacciato, J.C.; Motta, R.H.L.; Ferraresi, P.M.; Sartoratto, A. Antimicrobial Activity of Garlic against Oral Streptococci. Int. J. Dent. Hyg. 2007, 5, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, M.; Taheri, J.-B.; Shabestari, S.B.; Tanik, A.; Pahlevan, R. Comparison of Therapeutic Effect of Aqueous Extract of Garlic and Nystatin Mouthwash in Denture Stomatitis. Gerodontology 2012, 29, e680–e684. [Google Scholar] [CrossRef]
- Mendoza-Juache, A.; Aranda-Romo, S.; Bermeo-Escalona, J.R.; Gómez-Hernández, A.; Pozos-Guillén, A.; Sánchez-Vargas, L.O. The Essential Oil of Allium sativum as an Alternative Agent against Candida Isolated from Dental Prostheses. Rev. Iberoam. Micol. 2017, 34, 158–164. [Google Scholar] [CrossRef]
- Tang, F.-Y.; Chiang, E.-P.I.; Chung, J.-G.; Lee, H.-Z.; Hsu, C.-Y. S-Allylcysteine Modulates the Expression of E-Cadherin and Inhibits the Malignant Progression of Human Oral Cancer. J. Nutr. Biochem. 2009, 20, 1013–1020. [Google Scholar] [CrossRef]
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S.; Sarahroodi, S. Therapeutic Uses and Pharmacological Properties of Garlic, Shallot, and Their Biologically Active Compounds. Iran. J. Basic Med. Sci. 2013, 16, 1031–1048. [Google Scholar]
- Mangaiyarkarasi, S.P.; Manigandan, T.; Elumalai, M.; Cholan, P.K.; Kaur, R.P. Benefits of Aloe Vera in Dentistry. J. Pharm. Bioallied Sci. 2015, 7, S255–S259. [Google Scholar] [CrossRef]
- Choonhakarn, C.; Busaracome, P.; Sripanidkulchai, B.; Sarakarn, P. The Efficacy of Aloe Vera Gel in the Treatment of Oral Lichen Planus: A Randomized Controlled Trial. Br. J. Dermatol. 2008, 158, 573–577. [Google Scholar] [CrossRef]
- Babaee, N.; Zabihi, E.; Mohseni, S.; Moghadamnia, A.A. Evaluation of the Therapeutic Effects of Aloe Vera Gel on Minor Recurrent Aphthous Stomatitis. Dent. Res. J. (Isfahan) 2012, 9, 381–385. [Google Scholar]
- Sudarshan, R.; Annigeri, R.G.; Sree Vijayabala, G. Aloe Vera in the Treatment for Oral Submucous Fibrosis—A Preliminary Study. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2012, 41, 755–761. [Google Scholar] [CrossRef]
- Ahmadi, A. Potential Prevention: Aloe Vera Mouthwash May Reduce Radiation-Induced Oral Mucositis in Head and Neck Cancer Patients. Chin. J. Integr. Med. 2012, 18, 635–640. [Google Scholar] [CrossRef]
- Ajmera, N.; Chatterjee, A.; Goyal, V. Aloe Vera: It’s Effect on Gingivitis. J. Indian Soc. Periodontol. 2013, 17, 435–438. [Google Scholar] [CrossRef]
- Bhat, G.; Kudva, P.; Dodwad, V. Aloe Vera: Nature’s Soothing Healer to Periodontal Disease. J. Indian Soc. Periodontol. 2011, 15, 205–209. [Google Scholar] [CrossRef]
- Poor, M.R.; Hall, J.E.; Poor, A.S. Reduction in the Incidence of Alveolar Osteitis in Patients Treated with the SaliCept Patch, Containing Acemannan Hydrogel. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2002, 60, 374–379; discussion 379. [Google Scholar] [CrossRef]
- Fani, M.; Kohanteb, J. Inhibitory Activity of Aloe Vera Gel on Some Clinically Isolated Cariogenic and Periodontopathic Bacteria. J. Oral Sci. 2012, 54, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athiban, P.P.; Borthakur, B.J.; Ganesan, S.; Swathika, B. Evaluation of Antimicrobial Efficacy of Aloe Vera and Its Effectiveness in Decontaminating Gutta Percha Cones. J. Conserv. Dent. 2012, 15, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Ashwlayan, V.D.; Kumar, A.; Verma, M.; Garg, V.K.; Gupta, S. Therapeutic Potential of Calendula officinalis. Pharm. Pharmacol. Int. J. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Khairnar, M.S.; Pawar, B.; Marawar, P.P.; Mani, A. Evaluation of Calendula officinalis as an Anti-Plaque and Anti-Gingivitis Agent. J. Indian Soc. Periodontol. 2013, 17, 741–747. [Google Scholar] [CrossRef]
