The Efficient and Easy Micropropagation Protocol of Phyllanthus niruri
Abstract
1. Introduction
2. Results
2.1. Surface Sterilization
2.2. Shoot Multiplication Using Different Basal Medium and Its Strength
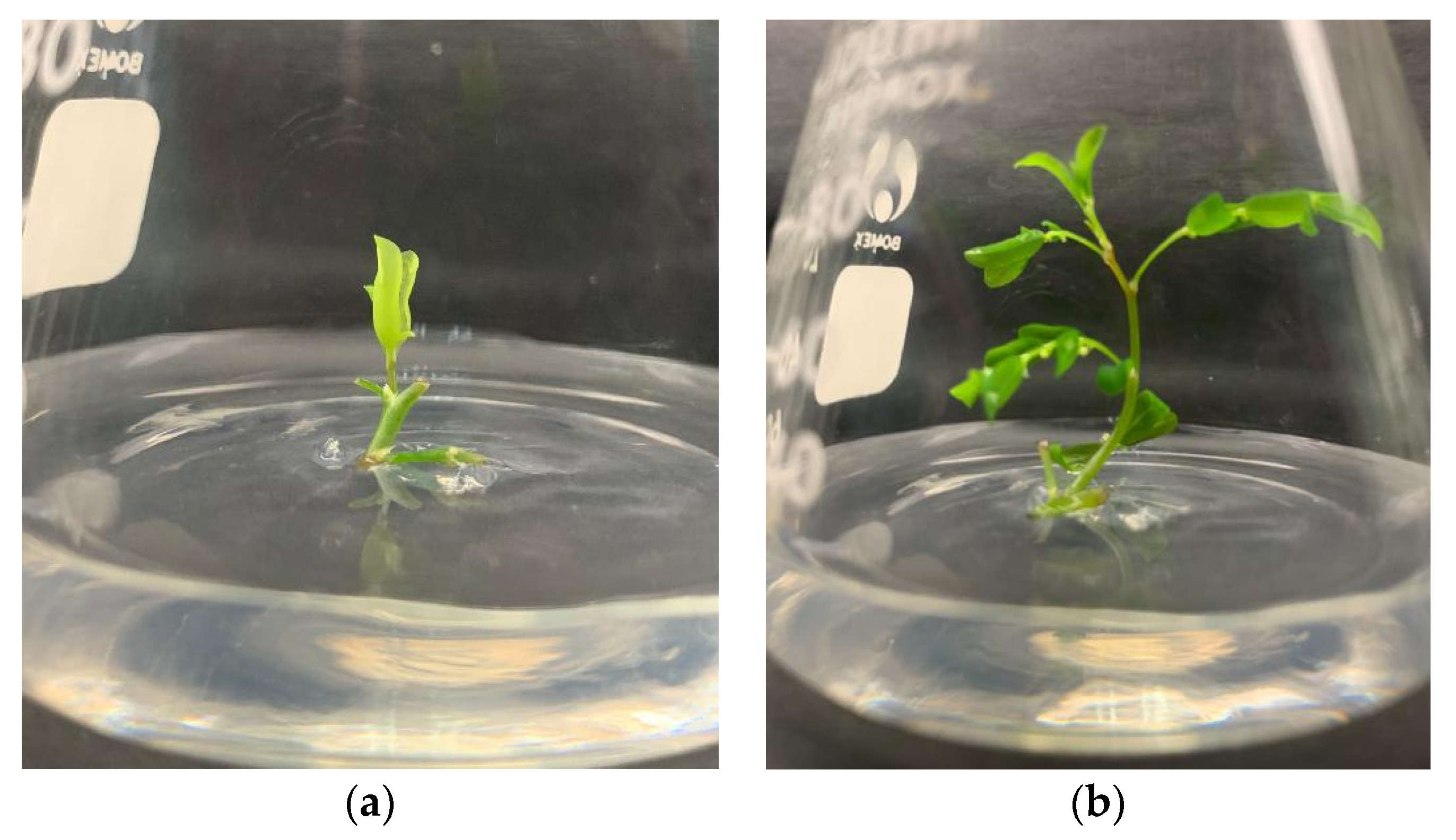
2.3. Shoot Multiplication Using Different Plant Growth Regulators and Its Concentrations
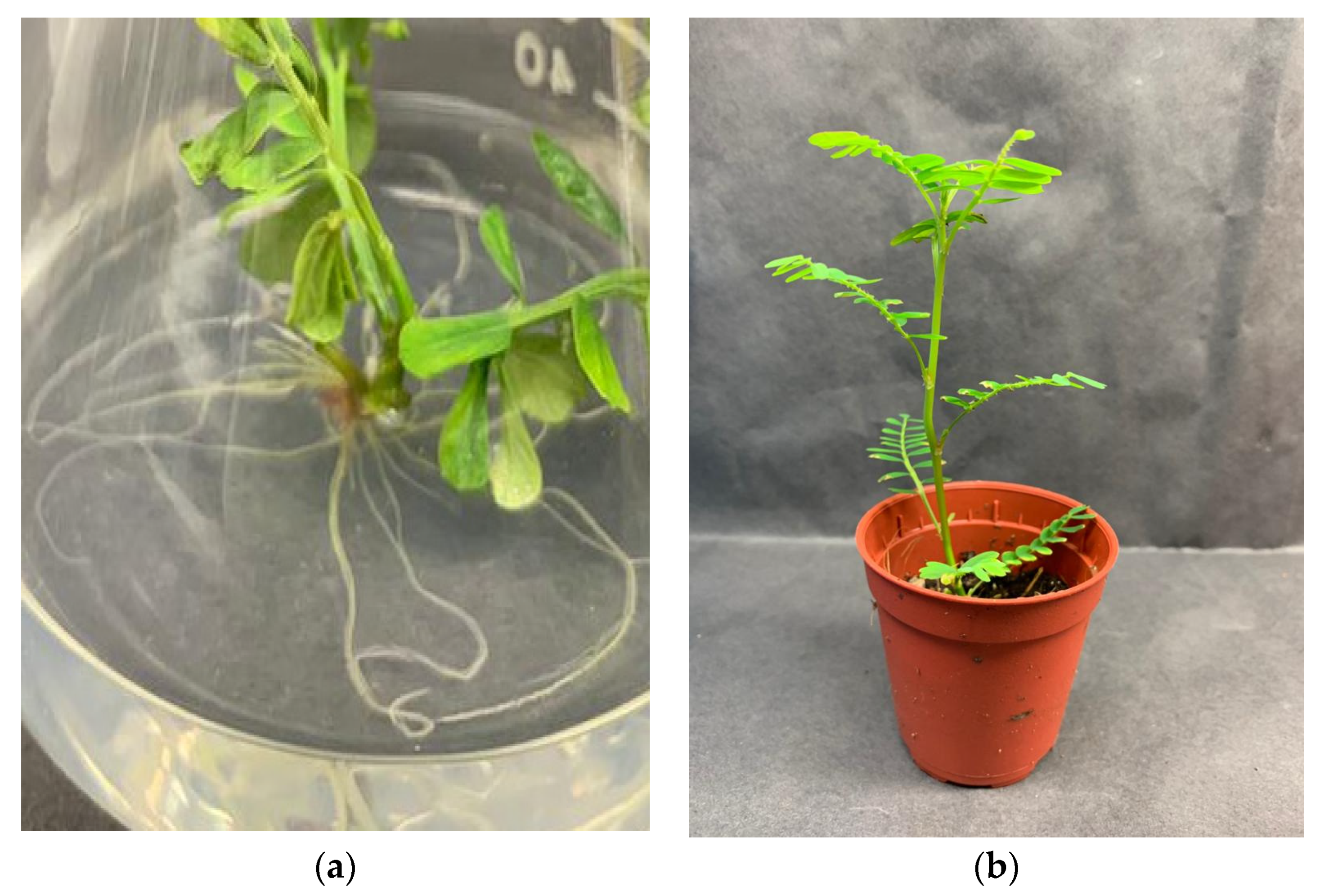
2.4. In Vitro Rooting and Acclimatization
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Maintenance
4.2. Surface Sterilization of Explants
4.3. Basal Media and Its Strength
4.4. Plant Growth Regulators and Its Concentrations
4.5. In Vitro Rooting and Acclimatization
4.6. Experimental Design and Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samat, N.M.A.A.; Ahmad, S.; Awang, Y.; Bakar, R.A.H.B.; Hakiman, M. Alterations in herbage yield, antioxidant acytivities, phytochemical contents, and bioactive compoubds of Sabah snake grass (Clinacanthus nutans L.) with regards to harvesting age and harvesting frequency. Molecules 2020, 25, 2833. [Google Scholar] [CrossRef]
- Haida, Z.; Hakiman, M. A review of therapeutic potentials of Clinacanthus nutans as source for alternative medicines. Sains Malays. 2019, 48, 2683–2691. [Google Scholar]
- DOA: Department of Agriculture. Reports on Progress Production for Organic Certified Farm; DOA: Washington, DC, USA, 2015. [Google Scholar]
- Farizah, A.; Azlan, S.Z.; Noorasiah, M.; Majid, S.A.A.; Ahmad, F.; Zaidi, F.A.S.; Majid, A.A.F. Issues and challenges in the development of the herbal industry in Malaysia. Prosiding Perkem. 2015, 10, 227–238. [Google Scholar]
- Unander, D.W.; Blumberg, B.S. In vitro activity of phyllanthus (Euphorbiaceae) species against the DNA polymerase of hepatitis viruses: Effects of growing environment and inter- and intra-specific differences. Econ. Bot. 1991, 45, 225–242. [Google Scholar] [CrossRef]
- Calixto, J.B.; Santos, A.R.S.; Cechinel Filho, V.; Yunes, R.A. A review of the plants of the genus phyllanthus: Their chemistry, pharmacology, and therapeutic potential. Med. Res. Rev. 1998, 18, 225–258. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Chandran, C.; Kulothungan, S. In vitro propagation of Phyllanthus niruri L.—A medicinal plant. Technovation 2008, 1, 131–133. [Google Scholar] [CrossRef]
- Chatterjee, M.; Sil, P.C. Hepatoprotective effect of aqueous extract of Phyllanthus niruri on nimesulide-induced oxidative stress in vivo. Indian J. Biochem. Biophys. 2006, 43, 299–305. [Google Scholar] [PubMed]
- Harish, R.; Shivanandappa, T. Antioxidant activity and hepatoprotective potential of Phyllanthus niruri. Food Chem. 2006, 95, 180–185. [Google Scholar] [CrossRef]
