Genome-Wide Analysis, Modeling, and Identification of Amino Acid Binding Motifs Suggest the Involvement of GH3 Genes during Somatic Embryogenesis of Coffea canephora
Abstract
:1. Introduction
2. Results
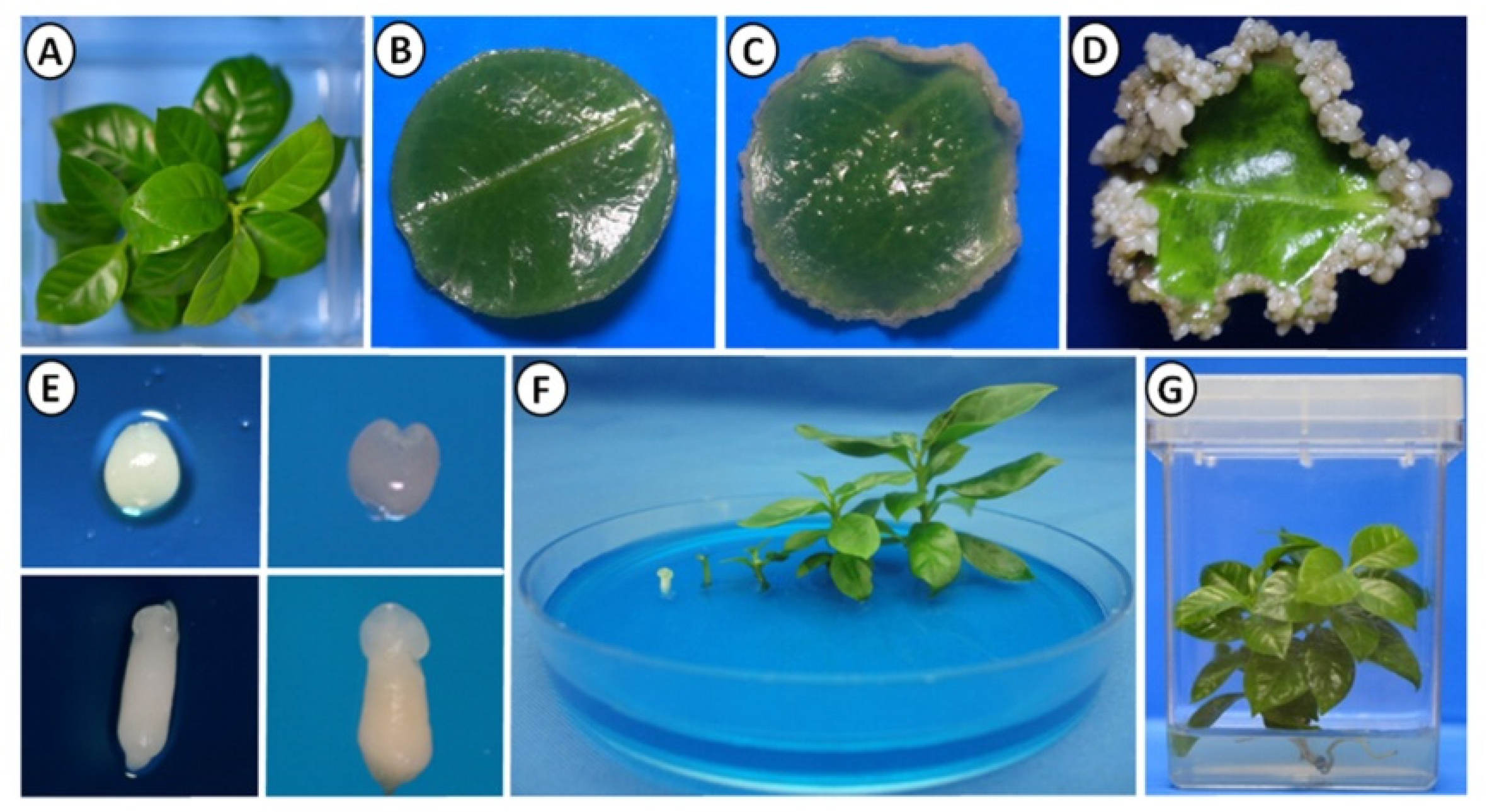
2.1. Somatic Embryogenesis Induction in C. canephora
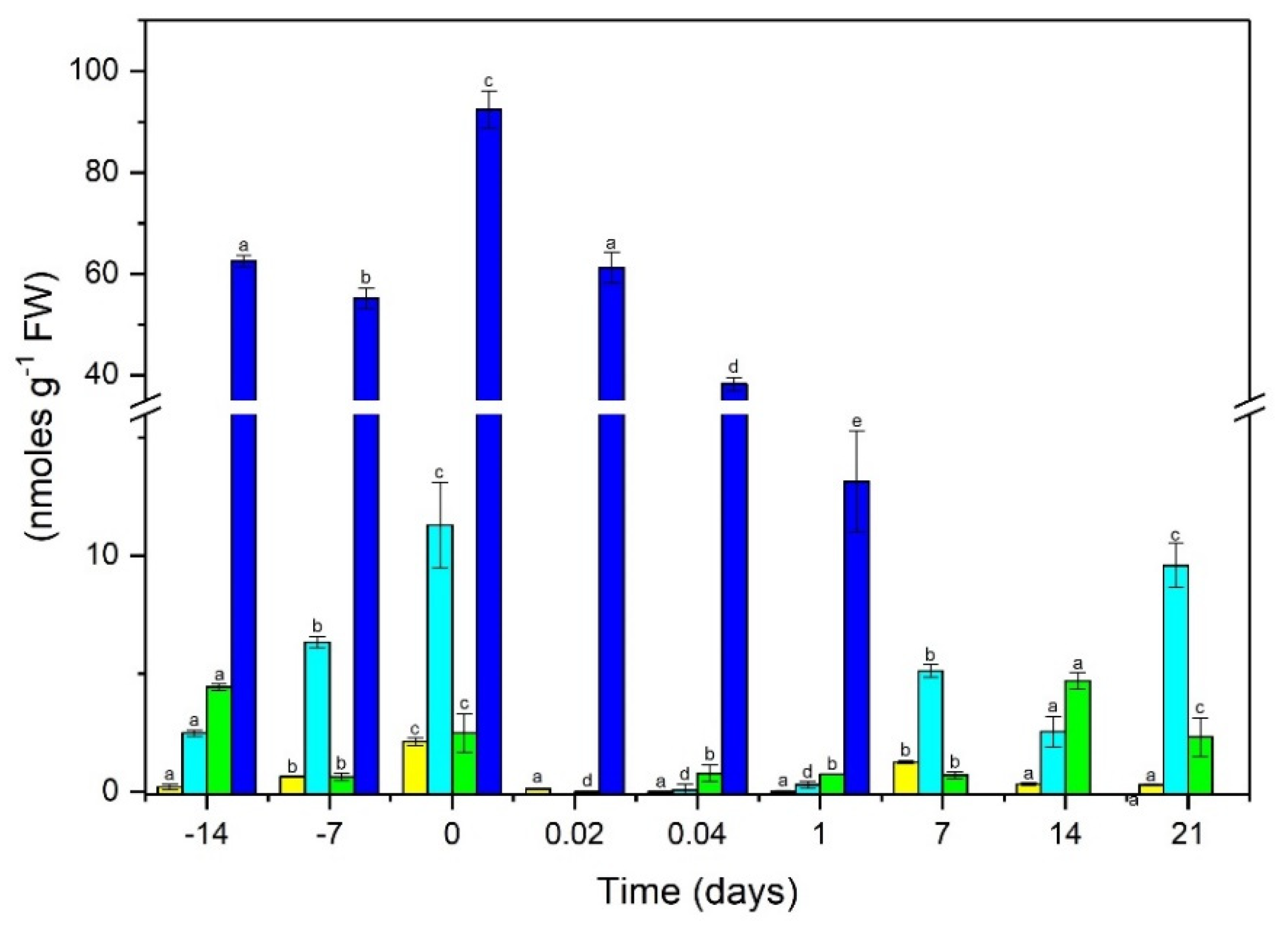
2.2. Ratio of Free and Conjugated Auxin
2.3. Genome-Wide Identification of the GH3 Gene Family in C. canephora
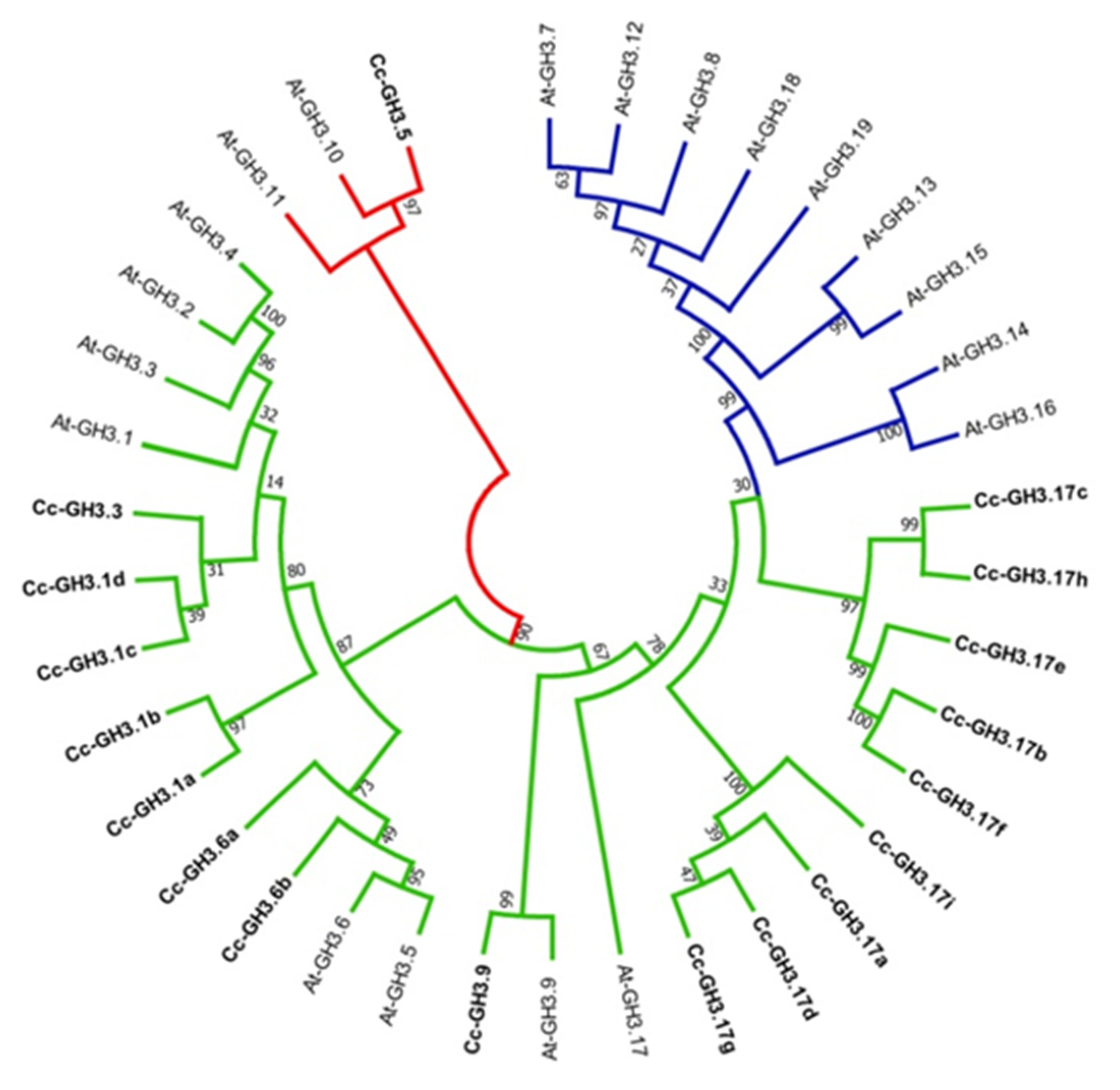
2.4. Phylogenetic Analysis of GH3 Genes in Several Species
2.5. Phylogenetic Analysis of C. canephora and A. thaliana GH3 Genes
2.6. Intron–Exon Structure for GH3 Genes in C. canephora
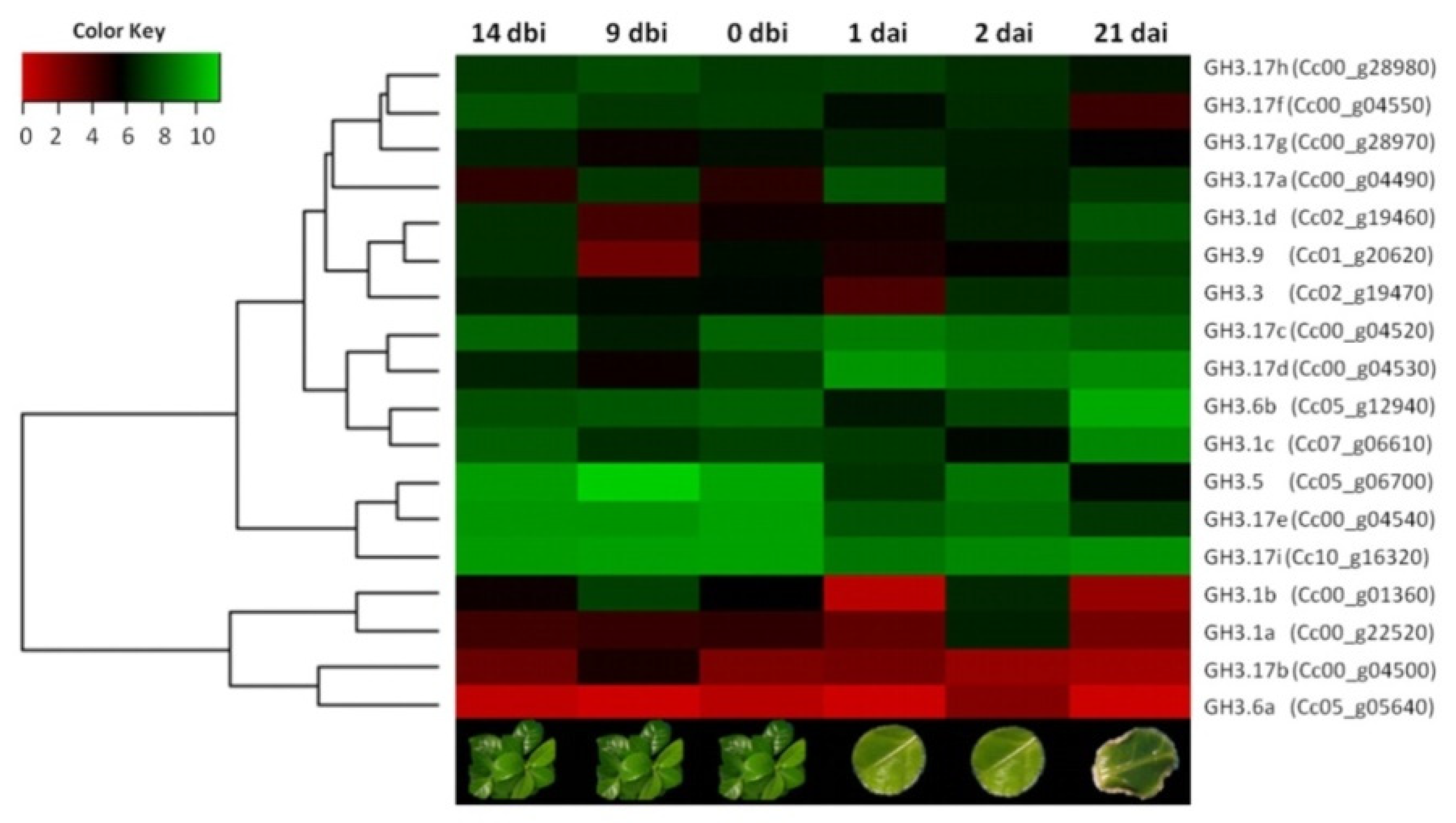
2.7. Expression Patterns of GH3 Genes Based on RNA-Seq Data and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.8. The Building of 3D Structures, Modelling and Molecular Docking of Selected CcGH3 Proteins
3. Discussion
4. Materials and Methods
4.1. Biological Material and Growth Conditions
4.2. Somatic Embryogenesis Induction
4.3. Auxins and Auxin Conjugates Extraction
4.4. High-Performance Liquid Chromatography
4.5. RNA Extraction and cDNA Synthesis
4.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.7. Genome- and Transcriptome-Wide Identification of GH3 Genes in C. canephora
4.8. Phylogenetic Analysis
4.9. Systematic Bioinformatics Analysis of C. canephora GH3 Genes
4.10. The Building of 3D Structures, Modelling, and Molecular Docking of Selected CcGH3 Proteins in C. canephora
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loyola-Vargas, V.M. The history of somatic embryogenesis. In Somatic Embryogenesis. Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: Cham, Switzerland, 2016; pp. 11–22. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. Somatic embryogenesis. An overview. In Somatic Embryogenesis. Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–10. [Google Scholar] [CrossRef]
- Nic-Can, G.I.; Loyola-Vargas, V.M. The role of the auxins during somatic embryogenesis. In Somatic Embryogenesis. Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: Cham, Switzerland, 2016; pp. 171–181. [Google Scholar] [CrossRef]
