Response of Three Miscanthus × giganteus Cultivars to Toxic Elements Stress: Part 1, Plant Defence Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Plannt Origins/Preparations
2.2. Experimental Design
2.3. Soil Sampling and Analysis
2.4. Plant Sampling and Analysis
2.4.1. Plant Growth Parameter Measurements, Leaf Sampling and Preparation
2.4.2. Toxic Element (TEs) Concentrations in Leaves
2.4.3. Antioxidant Enzymatic Activities
2.4.4. Secondary Metabolism Molecule and Photosynthetic Pigment Quantification
2.5. Statistical Analysis
3. Results
3.1. Granulometry, pH and Pseudototal Toxic Element (TE) Concentrations of Soils
3.2. Plant Growth Parameters and Toxic Elements (TE) Concentration in Leaves
3.3. Antioxidant Enzymatic Activity Assays
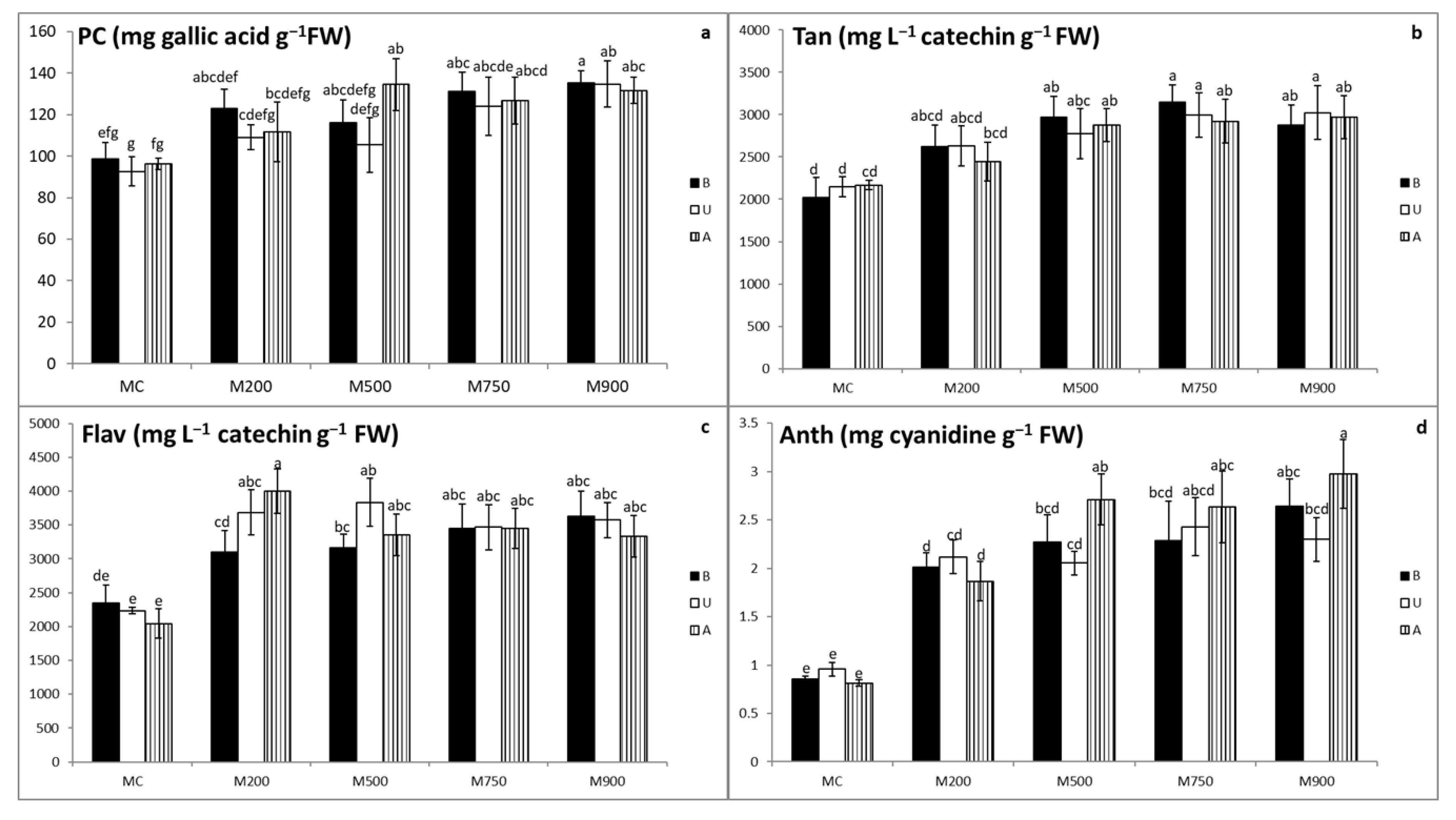
3.4. Secondary Metabolites Quantification
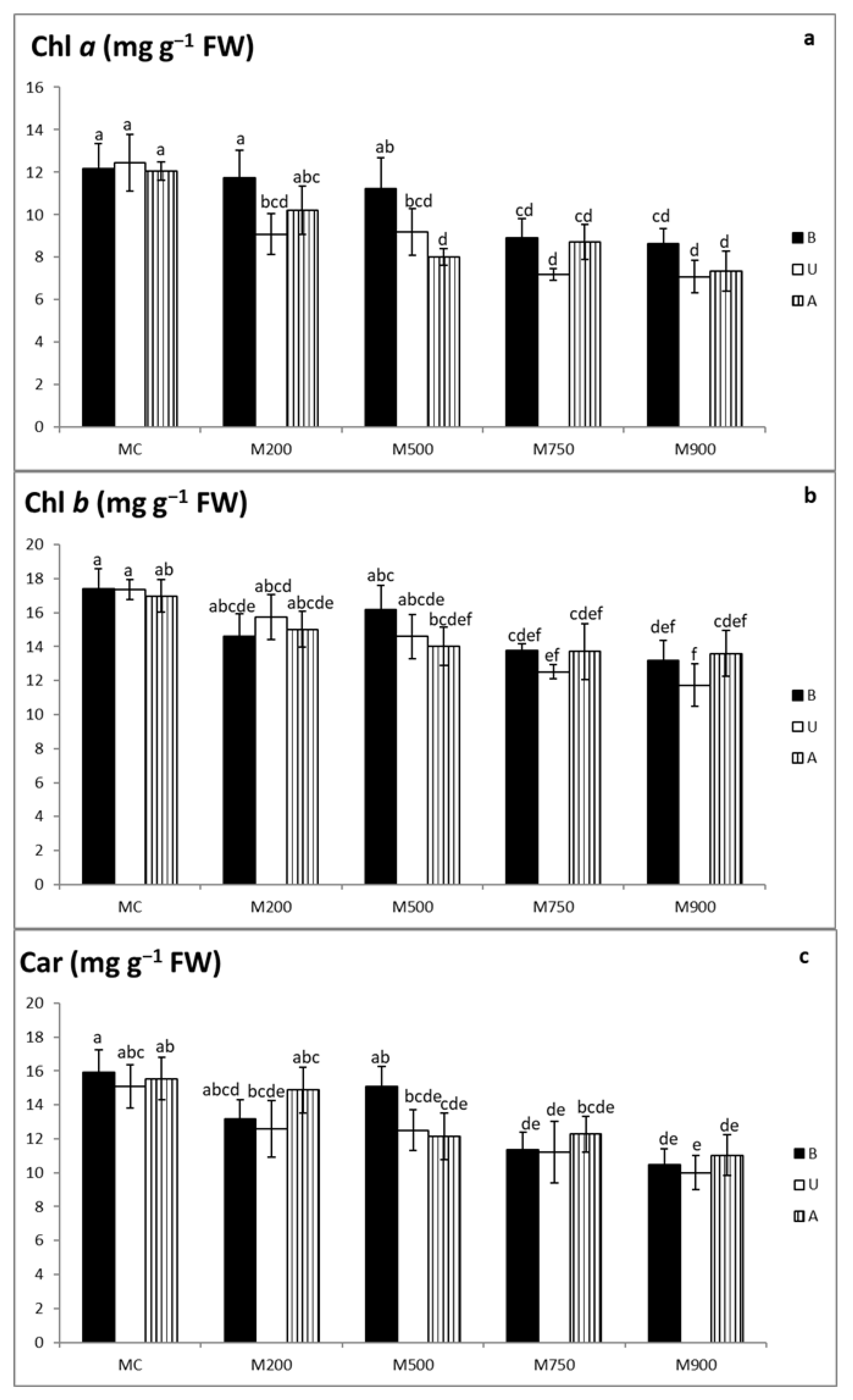
3.5. Photosynthetic Pigment Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomez-Sagasti, M.T.; Alkorta, I.; Becerril, J.M.; Epelde, L.; Anza, M.; Garbisu, C. Microbial Monitoring of the Recovery of Soil Quality During Heavy Metal Phytoremediation. Water Air Soil Pollut. 2012, 223, 3249–3262. [Google Scholar] [CrossRef]
- Panagos, P.; van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated Sites in Europe: Review of the Current Situation Based on Data Collected through a European Network. J. Environ. Public Health 2013, 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Al Souki, K.S.; Liné, C.; Louvel, B.; Waterlot, C.; Douay, F.; Pourrut, B. Miscanthus × giganteus Culture on Soils Highly Contaminated by Metals: Modelling Leaf Decomposition Impact on Metal Mobility and Bioavailability in the Soil–Plant System. Ecotoxicol. Environ. Saf. 2020, 199, 110654. [Google Scholar] [CrossRef] [PubMed]
- Al Souki, K.S.; Burdová, H.; Trubač, J.; Štojdl, J.; Kuráň, P.; Kříženecká, S.; Machová, I.; Kubát, K.; Popelka, J.; Auer Malinská, H.; et al. Enhanced Carbon Sequestration in Marginal Land Upon Shift towards Perennial C4 Miscanthus × giganteus: A Case Study in North-Western Czechia. Agronomy 2021, 11, 293. [Google Scholar] [CrossRef]