- Rajeshkumar, N.V.; Joy, K.L.; Kuttan, G.; Ramsewak, R.S.; Nair, M.G.; Kuttan, R. Antitumour and anticarcinogenic activity of Phyllanthus amarus extract. J. Ethnopharmacol. 2002, 81, 17–22. [Google Scholar] [CrossRef]
- Ishimaru, K.; Yoshimatsu, K.; Yamakawa, T.; Kamada, H.; Shimomura, K. Phenolic constituents in tissue cultures of Phyllanthus niruri. Phytochem. Rev. 1992, 31, 2015–2018. [Google Scholar] [CrossRef]
- Qian-Cutrone, J.; Huang, S.; Trimble, J.; Li, H.; Lin, P.F.; Alam, M.; Kadow, K.F. Niruriside, a new HIV REV/RRE binding inhibitor from Phyllanthus niruri. J. Nat. Prod. 1996, 59, 196–199. [Google Scholar] [CrossRef]
- Kunle, O.F.; Egharevba, H.O.; Ahmadu, P.O. Standardization of herbal medicines—A review. Biodivers. Conserv. 2012, 4, 101–112. [Google Scholar] [CrossRef]
- Wider, B.; Shang, H.; Li, X.; Ernst, E. Quality of herbal medicines: Challenges and solutions. Complement. Ther. Med. 2012, 20, 100–106. [Google Scholar]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- Hashim, Y.Z.; Salleh, H.M.; Puad, N.I.; Fuad, F.A.; Eissa, M.; Zainurin, N.A. Secondary metabolite research in Malaysia: Current status and future prospects. Second. Metab. Source Appl. 2018, 5, 101. [Google Scholar]
- Haida, Z.; Hakiman, M.A. comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 2019, 7, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Haida, Z.; Syahida, A.; Ariff, S.M.; Maziah, M.; Hakiman, M. Factors affecting cell biomass and flavonoid production of Ficus deltoidea var. kunstleri in cell suspension culture system. Sci. Rep. 2019, 9, 9533. [Google Scholar] [PubMed]
- Hussain, A.; Ahmed, I.; Nazir, H.; Ullah, I. Recent Advances in Plant In Vitro Culture, 2nd ed.; Annarita, L., Laura, M.R.R., Eds.; InTech: Rijeka, Croatia, 2012; pp. 1–28. [Google Scholar]
- Najhah, M.Y.; Jaafar, H.Z.E.; Nakasha, J.J.; Hakiman, M. Shoot multiplication and callus induction of Labisia pumila var. alata as influenced by different plant growth regulators treatments and its polyphenolic activities compared with the wild plant. Molecules 2021, 26, 3229. [Google Scholar]
- Hassan, S.A.M.; Zayed, N.S. Factor controlling micropropagation of fruit trees: A review. Sci. Int. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Padma, P.B.; Ilyas, M.M. In vitro plant regeneration and callus formation from the nodal explants of Phyllanthus niruri L. (Euphorbiaceae)—A medicinal herb. Int. J. Pharm. Technol. 2011, 3, 1958–1970. [Google Scholar]
- Gami, B.; Kothari, I.L. Antioxidant and antimicrobial activity of in vivo and in vitro grown plants of Phyllanthus niruri L. Int. J. Pharm. Bio. Sci. 2011, 2, 79–89. [Google Scholar]