- Turesky, S.; Gilmore, N.D.; Glickman, I. Reduced Plaque Formation by the Chloromethyl Analogue of Victamine C. J. Periodontol. 1970, 41, 41–43. [Google Scholar] [CrossRef] [Green Version]
- Gazim, Z.C.; Rezende, C.M.; Fraga, S.R.; Svidzinski, T.I.E.; Cortez, D.A.G. Antifungal Activity of the Essential Oil from Calendula officinalis L. (Asteraceae) Growing in Brazil. Braz. J. Microbiol. 2008, 39, 61–63. [Google Scholar] [CrossRef] [Green Version]
- Schmidgall, J.; Schnetz, E.; Hensel, A. Evidence for Bioadhesive Effects of Polysaccharides and Polysaccharide-Containing Herbs in an Ex Vivo Bioadhesion Assay on Buccal Membranes. Planta Med. 2000, 66, 48–53. [Google Scholar] [CrossRef]
- Babaee, N.; Moslemi, D.; Khalilpour, M.; Vejdani, F.; Moghadamnia, Y.; Bijani, A.; Baradaran, M.; Kazemi, M.T.; Khalilpour, A.; Pouramir, M.; et al. Antioxidant Capacity of Calendula officinalis Flowers Extract and Prevention of Radiation Induced Oropharyngeal Mucositis in Patients with Head and Neck Cancers: A Randomized Controlled Clinical Study. Daru 2013, 21, 18. [Google Scholar] [CrossRef] [Green Version]
- Patrick, K.F.; Kumar, S.; Edwardson, P.A.; Hutchinson, J.J. Induction of Vascularisation by an Aqueous Extract of the Flowers of Calendula officinalis L. the European Marigold. Phytomedicine 1996, 3, 11–18. [Google Scholar] [CrossRef]
- Preethi, K.C.; Kuttan, G.; Kuttan, R. Anti-Inflammatory Activity of Flower Extract of Calendula officinalis Linn. and Its Possible Mechanism of Action. Indian J. Exp. Biol. 2009, 47, 113–120. [Google Scholar]
- Preethi, K.C.; Kuttan, G.; Kuttan, R. Antioxidant Potential of an Extract of Calendula officinalis. Flowers in Vitro. and in Vivo. Pharm. Biol. 2006, 44, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Sultan, Z.; Zafar, M.; Shahab, S.; Najeeb, S.; Naseem, M. Green Tea (Camellia Sinensis): Chemistry and Oral Health. Open Dent. J. 2016, 10, 3–10. [Google Scholar] [CrossRef]
- Arab, H.; Maroofian, A.; Golestani, S.; Shafaee, H.; Sohrabi, K.; Forouzanfar, A. Review of the Therapeutic Effects of Camellia Sinensis (Green Tea) on Oral and Periodontal Health. J. Med. Plant Res. 2011, 5, 5465–5469. [Google Scholar]
- Makimura, M.; Hirasawa, M.; Kobayashi, K.; Indo, J.; Sakanaka, S.; Taguchi, T.; Otake, S. Inhibitory Effect of Tea Catechins on Collagenase Activity. J. Periodontol. 1993, 64, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Goenka, P.; Sarawgi, A.; Karun, V.; Nigam, A.G.; Dutta, S.; Marwah, N. Camellia Sinensis (Tea): Implications and Role in Preventing Dental Decay. Pharmacogn. Rev. 2013, 7, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Otake, S.; Makimura, M.; Kuroki, T.; Nishihara, Y.; Hirasawa, M. Anticaries Effects of Polyphenolic Compounds from Japanese Green Tea. Caries Res. 1991, 25, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Tahani, B.; Sabzian, R. Effect of Camellia Sinensis Plant on Decreasing the Level of Halitosis: A Systematic Review. Dent. Res. J. (Isfahan) 2018, 15, 379–384. [Google Scholar]
- Nata’ala, M.; Dalhat, M.; Omoye, B.; Isah, A.; Kabiru, S.; Bashiru, I.; Umar, F. Phytochemical Screening and Antibacterial Activity of Citrus sinensis (L.) Osbeck [Orange] and Citrus Aurantifolia (Cristm.) Swingle [Lime] Stem from Bacteria Associated with Dental Caries. J. Adv. Microbiol. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Aripin, D.; Julaeha, E.; Dardjan, M.; Cahyanto, A. Chemical Composition of Citrus Spp. and Oral Antimicrobial Effect of Citrus Spp. Peels Essential Oils against Streptococcus Mutans. Padjadjaran J. Dent. 2015, 27, 1–11. [Google Scholar] [CrossRef]
- Abdallah, E. Preliminary Phytochemical and Antibacterial Screening of Methanolic Leaf Extract of Citrus Aurantifolia. Pharm. Biotechnol. Curr. Res. 2016, 1, 2. [Google Scholar]