- Techer, D.; Martinez-Chois, C.; Laval-Gilly, P.; Henry, S.; Bennasroune, A.; D’Innocenzo, M.; Falla, J. Assessment of Miscanthus × giganteus for Rhizoremediation of Long Term PAH Contaminated Soils. Appl. Soil Ecol. 2012, 62, 42–49. [Google Scholar] [CrossRef]
- Técher, D.; Laval-Gilly, P.; Henry, S.; Bennasroune, A.; Martinez-Chois, C.; D’Innocenzo, M.; Falla, J. Prospects of Miscanthus × giganteus for PAH Phytoremediation: A Microcosm Study. Ind. Crop. Prod. 2012, 36, 276–281. [Google Scholar]
- Al Souki, K.S.; Burdová, H.; Mamirova, A.; Kuráň, P.; Kříženecká, S.; Oravová, L.; Tolaszová, J.; Nebeská, D.; Popelka, J.; Ust’ak, S.; et al. Evaluation of the Miscanthus × giganteus Short Term Impacts on Enhancing the Quality of Agricultural Soils Affected by Single and/or Multiple Contaminants. Environ. Technol. Innov. 2021, 24, 101890. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Pourrut, B.; Waterlot, C.; Louvel, B.; Bidar, G.; Labidi, S.; Fontaine, J.; Muchembled, J.; Sahraoui, A.L.-H.; Fourrier, H.; et al. Metal Accumulation and Shoot Yield of Miscanthus × giganteus Growing in Contaminated Agricultural Soils: Insights into Agronomic Practices. Agric. Ecosyst. Environ. 2015, 213, 61–71. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Waterlot, C.; Louvel, B.; Pourrut, B.; Douay, F. Metal, Nutrient and Biomass Accumulation During the Growing Cycle of Miscanthus Established on Metal-Contaminated Soils. J. Plant Nutr. Soil Sci. 2016, 179, 257–269. [Google Scholar] [CrossRef]
- Pelfrêne, A.; Kleckerová, A.; Pourrut, B.; Nsanganwimana, F.; Douay, F.; Waterlot, C. Effect of Miscanthus Cultivation on Metal Fractionation and Human Bioaccessibility in Metal-Contaminated Soils: Comparison Between Greenhouse and Field Experiments. Environ. Sci. Pollut. Res. 2015, 22, 3043–3054. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Louvel, B.; Douay, F.; Pourrut, B. Assessment of Miscanthus × giganteus Capacity to Restore the Functionality of Metal-Contaminated Soils: Ex Situ Experiment. Appl. Soil Ecol. 2017, 115, 44–52. [Google Scholar] [CrossRef]
- Küpper, H.; Andersen, E. Mechanisms of Metal Toxicity in Plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef]