- Islam, M.T.; Dembele, D.P.; Keller, E.J. Influence of explant, temperature and different culture vessels on in vitro culture for germplasm maintenance of four mint accessions. Plant Cell Tissue Organ Cult. 2005, 81, 123–130. [Google Scholar] [CrossRef]
- Ahmad, A.; Qamar, M.T.U.; Shoukat, A.; Aslam, M.M.; Tariq, M.; Hakiman, M.; Joyia, F.A. The effects of genotypes and media composition on callogenesis, regeneration and cell suspension culture of chamomile (Matricaria chamomilla L.). PeerJ 2021, 9, e11464. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Jones, A.M. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Appl. Microbiol. Biotechnol. 2020, 104, 9449–9485. [Google Scholar] [CrossRef] [PubMed]
- Zahid, N.A.; Jaafar, H.Z.E.; Hakiman, M. Micropropagation of ginger (Zingiber officinale Roscoe) ‘Bentong’ and evaluation of its secondary metabolites and antioxidant activities compared with the conventionally propagated plant. Plants 2021, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Preil, W. Plant Tissue Culture 100 Years Since Gottlied Haberlandt, 2nd ed.; Margit, L., Waltraud, R., Eds.; Thomson Press: Chennai, India, 2003; pp. 115–133. [Google Scholar]
- Su, Y.H.; Liu, Y.B.; Zhang, X.S. Auxin–cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Trigiano, R.; Gray, D. Development and Biotechnology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 91–98. [Google Scholar]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular mechanisms of plant regeneration. Annu. Rev. Plant Biol. 2019, 70, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R.; Samantaray, S.; Das, P. In vitro manipulation and propagation of medicinal plants. Biotechnol. Adv. 2000, 18, 91–120. [Google Scholar] [CrossRef]
- Martin, K.P.; Sunandakumari, C.; Chithra, M.; Madhusoodanan, P.V. Influence of auxins in direct in vitro morphogenesis of Euphorbia nivulia, a lectinaceous medicinal plant. Vitr. Cell Dev. Biol. Plant 2005, 41, 314–319. [Google Scholar] [CrossRef]
- Srinivasan, M.; Nachiappan, V.; Rajasekharan, R. Potential application of urea-derived herbicides as cytokinins in plant tissue culture. J. Biosci. 2006, 31, 599–605. [Google Scholar] [CrossRef]
- Azizi, P.; Rafii, M.Y.; Maziah, M.; Abdullah, S.N.A.; Hanafi, M.M.; Latif, M.A.; Sahebi, M. Understanding the shoot apical meristem regulation: A study of the phytohormones, auxin and cytokinin, in rice. Mech. Dev. 2015, 135, 1–15. [Google Scholar] [CrossRef]
- Haga, K.; Iino, M. Asymmetric distribution of auxin correlates with gravitropism and phototropism but not with autostraightening (autotropism) in pea epicotyls. J. Exp. Bot. 2006, 57, 837–847. [Google Scholar] [CrossRef]