- Kubeš, M.; Napier, R. Non-canonical auxin signalling: Fast and curious. J. Exp. Bot. 2019, 70, 2609–2614. [Google Scholar] [CrossRef]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-la-Peña, C.; Loyola-Vargas, V.M. Signaling overview of plant somatic embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Raggi, S.; Doyle, S.M.; Robert, S. Auxin: At the crossroads between chemistry and biology. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; John Wiley & Sons: New York, NY, USA, 2020; pp. 123–154. [Google Scholar] [CrossRef]
- Winnicki, K. The winner takes it all: Auxin—The main player during plant embryogenesis. Cells 2020, 9, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig-Müller, J. Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Peer, W.A. Auxin homeostasis: The DAO of catabolism. J. Exp. Bot. 2017, 68, 3145–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abel, S.; Theologis, A. Early genes and auxin action. Plant Physiol. 1996, 111, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Gallei, M.; Luschnig, C.; Friml, J. Auxin signalling in growth: Schrödinger’s cat out of the bag. Curr. Opin. Plant Biol. 2020, 53, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hagen, G.; Kleinschmidt, A.; Guilfoyle, T. Auxin-regulated gene expression in intact soybean hypocotyl and excised hypocotyl sections. Planta 1984, 162, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Hagen, G.; Guilfoyle, T.J. Rapid induction of selective transcription by auxins. Mol. Cell. Biol. 1985, 5, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Vielba, J.M. Identification and initial characterization of a new subgroup in the GH3 gene family in woody plants. J. Plant Biochem. Biotechnol. 2019, 28, 280–290. [Google Scholar] [CrossRef]
- Staswick, P.E.; Tiryaki, I.; Rowe, M.L. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 2002, 14, 1405–1415. [Google Scholar] [CrossRef] [Green Version]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef] [Green Version]
