Micropropagation and Cryopreservation of Yukon Draba (Draba yukonensis), a Special Concern Plant Species Endemic to Yukon Territory, Canada
Abstract
:1. Introduction
2. Results
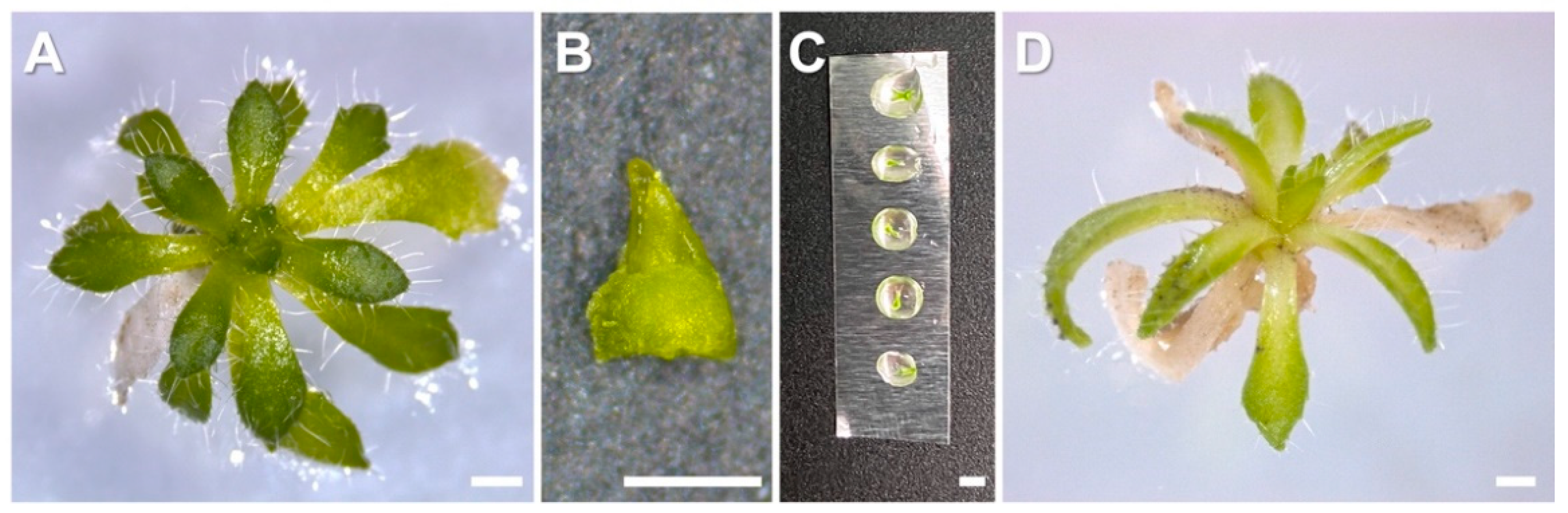
2.1. Micropropagation
2.1.1. Culture Initiation
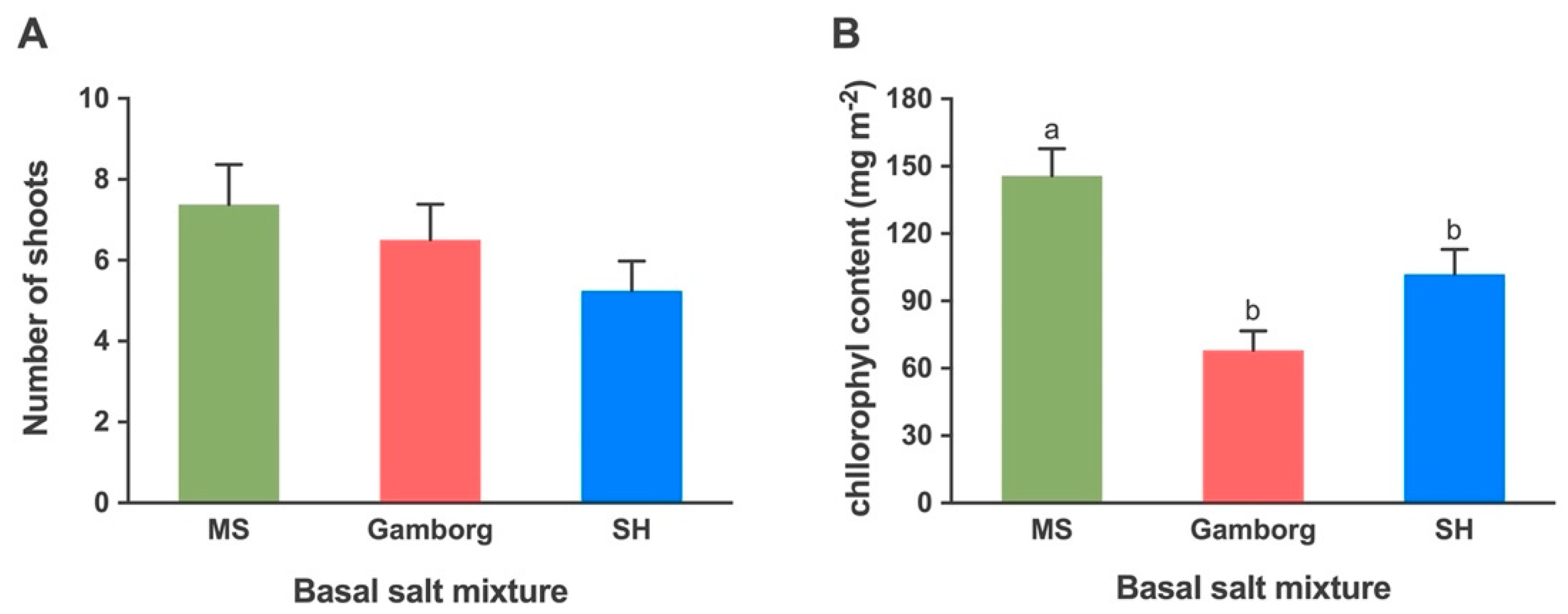
2.1.2. Shoot Proliferation: Basal Salts
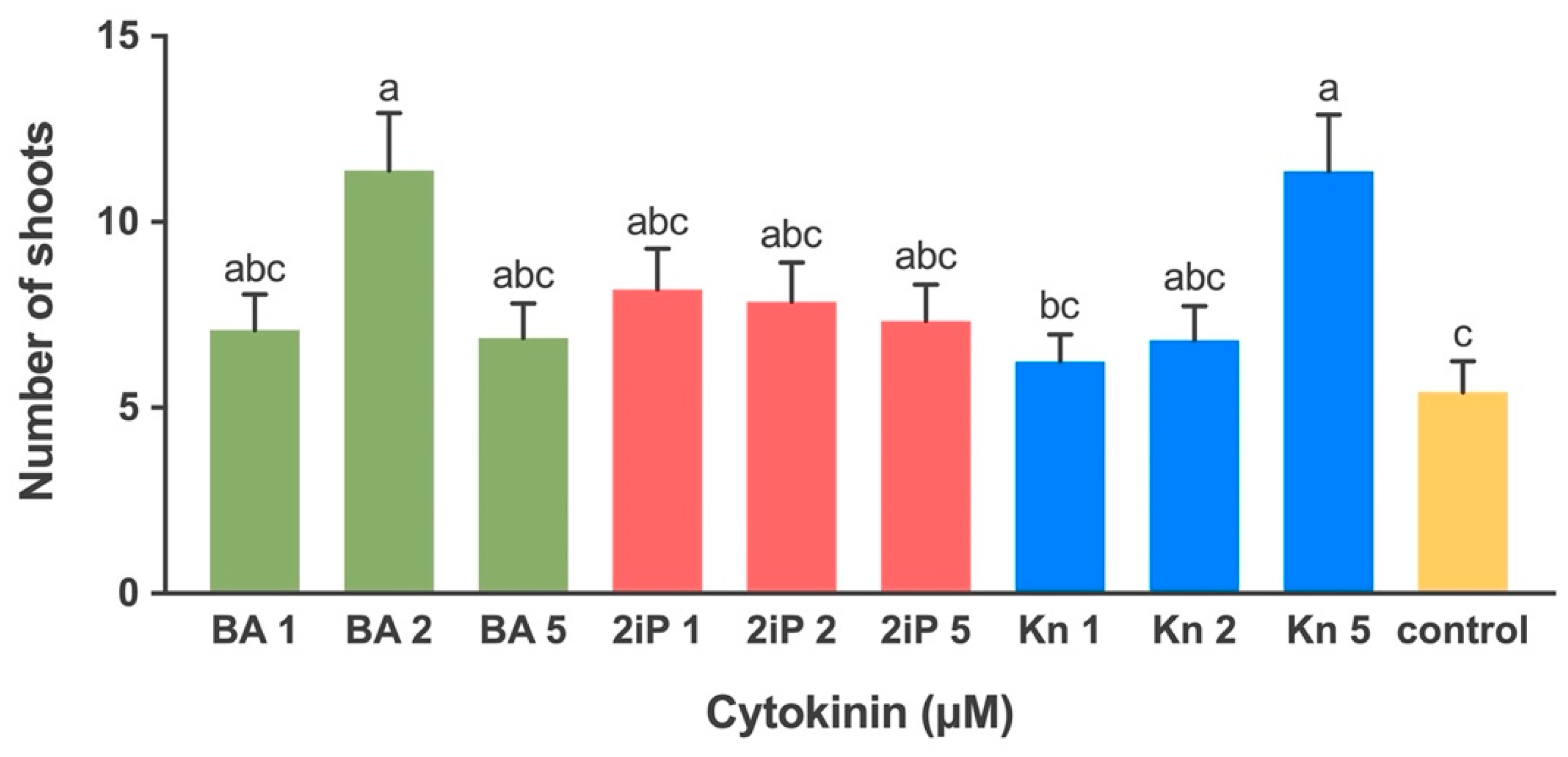
2.1.3. Effect of Cytokinin on Shoot Multiplication