- Zahid, N.A.; Jaafar, H.Z.E.; Hakiman, M. Alterations in microrhizome induction, shoot multiplication and rooting of ginger (Zingiber officinale Roscoe var. Bentong) with regards to sucrose and plant growth regulators application. Agronomy 2021, 11, 320. [Google Scholar] [CrossRef]
- Nadha, H.K.; Salwan, R.; Kasana, R.C.; Anand, M.; Sood, A. Identification and elimination of bacterial contamination during in vitro propagation of Guadua angustifolia Kunth. Pharmacogn. Mag. 2012, 8, 93–97. [Google Scholar]
- Bais, H.P.; Green, J.B.; Walker, T.S.; Okemo, P.O.; Vivanco, J.M. In vitro propagation of Spilanthes mauritiana DC., an endangered medicinal herb through axillary bud cultures. Vitr. Cell Dev. Biol. Plant 2002, 35, 598–601. [Google Scholar] [CrossRef]
- Karjee, S.; Singh, K.P.; Panwar, S. Development of in-vitro protocol for direct regeneration from thalamus ex-plant of Tagetes patula L. var. Pusa Deep. J. Pharmacogn. Phytochem. 2020, 9, 373–377. [Google Scholar]
- Poobathy, R.; Zakaria, R.; Murugaiyah, V.; Subramaniam, S. Surface sterilization and micropropagation of Ludisia discolor. Biocatal. Agric. Biotechnol. 2019, 22, 101–380. [Google Scholar] [CrossRef]
- Ana, K.P.; Ana, P.S.; Joselita, C.S.; Silvio, L.T.; Juliana, M.R.; Ana, R.P.; Cristiane, D.P. Sodium hypochlorite sterilization of culture medium in micropropagation of Gerbera hybrida cv. Essandre. Afr. J. Biotechnol. 2016, 15, 1995–1998. [Google Scholar] [CrossRef][Green Version]
- Parveen, S.; Mir, H.; Ranjan, T.; Pal, A.K.; Kundu, M. Effect of surface sterilants on in vitro establishment of pineapple (Ananas comosus (L.) Merill.) cv. Kew. Curr. J. Appl. Sci. Technol. 2019, 33, 1–6. [Google Scholar] [CrossRef]
- Warakagoda, P.S.; Subasinghe, S. In vitro culture establishment and shoot proliferation of Jatropha curcas L. Trop. Agric. 2009, 12, 77–80. [Google Scholar] [CrossRef][Green Version]
- Daud, N.H.; Jayaraman, S.; Mohamed, R. Methods paper: An improved surface sterilization technique for introducing leaf, nodal and seed explants of Aquilaria malaccensis from field sources into tissue culture. Asia Pac. J. Mol. Biol. Biotechnol. 2012, 20, 55–58. [Google Scholar]
- Mihaljevic, I.; Dugalic, K.; Tomas, V.; Viljevac, M.; Pranjic, A.; Cmelik, Z.; Jurkovic, Z. In vitro sterilization procedures for micropropagation of ‘oblacinska’ sour cherry. J. Agric. Sci. 2013, 58, 117–126. [Google Scholar] [CrossRef]
- Gałuszka, A.; Gustab, M.; Tuleja, M. In vitro morphogenetic responses from obligatory apomictic Taraxacum belorussicum Val. N. Tikhom seedlings explants. Plant Cell Tiss. Organ Cult. 2019, 139, 505–522. [Google Scholar] [CrossRef]