- Pathan, R.K.; Gali, P.R.; Pathan, P.; Gowtham, T.; Pasupuleti, S. In Vitro Antimicrobial Activity of Citrus Aurantifolia and Its Phytochemical Screening. Asian Pac. J. Trop. Dis. 2012, 2, S328–S331. [Google Scholar] [CrossRef]
- Kishore, N.; Verma, A.K. Coconut Palm (Cocos nucifera L.). Nat. Oral Care Dent. Ther. 2020, 271–284. [Google Scholar]
- Yong, J.W.H.; Ge, L.; Ng, Y.F.; Tan, S.N. The Chemical Composition and Biological Properties of Coconut (Cocos nucifera L.) Water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef] [Green Version]
- Loomba, S.; Jothi, V. Cocos nucifera: Its Properties and Contributions to Dentistry. Int. J. Sci. 2013, 1, 1–3. [Google Scholar]
- Peedikayil, F.C.; Sreenivasan, P.; Narayanan, A. Effect of Coconut Oil in Plaque Related Gingivitis—A Preliminary Report. Niger. Med. J. 2015, 56, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidu, S.; Suresh, A. EFFECTS OF TURMERIC (CURCUMA LONGA) IN DENTISTRY. Int. J. Dev. Res. 2018, 8, 21828–21831. [Google Scholar] [CrossRef]
- Mali, A.M.; Behal, R.; Gilda, S.S. Comparative Evaluation of 0.1% Turmeric Mouthwash with 0.2% Chlorhexidine Gluconate in Prevention of Plaque and Gingivitis: A Clinical and Microbiological Study. J. Indian Soc. Periodontol. 2012, 16, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Kim, B.S.; Keum, K.-S.; Yu, H.-H.; Kim, Y.-H.; Chang, B.-S.; Ra, J.-Y.; Moon, H.-D.; Seo, B.-R.; Choi, N.-Y.; et al. Essential Oil of Curcuma Longa Inhibits Streptococcus Mutans Biofilm Formation. J. Food Sci. 2011, 76, H226–H230. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Dinkar, C.; Vaishnav, L.K.; Rao, P.; Rai, M.P.; Fayad, R.; Baliga, M.S. The Indian Spice Turmeric Delays and Mitigates Radiation-Induced Oral Mucositis in Patients Undergoing Treatment for Head and Neck Cancer: An Investigational Study. Integr. Cancer Ther. 2014, 13, 201–210. [Google Scholar] [CrossRef]
- Suhag, A.; Dixit, J.; Prof, D. Role of Curcumin as a Subgingival Irrigant: A Pilot Study. Periodontal Pract. Today 2007, 4, 115–121. [Google Scholar]
- Das, A.D.; Balan, A.; KT, S. Comparative Study of the Efficacy of Curcumin and Turmeric Oil as Chemopreventive Agents in Oral Submucous Fibrosis: A Clinical and Histopathological Evaluation. J. Indian Acad. Oral Med. Radiol. 2010, 22, 88–92. [Google Scholar] [CrossRef]
- Singh, V.; Pal, M.; Gupta, S.; Tiwari, S.K.; Malkunje, L.; Das, S. Turmeric—A New Treatment Option for Lichen Planus: A Pilot Study. Natl. J. Maxillofac. Surg. 2013, 4, 198–201. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Debnath, K.; Rao, N.K.H. A Comparative Evaluation of the Efficacy of Curcumin and Chlorhexidine Mouthrinses on Clinical Inflammatory Parameters of Gingivitis: A Double-Blinded Randomized Controlled Clinical Study. J. Indian Soc. Periodontol. 2017, 21, 132–137. [Google Scholar] [CrossRef]
- Yadav, R.K.; Tikku, A.P.; Chandra, A.; Verma, P.; Bains, R.; Bhoot, H. A Comparative Evaluation of the Antimicrobial Efficacy of Calcium Hydroxide, Chlorhexidine Gel, and a Curcumin-Based Formulation against Enterococcus Faecalis. Natl. J. Maxillofac. Surg. 2018, 9, 52–55. [Google Scholar] [CrossRef]
- Madan, S.; Kashyap, S.; Mathur, G. Glycyrrhiza Glabra: An Efficient Medicinal Plant for Control of Periodontitis—A Randomized Clinical Trial. J. Int. Clin. Dent. Res. Organ. 2019, 11, 32–35. [Google Scholar] [CrossRef]
- Kaur, H. Glycyrrhiza Glabra: A Phytopharmacological Review. Int. J. Pharm. Sci. Res. 2013, 4, 2470–2477. [Google Scholar] [CrossRef]
- Jatav, V.; Singh, S.; Khatri, P.; Sharma, A. Recent Pharmacological Trends of Glycyrrhiza Glabra Linn. Unani Res. 2011, 1, 1–11. [Google Scholar] [CrossRef]