- Okrent, R.A.; Brooks, M.D.; Wildermuth, M.C. Arabidopsis GH3.12 (PBS3) conjugates amino acids to 4-substituted benzoates and is inhibited by salicylate. J. Biol. Chem. 2009, 284, 9742–9754. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, M.; Ciarkowska, A. Pea GH3 acyl acid amidosynthetase conjugates IAA to proteins in immature seeds of Pisum sativum L.—A new perspective on formation of high-molecular weight conjugates of auxin. J. Plant Physiol. 2021, 256, 153312. [Google Scholar] [CrossRef] [PubMed]
- Takase, T.; Nakazawa, M.; Ishikawa, A.; Manabe, K.; Matsui, M. DFL2, a new member of the Arabidopsis GH3 gene family, is involved in red light-specific hypocotyl elongation. Plant Cell Physiol. 2003, 44, 1071–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terol, J.; Domingo, C.; Talón, M. The GH3 family in plants: Genome wide analysis in rice and evolutionary history based on EST analysis. Gene 2006, 371, 279–290. [Google Scholar] [CrossRef]
- Böttcher, C.; Boss, P.K.; Davies, C. Acyl substrate preferences of an IAA-amido synthetase account for variations in grape (Vitis vinifera L.) berry ripening caused by different auxinic compounds indicating the importance of auxin conjugation in plant development. J. Exp. Bot. 2011, 62, 4267–4280. [Google Scholar] [CrossRef]
- Kumar, R.; Agarwal, P.; Tyagi, A.K.; Sharma, A.K. Genome-wide investigation and expression analysis suggest diverse roles of auxin-responsive GH3 genes during development and response to different stimuli in tomato (Solanum lycopersicum). Mol. Gen. Gen. 2012, 287, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, M.; Wu, X.; Wang, J. The gene structure and expression level changes of the GH3 gene family in Brassica napus relative to its diploid ancestors. Genes 2019, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Tian, C.E.; Duan, J.; Wu, K. Research progresses on GH3s, one family of primary auxin-responsive genes. Plant Growth Regul. 2008, 56, 225–232. [Google Scholar] [CrossRef]
- Westfall, C.S.; Zubieta, C.; Herrmann, J.; Kapp, U.; Nanao, M.H.; Jez, J.M. Structural basis for prereceptor modulation of plant hormones by GH3 proteins. Science 2012, 336, 1708–1711. [Google Scholar] [CrossRef]