- Catapan, E.; Otuki, M.; Viana, A.M. In vitro of Phyllanthus caroliniensis (Euphorbiaceace). Plant Cell Tiss. Organ Cult. 2000, 62, 195–202. [Google Scholar] [CrossRef]
- Emmanuel, E.; Keck, G.; Blanchard, J.M.; Vermande, P.; Perrodin, Y. Toxicological effects of disinfections using sodium hypochlorite on aquatic organisms and its contribution to AOX formation in hospital wastewater. Environ. Int. 2004, 30, 891–900. [Google Scholar] [CrossRef]
- Rostami, A.A.; Shahsavar, A. Nano-silver particles eliminate the in vitro contaminations of olive ‘Mission’ explants. Asian J. Plant Sci. 2009, 8, 505–509. [Google Scholar] [CrossRef]
- Nejatzadeh-Barandozi, F.; Darvishzadeh, F.; Aminkhani, A. Effect of nano silver and silver nitrate on seed yield of (Ocimum basilicum L.). Org. Med. Chem. Lett. 2014, 4, 11. [Google Scholar] [CrossRef]
- Rezali, N.I.; Jaafar Sidik, N.; Saleh, A.; Osman, N.I.; Mohd Adam, N.A. The effects of different strength of MS media in solid and liquid media on in vitro growth of Typhonium flagelliforme. Asian Pac. J. Trop. Biomed. 2017, 7, 151–156. [Google Scholar] [CrossRef]
- Cuba-Díaz, M.; Rivera-Mora, C.; Navarrete, E.; Klagges, M. Advances of native and non-native Antarcticspecies to in vitro conservation: Improvement of disinfection protocols. Sci. Rep. 2020, 10, 3845. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size-and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Da Silva, J.A.T.; Winarto, B.; Dobránszki, J.; Cardoso, J.C.; Zeng, S. Tissue disinfection for preparation of Dendrobium in vitro culture. Folia Hortic. 2016, 28, 57–75. [Google Scholar] [CrossRef]
- Catapan, E.; Luís, M.; Da Silva, B.; Netto Moreno, F.; Maria Viana, A. Micropropagation, callus and root culture of Phyllanthus urinaria (Euphorbiaceae). Plant Cell Tiss. Organ Cult. 2002, 70, 301–309. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, B.; Jha, S. In vitro tuberisation of Gloriosa superba L. on basal medium. Sci. Hortic. 2007, 114, 220–223. [Google Scholar] [CrossRef]
- Sadik, K.; Arinaitwe, G.; Rubaihayo, P.; Kiggundu, A.; Mukasa, S. TDZ and 4-CPPU in Gamborg B5 salts with MS vitamins doubles embryogenic 191 response from male flowers of EA-AAA banana. Afr. Crop. Sci. J. 2014, 22, 191–204. [Google Scholar]
- Antara, S.; Batra, A. Crucial role od nitrogen in in vitro regeneration of Phyllanthus amarus Schum. and Thonn. Int. J. Pharm. Sci. Res. 2011, 2, 2146–2151. [Google Scholar]
- Monfort, L.E.F.; Bertolucci, S.K.V.; Lima, A.F.; de Carvalho, A.A.; Mohammed, A.; Blank, A.F.; Pinto, J.E.B.P. Effects of plant growth regulators, different culture media and strength MS on production of volatile fraction composition in shoot cultures of Ocimum basilicum. Ind. Crops Prod. 2018, 116, 231–239. [Google Scholar] [CrossRef]