- Hambire, C.; Hambire, U. Glycyrrhiza Glabra: Its Role in Dentistry. SRM J. Res. Dent. Sci. 2020, 11, 106–109. [Google Scholar] [CrossRef]
- Sidhu, P.; Shankargouda, S.; Rath, A.; Hesarghatta Ramamurthy, P.; Fernandes, B.; Kumar Singh, A. Therapeutic Benefits of Liquorice in Dentistry. J. Ayurveda Integr. Med. 2020, 11, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Oh, S.; Kwon, H.-S.; Oh, Y.-S.; Lim, S.S.; Shin, H.-K. Anti-Inflammatory Effect of Roasted Licorice Extracts on Lipopolysaccharide-Induced Inflammatory Responses in Murine Macrophages. Biochem. Biophys. Res. Commun. 2006, 345, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Zhang, H. Phytochemical Constituents, Health Benefits, and Industrial Applications of Grape Seeds: A Mini-Review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delimont, N.M.; Carlson, B.N. Prevention of Dental Caries by Grape Seed Extract Supplementation: A Systematic Review. Nutr. Health 2020, 26, 43–52. [Google Scholar] [CrossRef]
- Singla, S.; Malhotra, R.; Nd, S.; Saxena, S. Antibacterial Efficacy of Mouthwash Prepared from Pomegranate, Grape Seed and Guava Extracts against Oral Streptococci: An in Vivo Study. J. Clin. Pediatr. Dent. 2018, 42, 109–113. [Google Scholar] [CrossRef]
- Süntar, I.P.; Akkol, E.K.; Yilmazer, D.; Baykal, T.; Kirmizibekmez, H.; Alper, M.; Yeşilada, E. Investigations on the in Vivo Wound Healing Potential of Hypericum perforatum L. J. Ethnopharmacol. 2010, 127, 468–477. [Google Scholar] [CrossRef]
- Süntar, I.; Oyardi, O.; Akkol, E.K.; Ozçelik, B. Antimicrobial Effect of the Extracts from Hypericum perforatum against Oral Bacteria and Biofilm Formation. Pharm. Biol. 2016, 54, 1065–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpag, O.F.; Duran, N.; Açikgül, F.C.; Türkmen, M. Comparison of Minimum Inhibitory Concentrations of Hypericum perforatum L. Essential Oils, 0.2% Chlorhexidine and 10 % Povidone-Iodine Over Aggregatibacter Actinomycetemcomitans and Porphyromonas Gingivalis. J. Essent. Oil Bear. Plants 2020, 23, 1192–1205. [Google Scholar] [CrossRef]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An Overview. Pharmacogn. Rev. 2011, 5, 82. [Google Scholar] [CrossRef] [Green Version]
- Miraj, S.; Alesaeidi, S. A Systematic Review Study of Therapeutic Effects of Matricaria Recuitta Chamomile (Chamomile). Electron. Phys. 2016, 8, 3024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Flaviana, M.; Morais-Braga, B.; Rocha, J.E.; Douglas, H.; Coutinho, M.; Salehi, B.; Tabanelli, G.; et al. Matricaria Genus as a Source of Antimicrobial Agents: From Farm to Pharmacy and Food Applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- Şener, B. Herbal Extracts Used in Dental Disorders. Biomed. J. Sci. Tech. Res. 2019, 19, 14107–14111. [Google Scholar] [CrossRef]
- Gomes, V.T.S.; Nonato Silva Gomes, R.; Gomes, M.S.; Joaquim, W.M.; Lago, E.C.; Nicolau, R.A. Effects of Matricaria recutita (L.) in the Treatment of Oral Mucositis. Sci. World J. 2018, 2018, 4392184. [Google Scholar] [CrossRef] [Green Version]
- Ghitu, A.; Schwiebs, A.; Radeke, H.H.; Avram, S.; Zupko, I.; Bor, A.; Pavel, I.Z.; Dehelean, C.A.; Oprean, C.; Bojin, F.; et al. A Comprehensive Assessment of Apigenin as an Antiproliferative, Proapoptotic, Antiangiogenic and Immunomodulatory Phytocompound. Nutrients 2019, 11, 858. [Google Scholar] [CrossRef] [Green Version]
- Mahendran, G.; Rahman, L. Ethnomedicinal, Phytochemical and Pharmacological Updates on Peppermint (Mentha × piperita L.)-A Review. Phytother. Res. 2020, 34, 2088–2139. [Google Scholar] [CrossRef]