2.1.4. In Vitro Rooting
2.1.5. Greenhouse Acclimatization
2.2. Cryopreservation
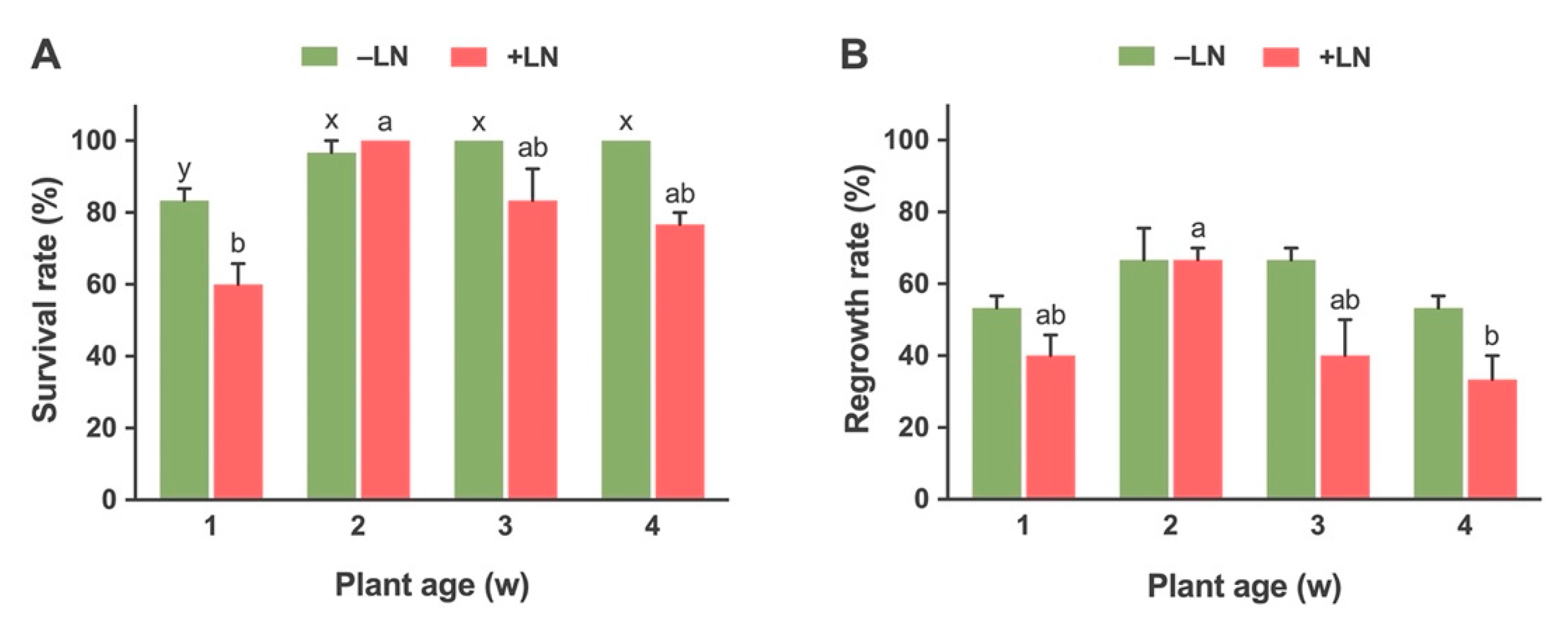
2.2.1. Effect of Stock Plant Age on Survival and Regrowth of Cryopreserved Shoot Tips
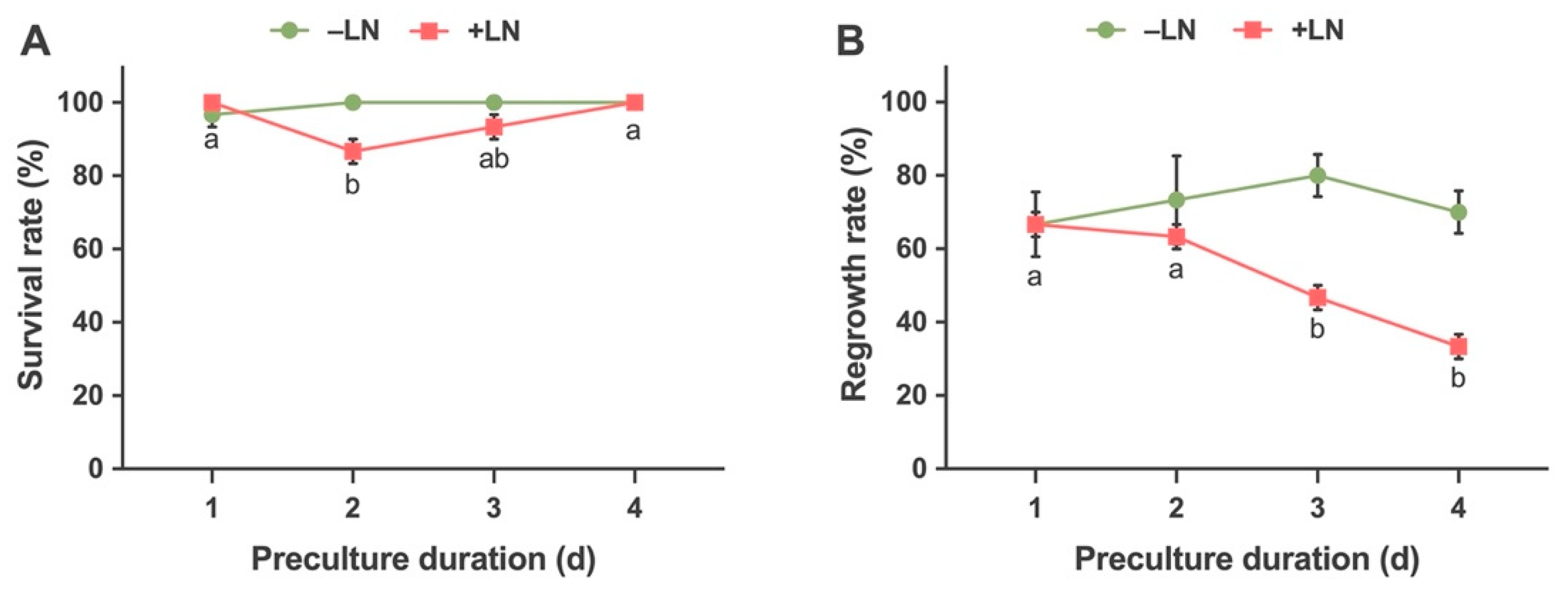
2.2.2. Effects of Preculture Duration on Survival and Regrowth
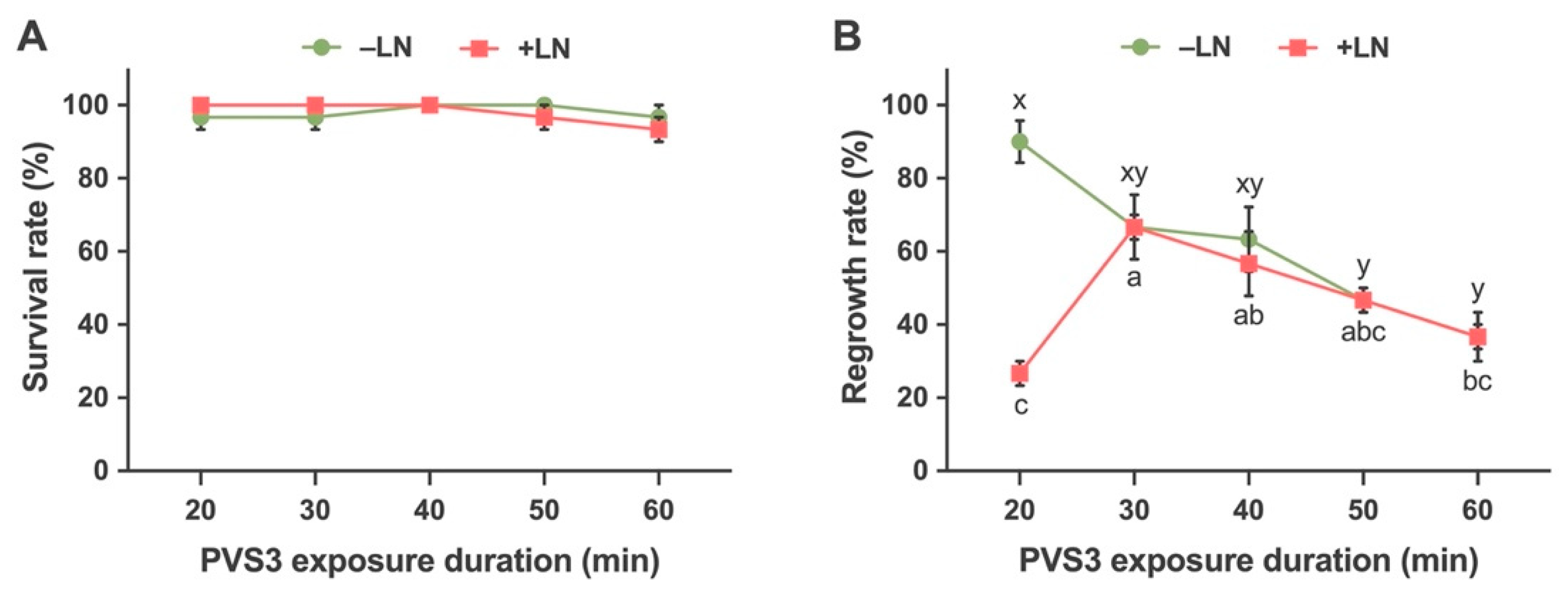
2.2.3. Effects of Time Durations of Exposure to Vitrification Solution (PVS3) on Survival and Regrowth