- Singh, A.; Jani, K.; Kumari, P.; Agarwal, P.K. Effect of MgCl2 and double concentration of Murashige and Skoog medium in vitro plantlet and root cultures generation in halophytic grasswort Salicornia brachiata. Plant Cell Tiss. Organ Cult. 2014, 120, 563–570. [Google Scholar] [CrossRef]
- Singh, P.; Dwivedi, P. Two-stage culture procedure using thidiazuron for efficient micropropagation of Stevia rebaudiana, an anti-diabetic medicinal herb. 3Biotech 2014, 4, 431–437. [Google Scholar] [CrossRef]
- Liang, O.P.; Keng, C.L. In vitro plant regeneration, flowering and fruiting of Phyllanthus niruri L. (Euphorbiaceae). Int. J. Bot. 2006, 2, 409–414. [Google Scholar] [CrossRef]
- Rajasubramaniam, S.; Saradhi, P.P. Rapid multiplication of Phyllanthus fraternus: A plant with anti-hepatitis viral activity. Ind. Crops Prod. 1997, 6, 35–40. [Google Scholar] [CrossRef]
- Gallavotti, A. The role of auxin in shaping shoot architecture. J. Exp. Bot. 2013, 64, 2593–2608. [Google Scholar] [CrossRef]
- Zazimalová, E.; Brezinova, A.; Holík, J.; Opatrny, Z. Partial auxin deprivation affects endogenous cytokinins in an auxin-dependent, cytokinin-independent tobacco cell strain. Plant Cell Rep. 1996, 16, 76–79. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; Garcia-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Hughes, K.W. In vitro ecology: Exogenous factors affecting growth and morphogenesis in plant culture systems. Environ. Exp. Bot. 1981, 21, 281–288. [Google Scholar] [CrossRef]
- Chen, C. Development of a heat transfer model for plant tissue culture vessels. Biosyst. Eng. 2003, 85, 67–77. [Google Scholar] [CrossRef]
- Waseem, K.; Jilani, M.S.; Khan, M.S.; Kiran, M.; Khan, G. Efficient in vitro Chrysanthemum morifolium L. plantlets from nodal segments. Afr. J. Biotechnol. 2011, 10, 1477–1484. [Google Scholar]
- Raad, M.K.; Zanjani, S.B.; Sayyad, A.R.; Maghsudi, M.; Kaviani, B. Effect of cultivar, type and age of explants, light conditions and plant growth regulators on callus formation of anthurium. Am. Eur. J. Agric. Environ. Sci. 2012, 12, 706–712. [Google Scholar]
- Jach, M.; Przywara, L. Somatic embryogenesis and organogenesis induced in immature zygotic embryos of selected sunflower (Helianthus annuus L.) genotypes. Acta Biol. Crac. Bot. 2000, 42, 83–86. [Google Scholar]
- Tanimoto, E. Regulation of root growth by plant hormones—Roles for auxin and gibberellin. Crit. Rev. Plant Sci. 2005, 24, 249–265. [Google Scholar] [CrossRef]