- Anwar, F.; Abbas, A.; Mehmood, T.; Gilani, A.-H.; Rehman, N. Mentha: A Genus Rich in Vital Nutra-Pharmaceuticals—A Review. Phyther. Res. 2019, 33, 2548–2570. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A Review of the Bioactivity and Potential Health Benefits of Peppermint Tea (Mentha piperita L.). Phyther. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Raghavan, R.; Shyamala Devi, M.; Varghese, M.; Joseph, A.; Madhavan, S.S.; Sreedevi, P.V.; Author, C. Effectiveness of Mentha piperita Leaf Extracts against Oral Pathogens: An in Vitro Study. J. Contemp. Dent. Pract. 2018, 19, 1042–1046. [Google Scholar] [CrossRef]
- Geraci, A.; Di Stefano, V.; Di Martino, E.; Schillaci, D.; Schicchi, R. Essential Oil Components of Orange Peels and Antimicrobial Activity. Nat. Prod. Res. 2017, 31, 653–659. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Phi, N.T.L.; Sawamura, M. Chemical Composition of Peel Essential Oils of Sweet Oranges (Citrus sinensis) from Uganda and Rwanda. J. Essent. Oil Bear. Plants 2013, 12, 26–33. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Phi, N.T.L.; Sawamura, M. Chemical Profile, Antifungal, Antiaflatoxigenic and Antioxidant Activity of Citrus Maxima Burm. and Citrus sinensis (L.) Osbeck Essential Oils and Their Cyclic Monoterpene, DL-Limonene. Food Chem. Toxicol. 2010, 48, 1734–1740. [Google Scholar] [CrossRef]
- Yadav, H.K.; Yadav, R.K.; Chandra, A.; Thakkar, R.R. The Effectiveness of Eucalyptus Oil, Orange Oil, and Xylene in Dissolving Different Endodontic Sealers. J. Conserv. Dent. 2016, 19, 332. [Google Scholar] [CrossRef] [Green Version]
- Arvanitoyannis, I.S.; Varzakas, T.H. Fruit/Fruit Juice Waste Management: Treatment Methods and Potential Uses of Treated Waste. Waste Manag. Food Ind. 2008, 569–628. [Google Scholar] [CrossRef]
- Amri, E.; Mamboya, F. Papain, a Plant Enzyme of Biological Importance: A Review. Am. J. Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.C.; Mascarini, R.C.; da Silva, B.M.C.G.; Flório, F.M.; Basting, R.T. Effect of a Papain-Based Gel for Chemomechanical Caries Removal on Dentin Shear Bond Strength. J. Dent. Child. 2007, 74, 93–97. [Google Scholar]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Duca, A.; Alexa, E.; Dehelean, C.A.; Soica, C.; Danciu, C.; Popescu, I.; Cocan, I.; Lalescu, D.; Muntean, D.M. Assessment of lipid profile of eight propolis samples from western romania. Farmacia 2019, 67, 126–132. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Król, W.; Bankova, V.; Sforcin, J.M.; Szliszka, E.; Czuba, Z.; Kuropatnicki, A.K. Propolis: Properties, Application, and Its Potential. Evid. Based. Complement. Altern. Med. 2013, 2013, 807578. [Google Scholar] [CrossRef]
- Sinha, D.; Sinha, A. Natural Medicaments in Dentistry. AYU (Int. Q. J. Res. Ayurveda) 2014, 35, 113. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, M.; Arimatsu, K.; Minagawa, T.; Matsuda, Y.; Sato, K.; Takahashi, N.; Nakajima, T.; Yamazaki, K. Brazilian Propolis Mitigates Impaired Glucose and Lipid Metabolism in Experimental Periodontitis in Mice. BMC Complementary Altern. Med. 2016, 16, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Auria, F.; Tecca, M.; Scazzocchio, F.; Renzini, V.; Strippoli, V. Effect of Propolis on Virulence Factors of Candida Albicans. J. Chemother. 2003, 15, 454–460. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An Update Review of Its Phytochemistry and Biological Activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (Rosemary) as Therapeutic and Prophylactic Agent. J. Biomed. Sci. 2019, 26, 1–22. [Google Scholar] [CrossRef]
- Begum, A.; Sandhya, S.; Ali, S.S.; Vinod, K.R.; Reddy, S.; Banji, D. An In-Depth Review on the Medicinal Flora Rosmarinus officinalis (Lamiaceae). Acta Sci. Polonorum Technol. Aliment. 2013, 12, 61–74. [Google Scholar]
- Al-Sereiti, M.R.; Abu-Amer, K.M.; Sen, P. Pharmacology of Rosemary (Rosmarinus officinalis Linn.) and Its Therapeutic Potentials; NISCAIR-CSIR: New Delhi, India, 1999. [Google Scholar]