- Nobuta, K.; Okrent, R.A.; Stoutemyer, M.; Rodibaugh, N.; Kempema, L.; Wildermuth, M.C.; Innes, R.W. The GH3 acyl adenylase family member PBS3 regulates salicylic acid-dependent defense responses in Arabidopsis. Plant Physiol. 2007, 144, 1144–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalczuk, L.; Cooke, T.J.; Cohen, J.D. Auxin levels at different stages of carrot somatic embryogenesis. Phytochemistry 1992, 31, 1097–1103. [Google Scholar] [CrossRef]
- Ayil-Gutiérrez, B.A.; Galaz-Ávalos, R.M.; Peña-Cabrera, E.; Loyola-Vargas, V.M. Dynamics of the concentration of IAA and some of its conjugates during the induction of somatic embryogenesis in Coffea canephora. Plant Signal. Behav. 2013, 8, e26998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uc-Chuc, M.Á.; Pérez-Hernández, C.A.; Galaz-Ávalos, R.M.; Brito-Argáez, L.; Aguilar-Hernández, V.; Loyola-Vargas, V.M. YUCCA-mediated biosynthesis of the auxin IAA is required during the somatic embryogenic induction process in Coffea canephora. Int. J. Mol. Sci. 2020, 21, 4751. [Google Scholar] [CrossRef] [PubMed]
- Ljung, K.; Hull, A.K.; Kowalczyk, M.; Marchant, A.; Celenza, J.; Cohen, J.D.; Sandberg, G. Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol. Biol. 2002, 50, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Rosquete, M.; Barbez, E.; Kleine-Vehn, J. Cellular auxin homeostasis: Gatekeeping is housekeeping. Mol. Plant 2012, 5, 772–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, L.W.; Heckert, M.J.; You, Y.; Albanese, N.; Fenwick, T.; Siehl, D.L.; Castle, L.A.; Tao, Y. Members of the GH3 family of proteins conjugate 2, 4-D and dicamba with aspartate and glutamate. Plant Cell Physiol. 2018, 59, 2366–2380. [Google Scholar] [CrossRef] [PubMed]
- Hangarter, R.P.; Good, N.E. Evidence that IAA conjugates are slow-release sources of free IAA in plant tissues. Plant Physiol. 1981, 68, 1424–1427. [Google Scholar] [CrossRef]
- Tang, Q.; Yu, P.; Tillmann, M.; Cohen, J.D.; Slovin, J.P. Indole-3-acetylaspartate and indole-3-acetylglutamate, the IAA-amide conjugates in the diploid strawberry achene, are hydrolyzed in growing seedlings. Planta 2019, 249, 1073–1085. [Google Scholar] [CrossRef]