- Andrejić, G.; Šinžar-Sekulić, J.; Prica, M.; Dželetović, Ž.; Rakić, T. Phytoremediation Potential and Physiological Response of Miscanthus × giganteus Cultivated on Fertilized and Non-Fertilized Flotation Tailings. Environ. Sci. Pollut. Res. 2019, 26, 34658–34669. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of Secondary Metabolites in Defense Mechanisms of Plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Lajayer, B.M.; Chorbanpour, M.; Nikabadi, S. Heavy Metals in Contaminated Environment: Destiny of Secondary Metabolite Biosynthesis, Oxidative Status and Phytoextraction in Medicinal Plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef]
- Firmin, S.; Labidi, S.; Fontaine, J.; Laruelle, F.; Tisserant, B.; Nsanganwimana, F.; Pourrut, B.; Dalpé, Y.; Grandmougin, A.; Douay, F.; et al. Arbuscular Mycorrhizal Fungal Inoculation Protects Miscanthus × giganteus against Trace Element Toxicity in a Highly Metal-Contaminated Site. Sci. Total Environ. 2015, 527–528, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Lopareva-Pohu, A.; Pourrut, B.; Waterlot, C.; Garcon, G.; Bidar, G.; Pruvot, C.; Shirali, P.; Douay, F. Assessment of Fly Ash-Aided Phytostabilisation of Highly Contaminated Soils After an 8-Year Field Trial: Part 1. Influence on Soil Parameters and Metal Extractability. Sci. Total Environ. 2011, 409, 647–654. [Google Scholar] [CrossRef]
- Douay, F.; Pelfrêne, A.; Planque, J.; Fourrier, H.; Richard, A.; Roussel, H.; Girondelot, B. Assessment of Potential Health Risk for Inhabitants Living Near a Former Lead Smelter. Part 1: Metal Concentrations in Soils, Agricultural Crops, and Homegrown Vegetables. Environ. Monit. Assess. 2013, 185, 3665–3680. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dringen, R.; Gutterer, J.M. Glutathione Reductase from Bovine Brain. Methods Enzymol. 2002, 348, 281–288. [Google Scholar]
- Nsanganwimana, F.; Al Souki, K.S.; Waterlot, C.; Douay, F.; Pelfrêne, A.; Ridošková, A.; Louvel, B.; Pourrut, B. Potentials of Miscanthus × giganteus for Phytostabilization of Trace Element-Contaminated Soils: Ex Situ Experiment. Ecotoxicol. Environ. Saf. 2021, 214, 112125. [Google Scholar] [CrossRef]
- Guo, H.; Hong, C.; Chen, X.; Xu, Y.; Liu, Y.; Jiang, D.; Zheng, B. Different Growth and Physiological Responses to Cadmium of the Three Miscanthus Species. PLoS ONE 2016, 11, e0153475. [Google Scholar]