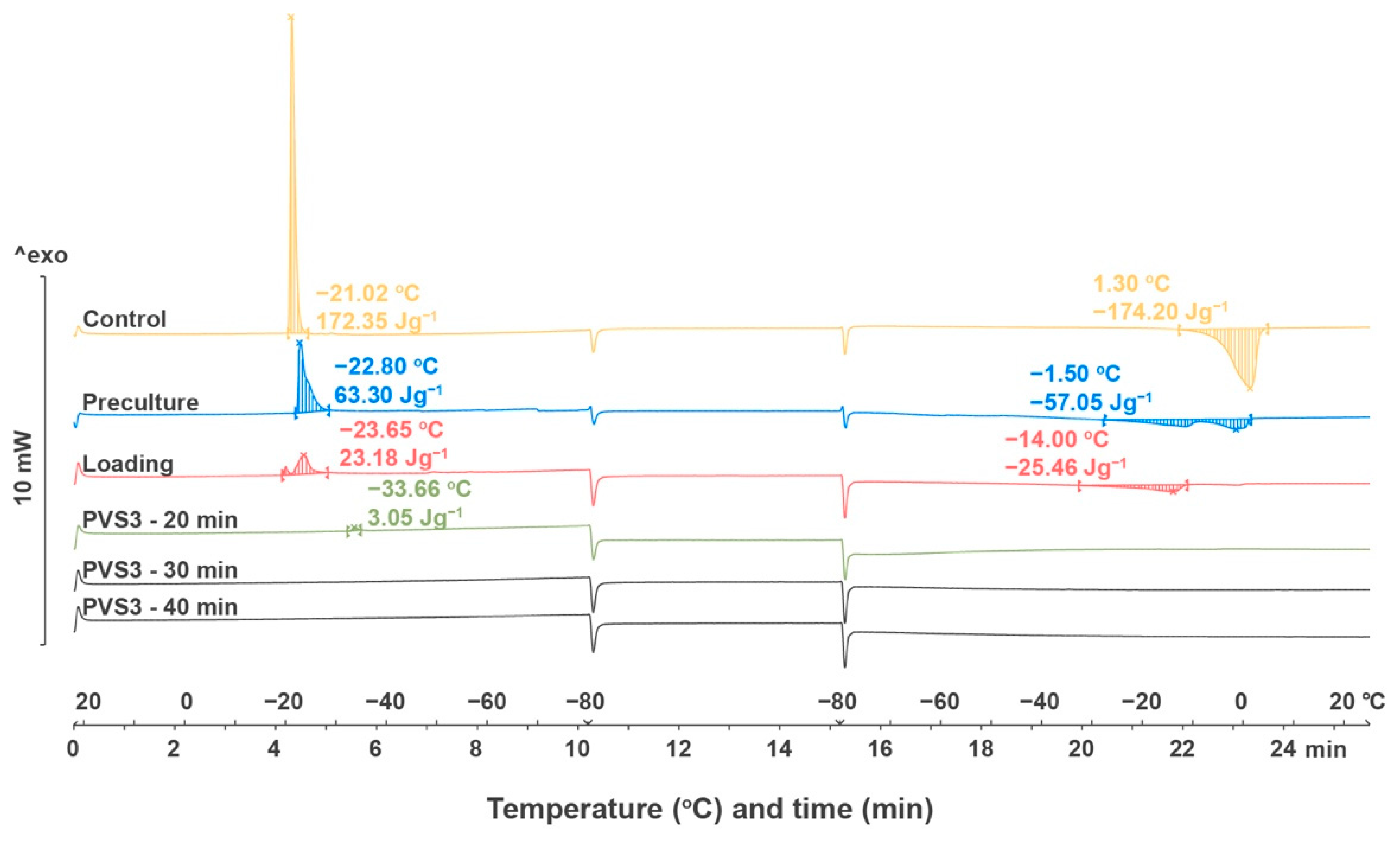
2.2.4. Thermal Analysis
3. Discussion
4. Materials and Methods
4.1. Micropropagation
4.1.1. Seed Germination and Culture Initiation
4.1.2. Growth Conditions
4.1.3. Shoot Multiplication
4.1.4. In Vitro Rooting
4.1.5. Greenhouse Acclimatization
4.2. Droplet-Vitrification Cryopreservation
4.2.1. Plant Material
4.2.2. Thermal Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COSEWIC. COSEWIC Assessment and Status Report on the Yukon Draba Draba yukonensis in Canada; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2018; Available online: https://publications.gc.ca/site/eng/9.874667/publication.html (accessed on 28 September 2021).
- Yukon Invasive Species Council—Success Stories. Available online: https://www.yukoninvasives.com/index.php/en/organization/success-stories (accessed on 24 September 2021).