- Liao, D.; Chen, X.; Chen, A.; Wang, H.; Liu, J.; Liu, J.; Gu, M.; Sun, S.; Xu, G. The characterization of six auxin-induced tomato GH3 genes uncovers a member, SlGH3.4, strongly responsive to arbuscular mycorrhizal symbiosis. Plant Cell Physiol. 2015, 56, 674–687. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.T.; Freitas, N.C.; Máximo, W.P.F.; Cardoso, T.B.; de Oliveira Prudente, D.; Paiva, L.V. Genome-wide analysis, transcription factor network approach and gene expression profile of GH3 genes over early somatic embryogenesis in Coffea spp. BMC Genom. 2019, 20, 812. [Google Scholar] [CrossRef]
- Quiroz-Figueroa, F.R.; Monforte-González, M.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. Direct somatic embryogenesis in Coffea canephora. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Vázquez-Flota, F.A., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 111–117. [Google Scholar] [CrossRef]
- Nic-Can, G.I.; López-Torres, A.; Barredo-Pool, F.A.; Wrobel, K.; Loyola-Vargas, V.M.; Rojas-Herrera, R.; De-la-Peña, C. New insights into somatic embryogenesis: LEAFY COTYLEDON1, BABY BOOM1 and WUSCHEL-RELATED HOMEOBOX4 are epigenetically regulated in Coffea canephora. PLoS ONE 2013, 8, e72160. [Google Scholar] [CrossRef] [Green Version]
- Nic-Can, G.I.; Galaz-Ávalos, R.M.; De-la-Peña, C.; Loyola-Vargas, V.M. Somatic embryogenesis: Identified factors that lead to embryogenic repression. A case of species of the same genus. PLoS ONE 2015, 10, e0126414. [Google Scholar] [CrossRef]
- Márquez-López, R.E.; Pérez-Hernández, C.A.; Kú-González, Á.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. Localization and transport of indole-3-acetic acid during somatic embryogenesis in Coffea canephora. Protoplasma 2018, 255, 695–708. [Google Scholar] [CrossRef]
- Quintana-Escobar, A.O.; Nic-Can, G.I.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M.; Góngora-Castillo, E. Transcriptome analysis of the induction of somatic embryogenesis in Coffea canephora and the participation of arf and AUX/IAA genes. PeerJ 2019, 7, e7752. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, T.; Fujii, Y.; Yamaguchi, T. Embryogenic callus induction from Coffea arabica leaf explants by benzyladenine. Plant Cell Physiol. 1985, 26, 595–597. [Google Scholar] [CrossRef]