- Andrejić, G.; Gajić, G.; Prica, M.; Dželetović, Ž.; Rakić, T. Zinc Accumulation, Photosynthetic Gas Exchange, and Chlorophyll a Fluorescence in Zn-Stressed Miscanthus × giganteus Plants. Photosynthetica 2018, 56, 1249–1258. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, X.; Fang, J.; Xiao, Y. Physiological Responses and Metal Uptake of Miscanthus Under Cadmium/Arsenic Stress. Environ. Sci. Pollut. Res. 2018, 25, 28275–28284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, S.; Huang, Y.; Zhou, S. The Tolerance and Accumulation of Miscanthus Sacchariflorus (Maxim.) Benth., an Energy Plant Species, to Cadmium. Int. J. Phytoremediat. 2015, 17, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Fernando, A.; Oliveira, J.S. Effects on Growth, Productivity and Biomass Quality of Miscanthus × giganteus of Soils Contaminated with Heavy Metals. In Proceedings of the Biomass for Energy, Industry and Climate Protection: 2nd World Biomass Conference, Rome, Italy, 10 May 2004. [Google Scholar]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead Uptake, Toxicity, and Detoxification in Plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and Redox Signalling in the Response of Plants to Abiotic Stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-Metal-Induced Reactive Oxygen Species: Phytotoxicity and Physicochemical Changes in Plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive Oxygen Species and Heavy Metal Stress in Plants: Impact on the Cell Wall and Secondary Metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants Under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Patra, J.K.; Thatoi, H. Antioxidative Response to Abiotic and Biotic Stresses in Mangrove Plants: A Review. Int. Rev. Hydrobiol. 2016, 101, 3–19. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-A.; Han, T.; Ahn, S.-K.; Kang, H.; Cho, M.R.; Lee, S.-C.; Im, K.-H. Effects of Heavy Metals on Plant Growths and Pigment Contents in Arabidopsis Thaliana. Plant Pathol. J. 2012, 28, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salope-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Martínez-Alcala, I.; Hernandez, L.E.; Esteban, E.; Walker, D.J.; Bernal, M.P. Responses of Noccaea Caerulescens and Lupinus Albus in Trace Elements-Contaminated Soils. Plant Physiol. Biochem. 