- Shruthi, S.; Vijayalaxmi, K. Antigenotoxic Effects of a Polyherbal Drug Septilin against the Genotoxicity of Cyclophosphamide in Mice. Toxicol. Rep. 2016, 3, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Daswani, B.R.; Yegnanarayan, R. Immunomodulatory Activity of Septilin, a Polyherbal Preparation. Phytother. Res. 2002, 16, 162–165. [Google Scholar] [CrossRef]
- Khanna, N.; Sharma, S.B. Anti-Inflammatory and Analgesic Effect of Herbal Preparation. Indian J. Med. Sci. 2001, 55, 195–202. [Google Scholar]
- Batiha, G.E.-S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Ben Kahla-Nakbi, A.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The Chemical Composition and Biological Activity of Clove Essential Oil, Eugenia Caryophyllata (Syzigium aromaticum L. Myrtaceae): A Short Review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Rojas, D.F.C.; Souza, C.R.F.; Oliveira, W.P. Clove (Syzygium aromaticum): A Precious Spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Popa, Z.; Rusu, L.; Susan, R.; Pinzaru, I.; Ardelean, E.; Borcan, F.; Voicu, M.; Sas, I.; Popovici, R.A.; Lazureanu, V. Obtaining and Characterization of a Polyurethane Carrier Used for Eugenol as a Possible Remedy in Oral Therapies. Mater. Plast. 2018, 55, 9–13. [Google Scholar] [CrossRef]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant Activity of Clove Oil—A Powerful Antioxidant Source. Arab. J. Chem. 2012, 4, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Sofia, P.K.; Prasad, R.; Vijay, V.K.; Srivastava, A.K. Evaluation of Antibacterial Activity of Indian Spices against Common Foodborne Pathogens. Int. J. Food Sci. Technol. 2007, 42, 910–915. [Google Scholar] [CrossRef]
- Buggapati, L. Herbs in Dentistry. Int. J. Pharm. Sci. Invent. 2016, 5, 7–12. [Google Scholar]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca Alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50. [Google Scholar] [CrossRef] [Green Version]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A.; Nader Pazyar, C. A Review of Applications of Tea Tree Oil in Dermatology. Int. J. Dermatol. 2012, 52, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.; Petermann, K.D.; Vedovello, S.A.S.; Degan, V.; Lucato, A.; Franzini, C.M. Antimicrobial Effect of Melaleuca Alternifolia Dental Gel in Orthodontic Patients. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 198–202. [Google Scholar] [CrossRef]
- Salehi, B.; Prakash Mishra, A.; Shukla, I.; Sharifi-Rad, M.; del Mar Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasri Nasrabadi, N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, Thyme, and Other Plant Sources: Health and Potential Uses. Phyther. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Soto-Mendívil, E.A.; Moreno-Rodríguez, J.F.; Estarrón-Espinosa, M.; García-Fajardo, J.A.; Obledo-Vázquez, E.N. Chemical composition and fungicidal activity of the essential oil Thymus vulgaris against Alternaria citr. e-Gnosis [Online] 2006, 4, 1–7. [Google Scholar]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.; Horhat, F. Thymus Vulgaris Essential Oil: Chemical Composition and Antimicrobial Activity. J. Med. Life 2014, 7, 56. [Google Scholar] [PubMed]
- Sas, I. Thymus Vulgaris Extract Formulated as Cyclodextrin Complexes: Synthesis, Characterization, Antioxidant Activity and in Vitro Cytotoxicity Assessment. Farmacia 2019, 67, 442–451. [Google Scholar] [CrossRef]
- Botelho, M.; Nogueira, N.A.P.; Bastos, G.; Fonseca, S.; Lemos, T.; Matos, F.; Montenegro, D.; Heukelbach, J.; Rao, V.S.; Brito, G. Antimicrobial Activity of the Essential Oil from Lippia Sidoides, Carvacrol and Thymol against Oral Pathogens. Braz. J. Med Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2007, 40, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vincenzi, M.; Stammati, A.; Silano, M. Constituents of Aromatic Plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef]