- Barnicoat, H.; Cripps, R.; Kendon, J.; Sarasan, V. Conservation in vitro of rare and threatened ferns—Case studies of biodiversity hotspot and island species. In Vitro Cell. Dev. Biol. Plant 2011, 47, 37–45. [Google Scholar] [CrossRef]
- Chavan, J.; Gaikwad, N.; Kshirsagar, P.; Umdale, S.; Bhat, K.; Dixit, G.; Yadav, S. Highly efficient in vitro proliferation and genetic stability analysis of micropropagated Ceropegiaevansii by RAPD and ISSR markers: A critically endangered plant of Western Ghats. Plant Biosyst. 2015, 149, 442–450. [Google Scholar] [CrossRef]
- Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K. Recent development in micropropagation techniques for rare plant species. Plants 2020, 9, 1733. [Google Scholar] [CrossRef]
- Patel, A.K.; Phulwaria, M.; Rai, M.K.; Gupta, A.K.; Shekhawat, S.; Shekhawat, N. In vitro propagation and ex vitro rooting of Caralluma edulis (Edgew.) Benth. & Hook. f.: An endemic and endangered edible plant species of the Thar Desert. Sci. Hortic. 2014, 165, 175–180. [Google Scholar]
- Pérez-Bermúdez, P.; Seitz, H.U.; Gavidia, I. A protocol for rapid micropropagation of endangered Isoplexis. In Vitro Cell. Dev. Biol.-Plant 2002, 38, 178–182. [Google Scholar] [CrossRef]
- Saxena, A.; Shukla, M.; Saxena, P. Synthetic Seeds: Relevance to Endangered Germplasm Conservation In Vitro. In Synthetic Seeds; Faisal, M., Alatar, A.A., Eds.; Springer: Cham, Switzerland, 2019; pp. 21–60. [Google Scholar]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Tsoktouridis, G.; Maloupa, E. In vitro propagation of medicinal and aromatic plants: The case of selected Greek species with conservation priority. In Vitro Cell. Dev. Biol. Plant 2019, 55, 635–646. [Google Scholar] [CrossRef]
- Cui, Y.; Deng, Y.; Zheng, K.; Hu, X.; Zhu, M.; Deng, X.; Xi, R. An efficient micropropagation protocol for an endangered ornamental tree species (Magnolia sirindhorniae Noot. & Chalermglin) and assessment of genetic uniformity through DNA markers. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Komakech, R.; Kim, Y.-G.; Kim, W.J.; Omujal, F.; Yang, S.; Moon, B.C.; Okello, D.; Rahmat, E.; Nambatya Kyeyune, G.; Matsabisa, M.G. A Micropropagation Protocol for the Endangered Medicinal Tree Prunus africana (Hook f.) Kalkman: Genetic Fidelity and Physiological Parameter Assessment. Front. Plant Sci. 2020, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Anandhan, S.; García-Pérez, L.M.; Ruiz-May, E.; Pérez, E.N.; Quiroz-Figueroa, F.R. An efficient protocol for in vitro propagation of the wild legume Cicer microphyllum Benth., a crop wild relative of chickpea (Cicer arietinum L.). In Vitro Cell. Dev. Biol. Plant 2019, 55, 9–14. [Google Scholar] [CrossRef]
- Iliev, I.; Gajdošová, A.; Libiaková, G.; Jain, S.M. Plant micropropagation. In Plant Cell Culture: Essential Methods; Davey, M.R., Anthony, P., Eds.; John Wiley & Sons: Chichester, UK, 2010; pp. 1–23. [Google Scholar]
- Benson, E.E. Cryopreservation of phytodiversity: A critical appraisal of theory & practice. Crit. Rev. Plant Sci. 2008, 27, 141–219. [Google Scholar]
- Bi, W.; Saxena, A.; Ayyanath, M.-M.; Harpur, C.; Shukla, M.R.; Saxena, P.K. Conservation, propagation, and redistribution (CPR) of Hill’s thistle: Paradigm for plant species at risk. Plant Cell Tissue Organ Cult. 2021, 145, 75–88. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Dev. Biol. Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Fabre, J.; Dereuddre, J. Encapsulation-dehydration: A new approach to cryopreservation of Solanum shoot-tips. CryoLetters 1990, 11, 413–426. [Google Scholar]
- Khoddamzadeh, A.A.; Sinniah, U.R.; Lynch, P.; Kadir, M.A.; Kadzimin, S.B.; Mahmood, M. Cryopreservation of protocorm-like bodies (PLBs) of Phalaenopsis bellina (Rchb. f.) Christenson by encapsulation-dehydration. Plant Cell Tissue Organ Cult. 2011, 107, 471–481. [Google Scholar] [CrossRef]
- Salama, A.; Popova, E.; Jones, M.P.; Shukla, M.R.; Fisk, N.S.; Saxena, P.K. Cryopreservation of the critically endangered golden paintbrush (Castilleja levisecta Greenm.): From nature to cryobank to nature. In Vitro Cell. Dev. Biol. Plant 2018, 54, 69–78. [Google Scholar] [CrossRef]
- Engelmann, F. In vitro conservation methods. In Biotechnology and Plant Genetic Resources: Conservation and Use; Biotechnology in Agriculture Series; Callow, J.A., Ford-Lloyd, B.V., Newbury, H.J., Eds.; CAB International: Oxford, UK, 1997; pp. 119–162. [Google Scholar]
- Wang, M.-R.; Chen, L.; da Silva, J.A.T.; Volk, G.M.; Wang, Q.-C. Cryobiotechnology of apple (Malus spp.): Development, progress and future prospects. Plant Cell Rep. 2018, 37, 689–709. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Panis, B.; Nagel, M.; Houwe, I.V.D. Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef]
- Reed, B.M. Plant cryopreservation: A continuing requirement for food and ecosystem security. In Vitro Cell. Dev. Biol. Plant 2017, 53, 285–288. [Google Scholar] [CrossRef]
- Benson, E.E. Cryopreservation theory. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 15–32. [Google Scholar]
- Whiteley, S.E.; Bunn, E.; Menon, A.; Mancera, R.L.; Turner, S.R. Ex situ conservation of the endangered species Androcalva perlaria (Malvaceae) by micropropagation and cryopreservation. Plant Cell Tissue Organ Cult. 2016, 125, 341–352. [Google Scholar] [CrossRef]