- Denoeud, F.; Carretero-Paulet, L.; Dereeper, A.; Droc, G.; Guyot, R.; Pietrella, M.; Zheng, C.; Alberti, A.; Anthony, F.; Aprea, G.; et al. The coffee genome provides insight into the convergent evolution of caffeine biosynthesis. Science 2014, 345, 1181–1184. [Google Scholar] [CrossRef] [Green Version]
- LeClere, S.; Tellez, R.; Rampey, R.A.; Matsuda, S.P.T.; Bartel, B. Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. J. Biol. Chem. 2002, 277, 20446–20452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peat, T.S.; Böttcher, C.; Newman, J.; Lucent, D.; Cowieson, N.; Davies, C. Crystal structure of an indole-3-acetic acid amido synthetase from grapevine involved in auxin homeostasis. Plant Cell 2012, 24, 4525–4538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westfall, C.S.; Sherp, A.M.; Zubieta, C.; Alvarez, S.; Schraft, E.; Marcellin, R.; Ramirez, L.; Jez, J.M. Arabidopsis thaliana GH3. 5 acyl acid amido synthetase mediates metabolic crosstalk in auxin and salicylic acid homeostasis. Proc. Natl. Acad. Sci. USA 2016, 113, 13917–13922. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Zhang, Y.; Li, M.; Jiao, X.; Zhou, L.; Ming, Z. Crystal structure of the acyl acid amido synthetase GH3-8 from Oryza sativa. Biochem. Biophys. Res. Commun. 2021, 534, 266–271. [Google Scholar] [CrossRef]
- Dereeper, A.; Bocs, S.; Rouard, M.; Guignon, V.; Ravel, S.; Tranchant-Dubreuil, C.; Poncet, V.; Garsmeur, O.; Lashermes, P.; Droc, G. The coffee genome hub: A resource for coffee genomes. Nucleic Acids Res. 2015, 43, D1028–D1035. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Vargas, V.M.; Avilez-Montalvo, J.R.; Avilez-Montalvo, R.N.; Márquez-López, R.E.; Galaz-Ávalos, R.M.; Mellado-Mojica, E. Somatic embryogenesis in Coffea spp. In Somatic Embryogenesis. Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: Cham, Switzerland, 2016; pp. 241–266. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, N.A.; Amaral-Silva, P.M.; Sattler, M.C.; de Oliveira, S.C.; Cesário, L.M.; Ferreira, A.; Carvalho, C.R.; Clarindo, W.R. Indirect somatic embryogenesis in Coffea with different ploidy levels: A revisiting and updating study. Plant Cell Tissue Organ Cult. 2019, 136, 255–267. [Google Scholar] [CrossRef]