- Nieves, J.W. Alternative Therapy through Nutrients and Nutraceuticals. Osteoporos. Fourth Ed. 2013, 1739–1749. [Google Scholar] [CrossRef]
- Faggion, C.M. Guidelines for Reporting Pre-Clinical In Vitro Studies on Dental Materials. J. Evid. Based Dent. Pract. 2012, 12, 182–189. [Google Scholar] [CrossRef]
- Guran, K.; Buzatu, R.; Pinzaru, I.; Boruga, M.; Marcovici, I.; Coricovac, D.; Avram, S.; Poenaru, M.; Susan, M.; Susan, R.; et al. In Vitro Pharmaco-Toxicological Characterization of Melissa Officinalis Total Extract Using Oral, Pharynx and Colorectal Carcinoma Cell Lines. Process 2021, 9, 850. [Google Scholar] [CrossRef]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. A Review of the Common Models Used in Mechanistic Studies on Demineralization-Remineralization for Cariology Research. Dent. J. 2017, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Amaechi, B.T.; Tenuta, L.M.A.; Ricomini Filho, A.P.; Cury, J.A. Protocols to Study Dental Caries In Vitro: Microbial Caries Models. Methods Mol. Biol. 2019, 1922, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.R.; Gomes, R.T.; Resende, M.; de Almeida, O.P.; Colleta, R. Della Isolation and Characterization of Gingival Fibroblasts Positive for Alkaline Phosphatase in Patients with Chronic Periodontitis and Drug-Induced Gingival Hyperplasia. Rev. Odonto Ciência 2010, 25, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Ara, T.; Kurata, K.; Hirai, K.; Uchihashi, T.; Uematsu, T.; Imamura, Y.; Furusawa, K.; Kurihara, S.; Wang, P.-L. Human Gingival Fibroblasts Are Critical in Sustaining Inflammation in Periodontal Disease. J. Periodontal Res. 2009, 44, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mountcastle, S.E.; Cox, S.C.; Sammons, R.L.; Jabbari, S.; Shelton, R.M.; Kuehne, S.A. A Review of Co-Culture Models to Study the Oral Microenvironment and Disease. J. Oral Microbiol. 2020, 12, 1773122. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulou, P.G.; Benakanakere, M.R.; Galicia, J.C.; Kinane, D.F. Epithelial Cell Pro-Inflammatory Cytokine Response Differs across Dental Plaque Bacterial Species. J. Clin. Periodontol. 2010, 37, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, M.; Kim, S.; Sethi, P.; DŘzgŘneş, N.; Konopka, K. Porphyromonas Gingivalis Stimulates IL-6 and IL-8 Secretion in GMSM-K, HSC-3 and H413 Oral Epithelial Cells. Anaerobe 2014, 28, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Ö.; Young, P.A.; Lamont, R.J.; Kenny, G.E. Gingival Epithelial Cell Signalling and Cytoskeletal Responses to Porphyromonas Gingivalis Invasion. Microbiology 2003, 149, 2417–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fives-Taylor, P.; Meyer, D.; Mintz, K. Characteristics of Actinobacillus Actinomycetemcomitans Invasion of and Adhesion to Cultured Epithelial Cells. Adv. Dent. Res. 1995, 9, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.W.; Shi, W.; Huang, G.T.-J.; Kinder Haake, S.; Park, N.-H.; Kuramitsu, H.; Genco, R.J. Interactions between Periodontal Bacteria and Human Oral Epithelial Cells: Fusobacterium Nucleatum Adheres to and Invades Epithelial Cells. Infect. Immun. 2000, 68, 3140–3146. [Google Scholar] [CrossRef] [Green Version]
- Bodet, C.; Chandad, F.; Grenier, D. Inflammatory Responses of a Macrophage/Epithelial Cell Co-Culture Model to Mono and Mixed Infections with Porphyromonas Gingivalis, Treponema Denticola, and Tannerella Forsythia. Microbes Infect. 2006, 8, 27–35. [Google Scholar] [CrossRef]