- Edesi, J.; Tolonen, J.; Ruotsalainen, A.L.; Aspi, J.; Häggman, H. Cryopreservation enables long-term conservation of critically endangered species Rubus humulifolius. Biodivers. Conserv. 2020, 29, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.-H.; Popova, E.; Lee, H.; Park, S.-U.; Ku, J.; Kang, J.-H.; Kim, H.-H. Cryopreservation of endangered wild species, Aster altaicus var. uchiyamae Kitam, using droplet-vitrification procedure. CryoLetters 2019, 40, 113–122. [Google Scholar]
- Popova, E.V.; Shukla, M.R.; McIntosh, T.; Saxena, P.K. In Vitro and Cryobiotechnology Approaches to Safeguard Lupinus rivularis Douglas ex Lindl., an Endangered Plant in Canada. Agronomy 2021, 11, 37. [Google Scholar] [CrossRef]
- Dinato, N.B.; Santos, I.R.I.; Vigna, B.B.Z.; de Paula, A.F.; Fávero, A.P. Pollen cryopreservation for plant breeding and genetic resources conservation. CryoLetters 2020, 41, 115–127. [Google Scholar] [PubMed]
- Kaczmarczyk, A.; Rokka, V.M.; Keller, E.J. Potato shoot tip cryopreservation. A review. Potato Res. 2011, 54, 45–79. [Google Scholar] [CrossRef] [Green Version]
- Panis, B.; Swennen, R.; Engelmann, F. Cryopreservation of plant germplasm. Acta Hortic. 2001, 560, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Perl, A. Cryopreservation in floricultural plants. In Floriculture Ornamental Biotech; Silva, J.A.T.D., Ed.; Global Science Books: Isleworth, UK, 2006; pp. 523–539. [Google Scholar]
- Griffith, M.; Timonin, M.; Wong, A.C.; Gray, G.R.; Akhter, S.R.; Saldanha, M.; Rogers, M.A.; Weretilnyk, E.A.; Moffatt, B. Thellungiella: An Arabidopsis-related model plant adapted to cold temperatures. Plant Cell Environ. 2007, 30, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Blamey, F.; Joyce, D.; Edwards, D.; Asher, C. Role of trichomes in sunflower tolerance to manganese toxicity. Plant Soil 1986, 91, 171–180. [Google Scholar] [CrossRef]
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric organic compounds in the soil–microorganism–plant system: Their role in iron availability. Eur. J. Soil Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- Driver, J.A.; Kuniyuki, A.H. In vitro propagation of Paradox walnut rootstock. HortScience 1984, 19, 507–509. [Google Scholar]
- Rathwell, R.; Shukla, M.R.; Jones, A.M.P.; Saxena, P.K. In vitro propagation of cherry birch (Betula lenta L.). Can. J. Plant Sci. 2016, 96, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Tetsumura, T.; Matsumoto, Y.; Sato, M.; Honsho, C.; Yamashita, K.; Komatsu, H.; Sugimoto, Y.; Kunitake, H. Evaluation of basal media for micropropagation of four highbush blueberry cultivars. Sci. Hortic. 2008, 119, 72–74. [Google Scholar] [CrossRef]
- Bajaj, Y.P.S.; Reghunath, B.R.; Gopalakrishnan, P.K. Elettaria cardamomum Maton (Cardamom): Aromatic compounds, in vitro culture studies, and clonal propagation. In Medicinal and Aromatic Plants IV.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 132–147. [Google Scholar]
- Jain, P.; Kachhwaha, S.; Kothari, S. Optimization of micronutrients for the improvement of in vitro plant regeneration of Stevia rebaudiana (Bert.) Bertoni. Indian J. Biotechnol. 2012, 11, 486–490. [Google Scholar]
- Sokolov, R.; Iakimova, E.; Atanassova, B. Effect of basal salt mixtures on growth performance of in vitro cultured Prunus ceracifera ‘Nigra’. Subtrop. Ornam. Hortic. 2015, 54, 78–85. [Google Scholar]
- Akin, M.; Eyduran, E.; Reed, B.M. Use of RSM and CHAID data mining algorithm for predicting mineral nutrition of hazelnut. Plant Cell Tissue Organ Cult. 2017, 128, 303–316. [Google Scholar] [CrossRef]
- Oberschelp, G.P.J.; Gonçalves, A.N. Assessing the effects of basal media on the in vitro propagation and nutritional status of Eucalyptus dunnii Maiden. In Vitro Cell. Dev. Biol. Plant 2016, 52, 28–37. [Google Scholar] [CrossRef]
- Hand, C.; Maki, S.; Reed, B.M. Modeling optimal mineral nutrition for hazelnut micropropagation. Plant Cell Tissue Organ Cult. 2014, 119, 411–425. [Google Scholar] [CrossRef]
- Srivastava, L.M. Plant Growth and Development: Hormones and Environment; Elsevier: New York, NY, USA, 2002. [Google Scholar]
- Skoog, F.; Miller, C. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–131. [Google Scholar]
- Sheikholeslami, B.; Shukla, M.; Turi, C.; Harpur, C.; Saxena, P.K. Saving threatened plant species: Reintroduction of Hill’s thistle (Cirsium hillii.(Canby) Fernald) to its natural habitat. PLoS ONE 2020, 15, e0231741. [Google Scholar] [CrossRef] [Green Version]
- Gailīte, A.; Kļaviņa, D.; Ievinsh, G. In vitro propagation of an endangered plant Saussurea esthonica. Environ. Exp. Bot. 2010, 8, 43–48. [Google Scholar]
- Martini, A.N.; Papafotiou, M. In vitro propagation and NaCl tolerance of the multipurpose medicinal halophyte Limoniastrum monopetalum. HortScience 2020, 55, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Abu-Romman, S.M.; Al-Hadid, K.A.; Arabiyyat, A.R. Kinetin is the most effective cytokinin on shoot multiplication from cucumber. J. Agric. Sci. 2015, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Choudhury, M.D.; Mazumdar, P.B. Micropropagation of Dioscorea alata L. through nodal segments. Afr. J. Biotechnol. 2013, 12, 6611–6617. [Google Scholar]
- Cassells, A.C.; Curry, R.F. Oxidative stress and physiological, epigenetic and genetic variability in plant tissue culture: Implications for micropropagators and genetic engineers. Plant Cell Tissue Organ Cult. 2001, 64, 145–157. [Google Scholar] [CrossRef]
- Debnath, S.C. Zeatin and TDZ-induced shoot proliferation and use of bioreactor in clonal propagation of medicinal herb, roseroot (Rhodiola rosea L). J. Plant Biochem. Biotechnol. 2009, 18, 245–248. [Google Scholar] [CrossRef]