2013, 66, 47–55. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics and Ionomics. Front. Plant Sci. 2016, 6, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative Damage and Antioxidative System in Algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Cadmium at High Dose Perturbs Growth, Photosynthesis and Nitrogen Metabolism While at Low Dose It up Regulates Sulfur Assimilation and Antioxidant Machinery in Garden Cress (Lepidium Sativum L.). Plant Sci. 2012, 182, 112–120. [Google Scholar] [CrossRef]
- Lopez-Orenes, A.; Bueso, M.C.; Conesa, H.M.; Calderon, A.A.; Ferrer, M.A. Seasonal Changes in Antioxidative/Oxidative Profile of Mining and Non-Mining Populations of Syrian Beancaper as Determined by Soil Conditions. Sci. Total Environ. 2017, 575, 437–447. [Google Scholar] [CrossRef]
- Pandey, N.; Pathak, G.C.; Pandey, D.K.; Pandey, R. Heavy Metals, Co, Ni, Cu, Zn and Cd, Produce Oxidative Damage and Evoke Differential Antioxidant Responses in Spinach. Braz. J. Plant Physiol. 2009, 21, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Bertrand, A.; Reis, R.; Mourato, M.P.; Martins, L.L.; Gonzalez, A. Growth and Physiological Responses to Cadmium Stress of Two Populations of Dittrichia viscosa (L.) Greuter. J. Hazard. Mater. 2013, 244–245, 555–562. [Google Scholar] [CrossRef] [PubMed]

| MC | M200 | M500 | M750 | M900 | |
|---|---|---|---|---|---|
| Clay (%) | 20.8 ± 1.2 | 17.8 ± 1.5 | 30.6 ± 2.7 | 19.2 ± 1.2 | 16.4 ± 1.1 |
| Silt (%) | 69.5 ± 3.5 | 57.3 ± 3.6 | 49.7 ± 2.9 | 56 ± 3.7 | 59.2 ± 2.2 |
| Sand (%) | 9.7 ± 0.7 | 24.9 | 19.7 ± 1.3 | 24.8 ± 1.9 | 24.4 ± 2.1 |
| pH | 6.4 ± 0.1 | 7.1 ± 0.3 | 7.6 ± 0.2 | 7.3 ± 0.5 | 7.1 ± 0.2 |
| Cd (mg kg−1) | 0.3 ± 0.0 | 3.8 ± 0.2 | 9.0 ± 0.2 | 13.5 ± 0.3 | 16.0 ± 0.3 |
| Pb (mg kg−1) | 37.3 ± 1.3 | 260.3 ± 2.0 | 528.6 ± 5.3 | 747.1 ± 16.9 | 898.6 ± 16.3 |
| Zn (mg kg−1) | 54.6 ± 3.1 | 388.0 ± 14.8 | 537.0 ± 10.9 | 906.0 ± 16.8 | 1116.0 ± 1.7 |
| Cd (mg kg−1) | Pb (mg kg−1) | Zn (mg kg−1) | Number of Tillers | Stem Diameter (mm) | ||