- Bates, A.M.; Fischer, C.L.; Abhyankar, V.P.; Johnson, G.K.; Guthmiller, J.M.; Progulske-Fox, A.; Brogden, K.A. Matrix Metalloproteinase Response of Dendritic Cell, Gingival Epithelial Keratinocyte, and T-Cell Transwell Co-Cultures Treated with Porphyromonas Gingivalis Hemagglutinin-B. Int. J. Mol. Sci. 2018, 19, 3923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancilio, S.; di Giacomo, V.; Di Giulio, M.; Gallorini, M.; Marsich, E.; Travan, A.; Tarusha, L.; Cellini, L.; Cataldi, A. Biological Responses of Human Gingival Fibroblasts (HGFs) in an Innovative Co-Culture Model with Streptococcus mitis to Thermosets Coated with a Silver Polysaccharide Antimicrobial System. PLoS ONE 2014, 9, e96520. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M.; D’ERCOLE, S.; Zara, S.; Cataldi, A.; Cellini, L. Streptococcus mitis/Human Gingival Fibroblasts Co-culture: The Best Natural Association in Answer to the 2-hydroxyethyl Methacrylate Release. Apmis 2012, 120, 139–146. [Google Scholar] [CrossRef] [PubMed]
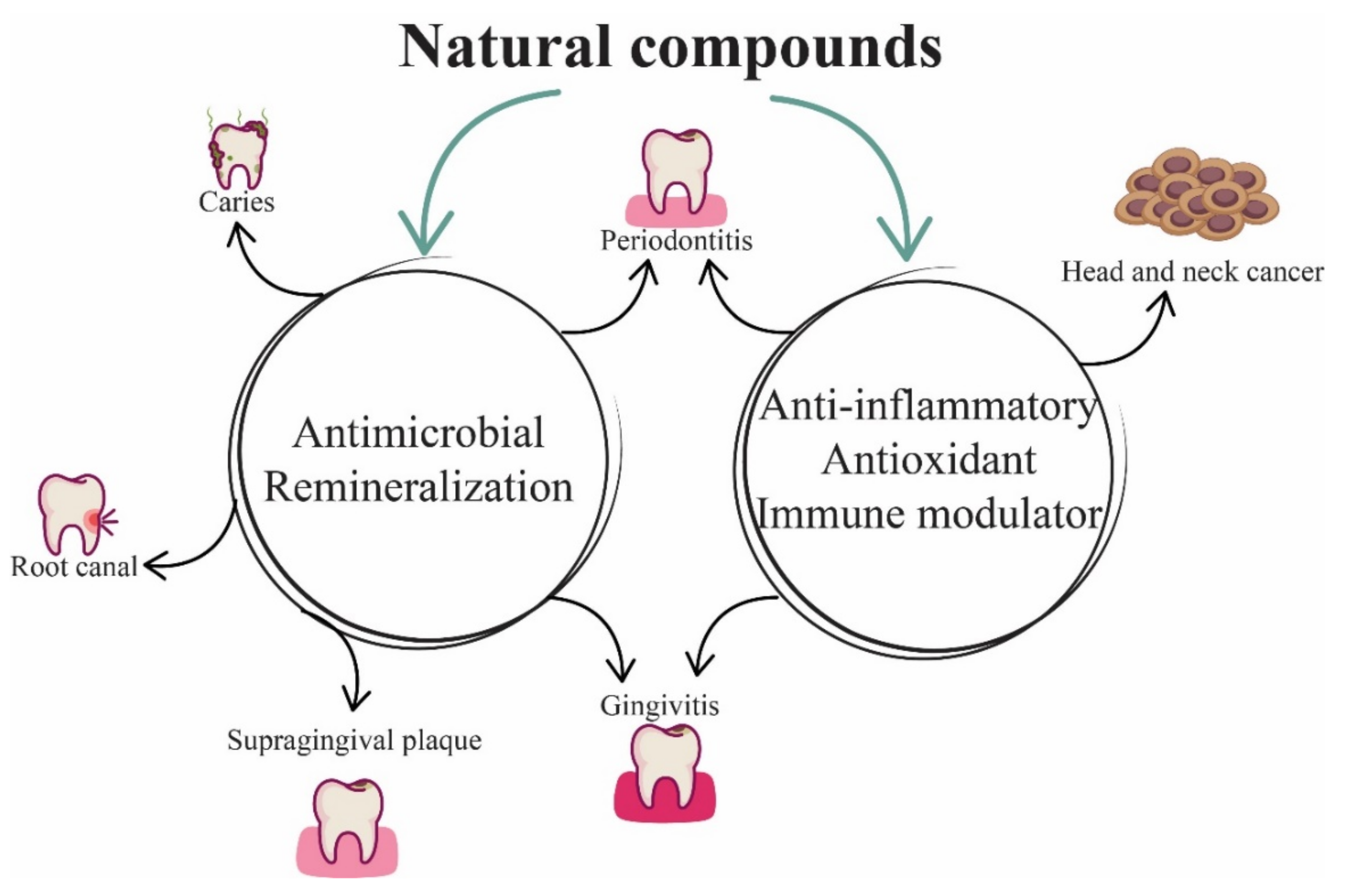






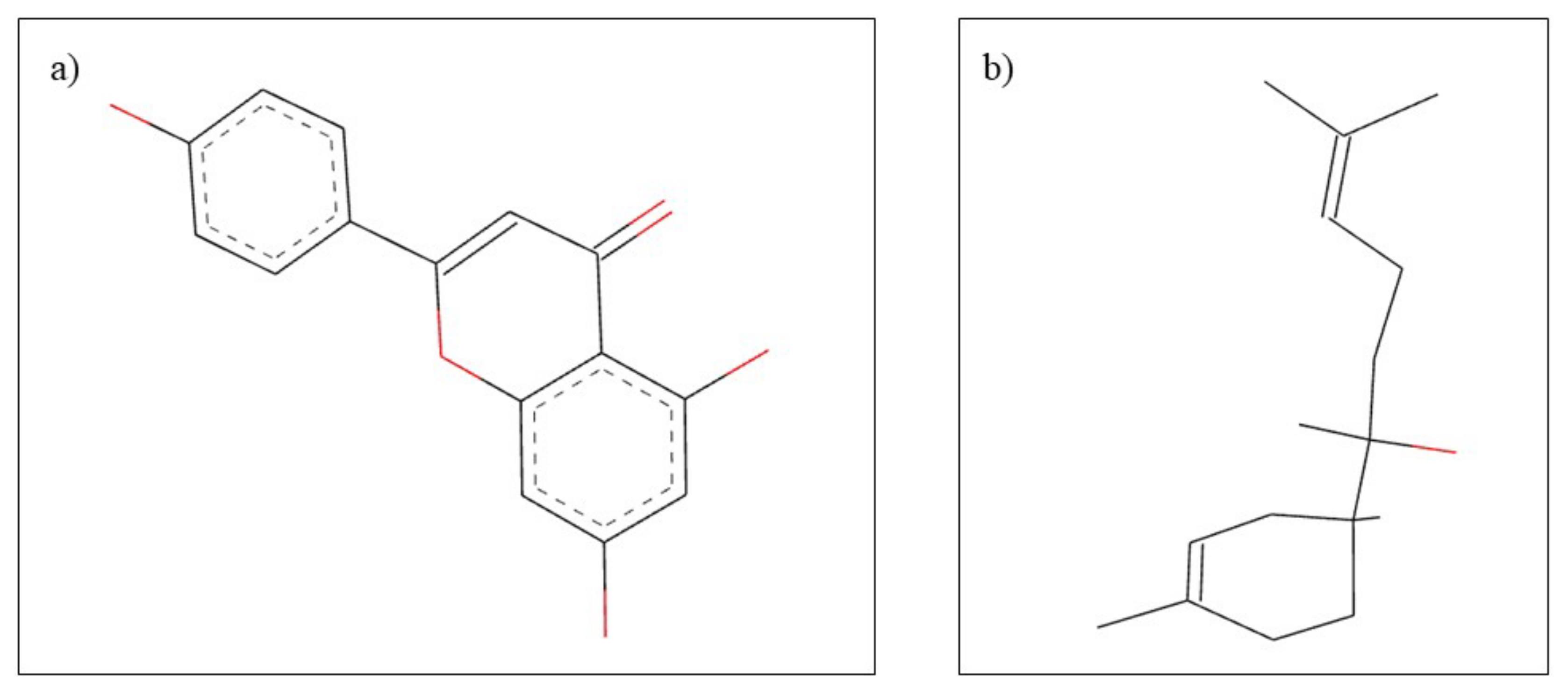










| Natural Compounds | Dentistry Applications | References |
|---|---|---|
| α-thujone, and β-thujone | Antimicrobial activity against S. mutans, L. rhamnosus and A. viscosus Anticandidal activities Antiplaque activity | [36] |
| Allicin | Antimicrobial activity against S. mutans, S.sobrinus, and A. oris | [37] |
| Carvone | Treatment of gingivitis and periodontal disease | [5] |
| Catechins | Antibacterial activity against S. mutans and S. sobrinus | [38] |
| Chitosan | Antihemorrhagic Analgesic Anti-inflamatory activity Antibacterial and antifungal activity | [39] |
| Cinnamonic acid | Periodontitis treatment Decreases periodontal inflammation Antibacterial activity against S. mutans and L. casei | [40,41] |
| Curcumin | Antioxidant and anti-inflamatory activity Analgesic in dental pain | [42] |
| Eugenol | Antibacterial activity against P. gingivalis Antibiofilm activity against C. albicans and S. mutans Anti-inflammatory activity | [41,43] |
| Gingerol | Anti-inflamatory activity Antioxidant activity Antimicrobial activity against P. gingivalis and P. endodontalis Treatment of recurrent apthous stomatitis, xerostomia, dental caries and gingivitis | [44] |
| Linalool | Antibacterial activity against P. gingivalis and S. mutans | [41] |
| Menthol | Antiplaque and anti-gingivitis agent | [45] |
| Sanguinarine | Gingivitis and periodontal disease | [46] |
| tt-Farnesol | Antimicrobial activity against S. mutans | [47] |
| Rosmarinic acid | Antimicrobial activity on Gram-negative and positive bacteria (S. aureus, S. albus, V. cholerae and E. coli) | [5] |
| Thymol | Antibacterial activity against S. mutans | [48] |
| Quercetin | Treatment of cancers, periodontal disease, oral lesions, tooth decay, and oral infections | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milutinovici, R.-A.; Chioran, D.; Buzatu, R.; Macasoi, I.; Razvan, S.; Chioibas, R.; Corlan, I.V.; Tanase, A.; Horia, C.; Popovici, R.A.; et al. Vegetal Compounds as Sources of Prophylactic and Therapeutic Agents in Dentistry. Plants 2021, 10, 2148. https://doi.org/10.3390/plants10102148
Milutinovici R-A, Chioran D, Buzatu R, Macasoi I, Razvan S, Chioibas R, Corlan IV, Tanase A, Horia C, Popovici RA, et al. Vegetal Compounds as Sources of Prophylactic and Therapeutic Agents in Dentistry. Plants. 2021; 10(10):2148. https://doi.org/10.3390/plants10102148
Chicago/Turabian StyleMilutinovici, Raluca-Adriana, Doina Chioran, Roxana Buzatu, Ioana Macasoi, Susan Razvan, Raul Chioibas, Ion Virgil Corlan, Alina Tanase, Calniceanu Horia, Ramona Amina Popovici, and et al. 2021. "Vegetal Compounds as Sources of Prophylactic and Therapeutic Agents in Dentistry" Plants 10, no. 10: 2148. https://doi.org/10.3390/plants10102148