- Kadota, M.; Niimi, Y. Effects of cytokinin types and their concentrations on shoot proliferation and hyperhydricity in in vitro pear cultivar shoots. Plant Cell Tissue Organ Cult. 2003, 72, 261–265. [Google Scholar] [CrossRef]
- Chukwujekwu, J.; Fennell, C.; Van Staden, J. Optimisation of the tissue culture protocol for the endangered Aloe polyphylla. S. Afr. J. Bot. 2002, 68, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Bornman, C.H.; Vogelmann, T.C. Effect of rigidity of gel medium on benzyladenine-induced adventitious bud formation and vitrification in vitro in Picea abies. Physiol. Plant. 1984, 61, 505–512. [Google Scholar] [CrossRef]
- Williams, R.R.; Taji, A.M. Effect of temperature, gel concentration and cytokinins on vitrification of Olearia microdisca (J.M. Black) in vitro shoot cultures. Plant Cell Tissue Organ Cult. 1991, 26, 1–6. [Google Scholar] [CrossRef]
- Hamdi, S.; Crèche, J.; Garnier, F.; Mars, M.; Decendit, A. Cytokinin involvment in the control of coumarin accumulation in Nicotiana tabacum. Investigations with normal and transformed tissues carrying the isopentenyl transferase gene. Plant Physiol. Biochem. 1995, 33, 283–288. [Google Scholar]
- Harding, S.; Smigocki, A. Cytokinins modulate stress response genes in isopentenyl transferase-transfornied Nicotiana plumbaginifolia plants. Physiol. Plant. 1994, 90, 327–333. [Google Scholar] [CrossRef]
- Memelink, J.; Hoge, J.H.C.; Schilperoort, R.A. Cytokinin stress changes the developmental regulation of several defence-related genes in tobacco. EMBO J. 1987, 6, 3579–3583. [Google Scholar] [CrossRef]
- Dilworth, L.; Riley, C.; Stennett, D. Plant constituents: Carbohydrates, oils, resins, balsams, and plant hormones. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 61–80. [Google Scholar]
- Laubscher, C.P.; Ndakidemi, P.A. Rooting success using IBA auxin on endangered Leucadendron laxum (PROTEACEAE) in different rooting mediums. Afr. J. Biotechnol. 2008, 7, 3437–3442. [Google Scholar]
- Martins, J.; Moreira, O.; Silva, L.; Moura, M. Vegetative propagation of the endangered Azorean tree, Picconia azorica. Arquipel. Life Mar. Sci. 2011, 28, 39–46. [Google Scholar]
- Poston, A.L. Cutting Propagation and Container Production of Rudy Haag Burning Bush (Euonymus alatus Rudy Haag). Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2007. [Google Scholar]
- Kaviani, B.; Negahdar, N. Propagation, micropropagation and cryopreservation of Buxus hyrcana Pojark., an endangered ornamental shrub. S. Afr. J. Bot. 2017, 111, 326–335. [Google Scholar] [CrossRef]
- Cancino-García, V.J.; Ramírez-Prado, J.H.; De-la-Peña, C. Auxin perception in Agave is dependent on the species’ Auxin Response Factors. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dewir, Y.H.; El-Mahrouk, M.E.; Murthy, H.N.; Paek, K.Y. Micropropagation of Cattleya: Improved in vitro rooting and acclimatization. Hortic. Environ. Biotechnol. 2015, 56, 89–93. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Vertocnik, A.; Town, C.D. Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. J. Exp. Bot. 2005, 56, 2095–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, J.S.; Ten Tusscher, K. The systems biology of lateral root formation: Connecting the dots. Mol. Plant 2019, 12, 784–803. [Google Scholar] [CrossRef] [PubMed]
- Pence, V.C. The possibilities and challenges of in vitro methods for plant conservation. Kew Bull. 2010, 65, 539–547. [Google Scholar] [CrossRef]
- Popova, E.; Shukla, M.; Kim, H.H.; Saxena, P.K. Plant cryopreservation for biotechnology and breeding. In Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools; Al-Khayri, J., Jain, S., Johnson, D., Eds.; Springer: Berlin, Germany, 2015; pp. 63–93. [Google Scholar]
- Uchendu, E.; Lata, H.; Chandra, S.; Khan, I.A.; ElSohly, M.A. Cryopreservation of shoot tips of elite cultivars of Cannabis sativa L. by droplet vitrification. Med. Cannabis Cannabinoids 2019, 2, 29–34. [Google Scholar] [CrossRef]
- Coelho, N.; González-Benito, M.E.; Romano, A. Cryopreservation of shoot tips from the endangered endemic species Tuberaria major. Acta Physiol. Plant. 2014, 36, 3333–3336. [Google Scholar] [CrossRef]
- Wang, M.-R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.-C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tissue Organ Cult. 2021, 144, 7–20. [Google Scholar] [CrossRef]
- Sakai, A. Development of cryopreservation techniques. In Proceedings of the Cryopreservation of Tropical Plant Germplasm—Current Research Progress and Applications; JIRCAS: Usukuba, Japan; IPGRI: Rome, Italy, 2000; pp. 1–7. [Google Scholar]
- Condello, E.; Caboni, E.; Andrè, E.; Piette, B.; Druart, P.; Swennen, R.; Panis, B. Cryopreservation of apple in vitro axillary buds using droplet-vitrification. CryoLetters 2011, 32, 175–185. [Google Scholar] [PubMed]
- Zhao, C.; Wu, Y.; Engelmann, F.; Zhou, M. Cryopreservation of axillary buds of grape (Vitis vinifera) in vitro plantlets. CryoLetters 2001, 22, 321–328. [Google Scholar] [PubMed]
- Chang, Y.; Reed, B.M. Preculture conditions influence cold hardiness and regrowth of Pyrus cordata shoot tips after cryopreservation. HortScience 2001, 36, 1329–1333. [Google Scholar] [CrossRef]