|---|---|---|---|---|---|---|
| B | MC | <LD * | <LD * | 32.2 + 2.8 fg | 8.0 + 1.0 b | 8.2 + 0.6 a |
| M200 | 0.5 + 0.0 e | <LD * | 60.5 + 2.6 bcd | 4.0 + 1.6 bc | 7.1 + 0.8 ab | |
| M500 | 1.0 + 0.1 d | <LD * | 59.7 + 7.1 cd | 5.0 + 1.6 bc | 5.1 + 0.7 c | |
| M750 | 1.8 + 0.3 c | <LD * | 72.9 + 8.5 abc | 4.0 + 0.9 bc | 5.3 + 0.6 bc | |
| M900 | 2.2 + 0.1 ab | <LD * | 74.0 + 8.9 a | 4.0 + 0.4 bc | 4.9 + 0.8 c | |
| U | MC | <LD * | <LD * | 21.6 + 2.1 g | 14.0 + 1.6 a | 6.1 + 0.4 abc |
| M200 | 0.5 + 0.1 e | <LD * | 54.7 + 5.7 d | 7.0 + 1.2 b | 6.2 + 0.9 abc | |
| M500 | 0.9 + 0.1 d | <LD * | 52.1 + 7.2 de | 8.0 + 1.3 b | 5.7 + 0.6 bc | |
| M750 | 2.1 + 0.3 abc | <LD * | 64.8 + 5.5 abcd | 5.0 + 0.8 bc | 6.1 + 1.0 abc | |
| M900 | 2.3 + 0.2 a | <LD * | 68.8 + 5.7 abc | 5.0 + 1.6 bc | 6.2 + 0.3 abc | |
| A | MC | <LD * | <LD * | 39.0 + 1.7 ef | 8.0 + 1.7 b | 7.4 + 1.2 ab |
| M200 | 0.5 + 0.0 e | <LD * | 60.0 + 5.8 cd | 4.0 + 1.1 bc | 5.9 + 0.7 abc | |
| M500 | 1.1 + 0.1 d | <LD * | 62.6 + 3.3 abcd | 4.0 + 1.0 bc | 7.1 + 0.3 ab | |
| M750 | 1.9 + 0.1 bc | <LD * | 71.2 + 5.7 abc | 4.0 + 1.0 bc | 5.7 + 0.5 bc | |
| M900 | 2.2 + 0.1 ab | <LD * | 73.5 + 6.6 ab | 4.0 + 0.7 bc | 5.2 + 0.7 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Souki, K.S.; Liné, C.; Douay, F.; Pourrut, B. Response of Three Miscanthus × giganteus Cultivars to Toxic Elements Stress: Part 1, Plant Defence Mechanisms. Plants 2021, 10, 2035. https://doi.org/10.3390/plants10102035
Al Souki KS, Liné C, Douay F, Pourrut B. Response of Three Miscanthus × giganteus Cultivars to Toxic Elements Stress: Part 1, Plant Defence Mechanisms. Plants. 2021; 10(10):2035. https://doi.org/10.3390/plants10102035
Chicago/Turabian StyleAl Souki, Karim Suhail, Clarisse Liné, Francis Douay, and Bertrand Pourrut. 2021. "Response of Three Miscanthus × giganteus Cultivars to Toxic Elements Stress: Part 1, Plant Defence Mechanisms" Plants 10, no. 10: 2035. https://doi.org/10.3390/plants10102035
APA StyleAl Souki, K. S., Liné, C., Douay, F., & Pourrut, B. (2021). Response of Three Miscanthus × giganteus Cultivars to Toxic Elements Stress: Part 1, Plant Defence Mechanisms. Plants, 10(10), 2035. https://doi.org/10.3390/plants10102035