- Van der Krieken, W.M.; Breteler, H.; Visser, M.H.M.; Mavridou, D. The role of the conversion of IBA into IAA on root regeneration in apple: Introduction of a test system. Plant Cell Rep. 1993, 12, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005, 17, 616–627. [Google Scholar] [CrossRef]
- Kalidass, C.; Mohan, V.R. In vitro clonal propagation of Phyllanthus urinaria (Euphorbiaceae)—A medicinal plant. Researcher 2009, 1, 56–61. [Google Scholar]
- De Klerk, G.J.; Ter Brugge, J.; Marinova, S. Effectiveness of indoleacetic acid, indolebutyric acid and naphthaleneacetic acid during adventitious root formation in vitro in Malus “Jork 9”. Plant Cell Tiss. Organ Cult. 1997, 49, 39–44. [Google Scholar] [CrossRef]
- Ansar, A.; Ahmad, T.; Abbasi, N.A.; Hafiz, I.A. Effect of different concentrations of auxins on in vitro rooting of olive cultivar “Moraiolo”. Pak. J. Bot. 2009, 41, 1223–1231. [Google Scholar]
- Wada, S.; Tanimoto, E.; Masuda, Y. Cell elongation and metabolic turnover of the cell wall as affected by auxin and cell wall degrading enzymes. Plant Cell Physiol. 1968, 9, 369–376. [Google Scholar]
- Ludwig-Müller, J. Indole-3-butyric acid in plant growth and development. Plant Growth Regul. 2000, 32, 219–230. [Google Scholar] [CrossRef]
- Kollmeier, M.; Felle, H.H.; Horst, W.J. Is basipetal auxin floe involed in inhibition of root elongation. Plant Physiol. 2000, 122, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Kirdmanee, C.; Kitaya, Y.; Kozai, T. Effect of CO2 enrichment and supporting material in vitro on photoautotrophic growth of eucalyptus plantlets in vitro and ex vitro. Vitr. Cell. Dev. Biol. Plant 1995, 31, 144–149. [Google Scholar] [CrossRef]
- Manjusha, A.V.M.; Sathyanarayana, B.N. Acclimatization studies in stevia (Stevia rebaudiana Bert.). Acta. Hortic. 2010, 865, 129–134. [Google Scholar] [CrossRef]
- Modgil, M.; Sharma, T.; Thakur, M. Commercially feasible protocol for rooting and acclimatization of micropropagated apple rootstocks. Acta Hortic. 2009, 839, 209–214. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid grwoth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
| Treatment/Chemical Sterilants | Concentration | Percentage of Contamination (%) |
|---|---|---|
| Clorox® (%) | 10 | 10.22 ± 2.28 ab |
| 20 | 6.67 ± 0.7 bc | |
| 30 | 4.44 ± 1.21 c | |
| Nano Silver (ppm) | 10 | 14.22 ± 1.13 a |
| 20 | 12.44 ± 1.13 a | |
| 30 | 12.00 ± 2.28 a |
| Treatment | Number of Shoots | Length of Shoot (cm) | Number of Leaves |
|---|---|---|---|
| MSH | 2.00 ± 0.57 bc | 2.79 ± 0.14 bc | 23.11 ± 0.94 a |
| MSF | 2.33 ± 0.33 ab | 3.11 ± 0.43 b | 27.91 ± 0.90 a |
| MSD | 0.67 ± 0.33 c | 1.5 ± 0.23 c | 4.88 ± 0.89 b |
| B5H | 3.67 ± 0.33 a | 3.52 ± 0.12 ab | 24.25 ± 0.47 a |