- Wilms, H.; Sleziak, N.F.; Van der Auweraer, M.; Brands, M.; Verleije, M.; Hardeman, D.; Andre, E.; Panis, B. Development of a fast and user-friendly cryopreservation protocol for sweet potato genetic resources. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Panis, B.; Lambardi, M. Status of cryopreservation technologies in plants (crops and forest trees). In The Role of Biotechnology in Exploring and Protecting Agricultural Genetic Resources; Ruane, J., Sonnino, A., Eds.; United Nations Food and Agriculture Organization (FAO): Rome, Italy, 2006; Volume 5, pp. 61–78. [Google Scholar]
- González-Benito, M.E.; Pérez, C.; Viviani, A.B. Cryopreservation of nodal explants of an endangered plant species (Centaurium rigualii Esteve) using the encapsulation–dehydration method. Biodivers. Conserv. 1997, 6, 583–590. [Google Scholar] [CrossRef]
- Turner, S.; Senaratna, T.; Bunn, E.; Tan, B.; Dixon, K.; Touchell, D. Cryopreservation of shoot tips from six endangered Australian species using a modified vitrification protocol. Ann. Bot. 2001, 87, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Kaviani, B. Conservation of plant genetic resources by cryopreservation. Aust. J. Crop Sci. 2011, 5, 778–800. [Google Scholar]
- Kulus, D.; Serocka, M.; Mikuła, A. Effect of various preculture and osmotic dehydration conditions on cryopreservation efficiency and morphogenetic response of chrysanthemum shoot tips. Acta Sci. Pol. Hort. Cult 2018, 17, 139–147. [Google Scholar] [CrossRef]
- Benelli, C.; De Carlo, A.; Engelmann, F. Recent advances in the cryopreservation of shoot-derived germplasm of economically important fruit trees of Actinidia, Diospyros, Malus, Olea, Prunus, Pyrus and Vitis. Biotechnol. Adv. 2013, 31, 175–185. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Shepherd, A.N.; Kretzschmar, A.A.; Volk, G.M. Modifications to a Vitis shoot tip cryopreservation procedure: Effect of shoot tip size and use of cryoplates. CryoLetters 2019, 40, 103–112. [Google Scholar]
- Wang, Q.; Li, P.; Batuman, Ö.; Gafny, R.; Mawassi, M. Effect of benzyladenine on recovery of cryopreserved shoot tips of grapevine and citrus cultured in vitro. CryoLetters 2003, 24, 293–302. [Google Scholar]
- Yoon, J.-W.; Kim, H.-H.; Ko, H.-C.; Hwang, H.-S.; Hong, E.-S.; Cho, E.-G.; Engelmann, F. Cryopreservation of cultivated and wild potato varieties by droplet vitrification: Effect of subculture of mother-plants and of preculture of shoot tips. CryoLetters 2006, 27, 211–222. [Google Scholar]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Volk, G.M.; Harris, J.L.; Rotindo, K.E. Survival of mint shoot tips after exposure to cryoprotectant solution components. Cryobiology 2006, 52, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; Han, L.; Lu, X.-X.; Volk, G.M.; Xin, X.; Yin, G.-K.; He, J.-J.; Wang, L.; Chen, X.-L. Cryopreservation of Jerusalem artichoke cultivars using an improved droplet-vitrification method. Plant Cell Tissue Organ Cult. 2017, 128, 577–587. [Google Scholar] [CrossRef]
- Chen, X.-L.; Li, J.-H.; Xin, X.; Zhang, Z.-E.; Xin, P.-P.; Lu, X.-X. Cryopreservation of in vitro-grown apical meristems of Lilium by droplet-vitrification. S. Afr. J. Bot. 2011, 77, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Marković, Z.; Chatelet, P.; Sylvestre, I.; Kontić, J.K.; Engelmann, F. Cryopreservation of grapevine (Vitis vinifera L.) in vitro shoot tips. Cent. Eur. J. Biol. 2013, 8, 993–1000. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Kretzschmar, A.A.; Bonnart, R.; Shepherd, A.; Volk, G.M. Cryopreservation of 12 Vitis species using apical shoot tips derived from plants grown in vitro. HortScience 2019, 54, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Arnao, M.T.; Lazaro-Vallejo, C.E.; Engelmann, F.; Gamez-Pastrana, R.; Martinez-Ocampo, Y.M.; Pastelin-Solano, M.C.; Diaz-Ramos, C. Multiplication and cryopreservation of vanilla (Vanilla planifolia ‘Andrews’). In Vitro Cell. Dev. Biol. Plant 2009, 45, 574–582. [Google Scholar] [CrossRef]
- Kulus, D. Effect of bead composition, PVS type, and recovery medium in cryopreservation of bleeding heart ‘Valentine’—Preliminary Study. Agronomy 2020, 10, 891. [Google Scholar] [CrossRef]
- Kim, H.-H.; Lee, Y.-G.; Shin, D.-J.; Ko, H.-C.; Gwag, J.-G.; Cho, E.-G.; Engelmann, F. Development of alternative plant vitrification solutions in droplet-vitrification procedures. CryoLetters 2009, 30, 320–334. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saxena, A.; Bi, W.-L.; Shukla, M.R.; Cannings, S.; Bennett, B.; Saxena, P.K. Micropropagation and Cryopreservation of Yukon Draba (Draba yukonensis), a Special Concern Plant Species Endemic to Yukon Territory, Canada. Plants 2021, 10, 2093. https://doi.org/10.3390/plants10102093
Saxena A, Bi W-L, Shukla MR, Cannings S, Bennett B, Saxena PK. Micropropagation and Cryopreservation of Yukon Draba (Draba yukonensis), a Special Concern Plant Species Endemic to Yukon Territory, Canada. Plants. 2021; 10(10):2093. https://doi.org/10.3390/plants10102093
Chicago/Turabian StyleSaxena, Akansha, Wen-Lu Bi, Mukund R. Shukla, Syd Cannings, Bruce Bennett, and Praveen K. Saxena. 2021. "Micropropagation and Cryopreservation of Yukon Draba (Draba yukonensis), a Special Concern Plant Species Endemic to Yukon Territory, Canada" Plants 10, no. 10: 2093. https://doi.org/10.3390/plants10102093
APA StyleSaxena, A., Bi, W.-L., Shukla, M. R., Cannings, S., Bennett, B., & Saxena, P. K. (2021). Micropropagation and Cryopreservation of Yukon Draba (Draba yukonensis), a Special Concern Plant Species Endemic to Yukon Territory, Canada. Plants, 10(10), 2093. https://doi.org/10.3390/plants10102093