| B5F | 3.00 ± 0.57 ab | 4.84 ± 0.33 a | 35.94 ± 1.81 a |
| B5D | 1.67 ± 0.66 bc | 3.58 ± 0.69 ab | 22.83 ± 0.92 a |
| Cytokinin (µM) | Number of Shoots | Length of Shoot (cm) | Number of Leaves |
|---|---|---|---|
| Control | 5.0 ± 0.50 ab | 3.68 ± 0.60 ab | 27.33 ± 1.15 a |
| BAP 2.5 | 4.45 ± 0.96 abc | 3.09 ± 0.45 bcd | 20.89 ± 1.29 abcd |
| BAP 5.0 | 5.33 ± 0.57 a | 2.5 ± 0.51 bcde | 24.11 ± 2.00 abc |
| BAP 7.5 | 4.45 ± 0.77 abc | 2.47 ± 0.44 bcde | 23.22 ± 1.83 abc |
| BAP 10.0 | 2.55 ± 0.61 e | 1.67 ± 0.15 e | 16.00 ± 1.83 bcd |
| Kn 2.5 | 5.33 ± 0.77 a | 3.38 ± 0.42 abc | 25.33 ± 0.62 ab |
| Kn 5.0 | 4.34 ± 0.33 abcd | 4.31 ± 0.89 a | 22.45 ± 0.49 abc |
| Kn 7.5 | 4.1 1± 0.29 abcd | 2.38 ± 0.30 cde | 18.67 ± 2.05 abcd |
| Kn 10.0 | 3.67 ± 0.50 bcde | 2.73 ± 0.58 bcde | 18.33 ± 1.50 abcd |
| Zn 2.5 | 3.55 ± 0.39 bcde | 2.52 ± 0.07 bcde | 15.46 ± 1.61 cd |
| Zn 5.0 | 2.89 ± 0.11 de | 2.01 ± 0.09 de | 17.00 ± 2.71 bcd |
| Zn 7.5 | 3.22 ± 0.29 cde | 1.99 ± 0.06 de | 14.67 ± 1.64 cd |
| Zn 10.0 | 4.22 ± 0.11 abcd | 2.56 ± 0.09 bcde | 2.22 ± 1.44 abc |
| 2iP 2.5 | 3.89 ± 0.72 abcde | 2.71± 0.36 bcde | 15.22 ± 1.36 cd |
| 2iP 5.0 | 3.0 ± 0.33 cde | 1.63 ± 0.20 e | 12.33 ± 0.38 d |
| 2iP 7.5 | 3.45 ± 0.22 cde | 2.43 ± 0.56 cde | 16.00 ± 2.54 bcd |
| 2iP 10.0 | 4.00 ± 0.57 abcde | 2.47 ± 0.16 bcde | 15.89 ± 1.89 bcd |
| Auxin (µM) | Number of Roots | Length of Root (cm) |
|---|---|---|
| Control | 1.0 ± 0.40 h | 0.13 ± 0.03 f |
| IBA (1.25) | 9.4 ± 1.11 cd | 1.07 ± 0.03 abc |
| IBA (2.5) | 17.92 ± 1.38 a | 1.29 ± 0.11 a |
| IBA (5) | 10.53 ± 0.67 bc | 0.79 ± 0.19 cde |
| IAA (1.25) | 7.27 ± 0.40 de | 0.97 ± 0.09 bcd |
| IAA (2.5) | 3.47 ± 0.24 g | 0.85 ± 0.05 cde |
| IAA (5) | 4.87 ± 0.33 fg | 0.86 ± 0.06 cde |
| NAA (1.25) | 6.93 ± 0.94 ef | 0.65 ± 0.11 e |
| NAA (2.5) | 12.47 ± 0.88 b | 1.22 ± 0.08 ab |
| NAA (5) | 11.87 ± 0.43 b | 0.77 ± 0.06 de |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suraya, A.A.; Misran, A.; Hakiman, M. The Efficient and Easy Micropropagation Protocol of Phyllanthus niruri. Plants 2021, 10, 2141. https://doi.org/10.3390/plants10102141
Suraya AA, Misran A, Hakiman M. The Efficient and Easy Micropropagation Protocol of Phyllanthus niruri. Plants. 2021; 10(10):2141. https://doi.org/10.3390/plants10102141
Chicago/Turabian StyleSuraya, Azal Anis, Azizah Misran, and Mansor Hakiman. 2021. "The Efficient and Easy Micropropagation Protocol of Phyllanthus niruri" Plants 10, no. 10: 2141. https://doi.org/10.3390/plants10102141
APA StyleSuraya, A. A., Misran, A., & Hakiman, M. (2021). The Efficient and Easy Micropropagation Protocol of Phyllanthus niruri. Plants, 10(10), 2141. https://doi.org/10.3390/plants10102141