- Zhang, H.; Ali, A.; Hou, F.; Wu, T.; Guo, D.; Zeng, X.; Wang, F.; Zhao, H.; Chen, X.; Xu, P. Effects of ploidy variation on promoter DNA methylation and gene expression in rice (Oryza sativa L.). BMC Plant Biol. 2018, 18, 314. [Google Scholar] [CrossRef]
- Westfall, C.S.; Herrmann, J.; Chen, Q.; Wang, S.; Jez, J.M. Modulating plant hormones by enzyme action. The GH3 family of acyl acid amido synthetases. Plant Signal. Behav. 2010, 5, 1607–1612. [Google Scholar] [CrossRef] [Green Version]
- Jain, M.; Kaur, N.; Tyagi, A.K.; Khurana, J.P. The auxin-responsive GH3 gene family in rice (Oryza sativa). Funct. Integr. Genom. 2006, 6, 36–46. [Google Scholar] [CrossRef]
- Staswick, P.E.; Tiryaki, I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, C.; Keyzers, R.A.; Boss, P.K.; Davies, C. Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J. Exp. Bot. 2010, 61, 3615–3625. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Westfall, C.S.; Hicks, L.M.; Wang, S.; Jez, J.M. Kinetic basis for the conjugation of auxin by a GH3 family indole-acetic acid-amido synthetase. J. Biol. Chem. 2010, 285, 29780–29786. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Cao, Y.; Huang, L.; Zhao, J.; Xu, C.; Li, X.; Wang, S. Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell 2008, 20, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.W.; Li, C.H.; Cao, J.; Zhang, Y.C.; Zhang, S.Q.; Xia, Y.F.; Sun, D.Y.; Sun, Y. Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3.13 activation. Plant Physiol. 2009, 151, 1889–1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazawa, M.; Yabe, N.; Ichikawa, T.; Yamamoto, Y.Y.; Yoshizumi, T.; Hasunuma, K.; Matsui, M. DFL1, an auxin-responsive GH3 gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J. 2001, 25, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 2007, 19, 2430–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Barnewall, R.J.; Marsh, I.B.; Szentirmay, A.N.; Quinn, J.C.; van Houdt, R.; Gunst, Q.D.; van den Hoff, M.J.B. Efficiency correction is required for accurate quantitative PCR analysis and reporting. Clin. Chem. 2021, 67, 829–842. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Goulao, L.F.; Fortunato, A.S.; Ramalho, J.C. Selection of reference genes for normalizing quantitative real-time PCR gene expression data with multiple variables in Coffea spp. Plant Mol. Biol. Rep. 2012, 30, 741–759. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Magalhäes, C.S.; Almeida, D.M.; Barbosa, H.J.C.; Dardenne, L.E. A dynamic niching genetic algorithm strategy for docking highly flexible ligands. Inf. Sci. 2014, 289, 206–224. [Google Scholar] [CrossRef]



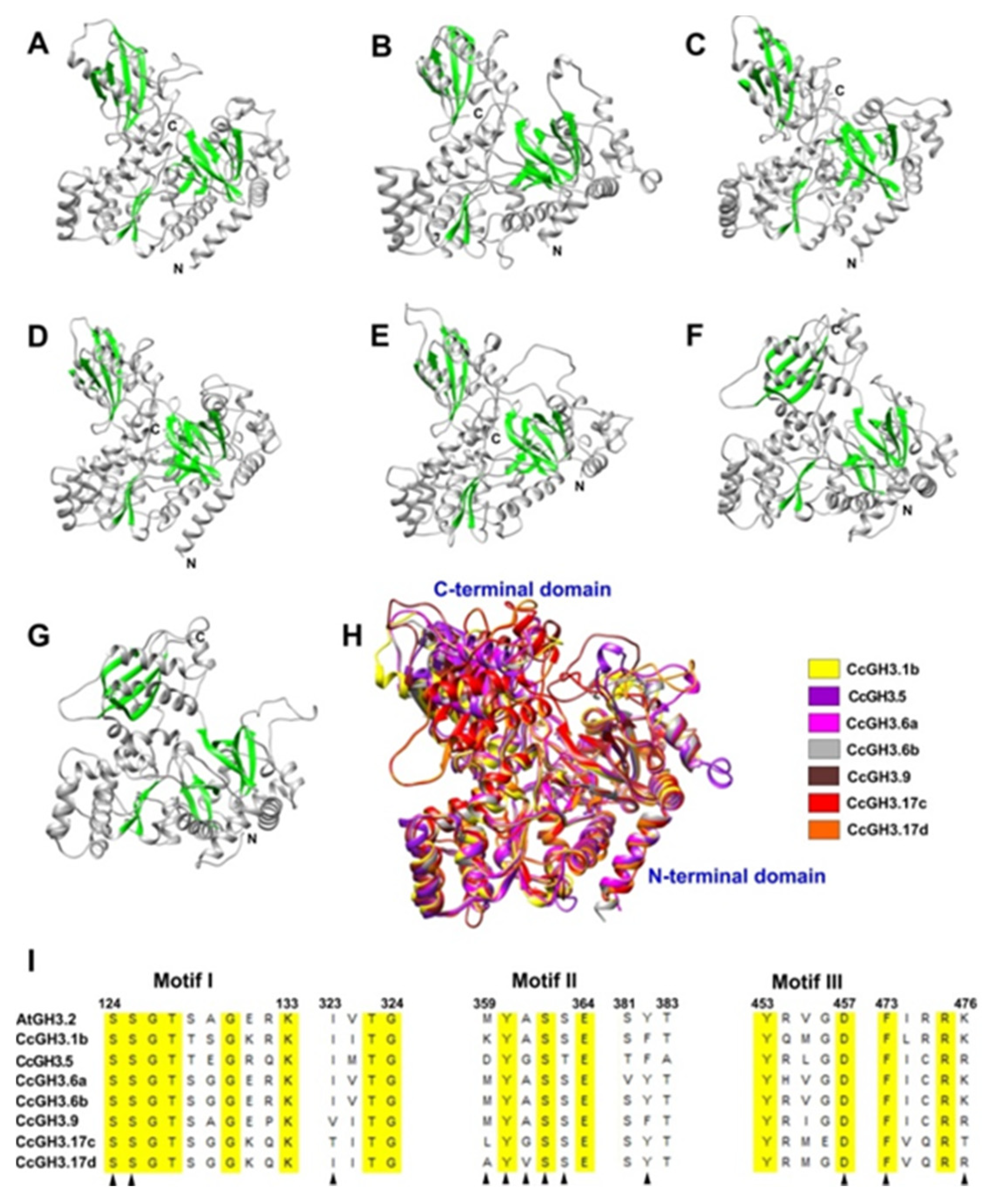
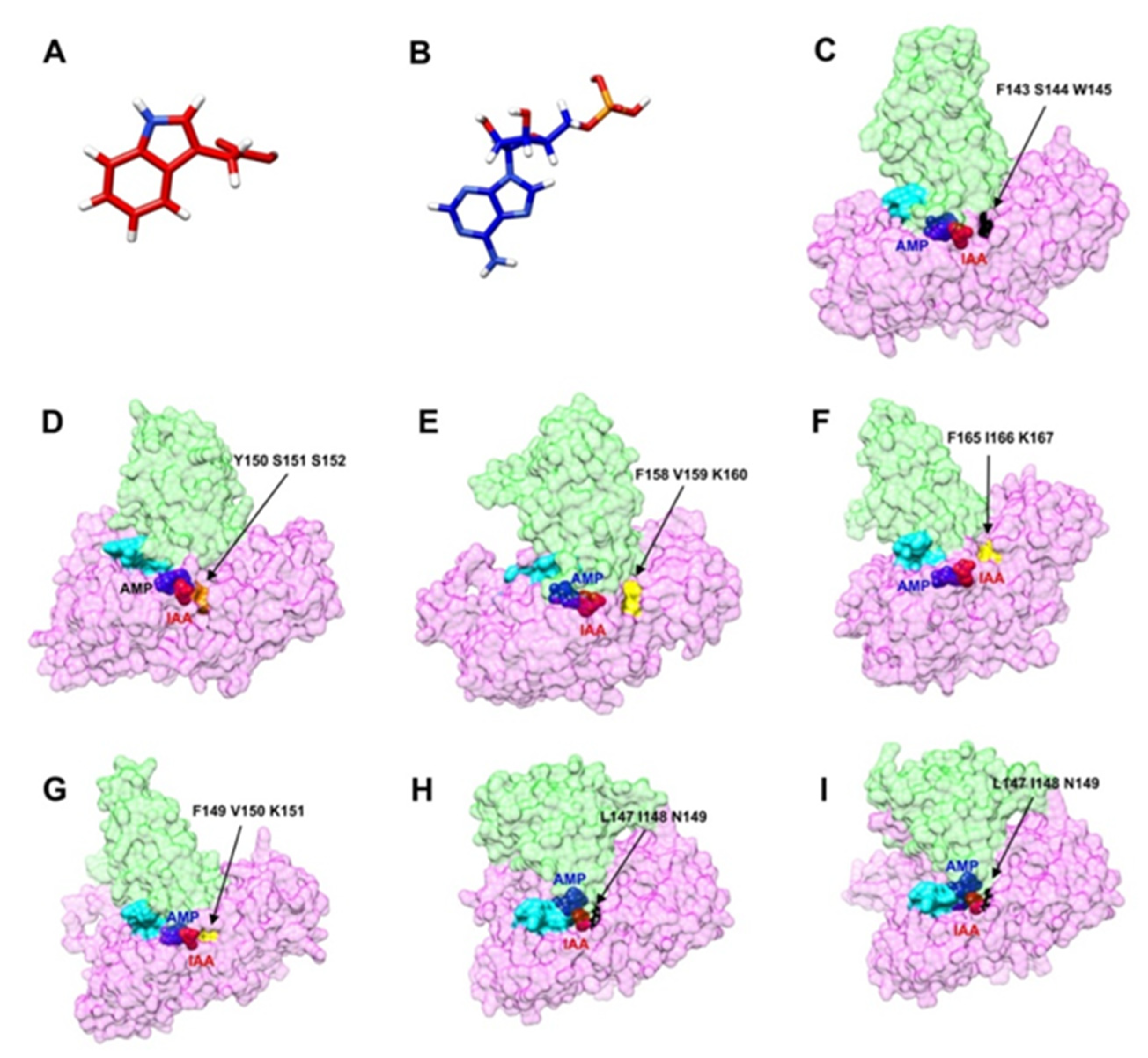
| Gene | Locus ID | ORF (bp) | TAIR Locus ID | No. Exons | Start | End | Deduced Polypeptide | Predicted Subcellular Localization | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Length (aa) | MW (Da) | pI | ||||||||
| CcGH3.1a | Cc00_g22520 | 2581 | AT2G14960 | 4 | 142,656,917 | 142,659,058 | 569 | 64,641.15 | 5.69 | Cytoplasmic |
| CcGH3.1b | Cc00_g01360 | 2740 | AT2G14960 | 3 | 8,822,291 | 8,824,450 | 593 | 66,898.26 | 5.35 | Cytoplasmic |
| CcGH3.1c | Cc07_g06610 | 2750 | AT2G14960 | 4 | 4,821,858 | 4,824,041 | 528 | 59,892.74 | 6.50 | Cytoplasmic |
| CcGH3.1d | Cc02_g19460 | 1367 | AT2G14960 | 4 | 17,549,243 | 17,550,429 | 271 | 30,560.21 | 8.26 | Cytoplasmic |
| CcGH3.3 | Cc02_g19470 | 862 | AT4G37390AtGH3.2/YDK1 | 1 | 17,550,591 | 17,551,298 | 236 | 26,644.15 | 5.61 | Cytoplasmic |
| CcGH3.5 | Cc05_g06700 | 3771 | AT2G46370AtGH3.11/JAR1 | 4 | 21,465,217 | 21,468,524 | 591 | 67,271.46 | 5.91 | Cytoplasmic |
| CcGH3.6a | Cc05_g05640 | 2379 | AT5G54510AtGH3.6/DFL1 | 3 | 20,228,391 | 20,230,769 | 607 | 68,277.83 | 5.53 | Cytoplasmic |
| CcGH3.6b | Cc05_g12940 | 2430 | AT5G54510AtGH3.6/DFL1 | 3 | 26,669,847 | 26,672,091 | 622 | 69,873.15 | 6.09 | Cytoplasmic |
| CcGH3.9 | Cc01_g20620 | 2990 | AT2G47750AtGH3.9 | 4 | 37,172,864 | 37,175,503 | 606 | 68,693.72 | 5.93 | Cytoplasmic |
| CcGH3.17a | Cc00_g04490 | 6755 | AT1G28130AtGH3.17 | 4 | 34,209,474 | 34,211,882 | 583 | 65,979.47 | 5.66 | Cytoplasmic |
| CcGH3.17b | Cc00_g04500 | 1516 | AT1G28130AtGH3.17 | 3 | 34,230,766 | 34,232,281 | 371 | 42,536.80 | 6.19 | --- |
| CcGH3.17c | Cc00_g04520 | 2705 | AT1G28130AtGH3.17 | 4 | 34,265,456 | 34,267,828 | 583 | 65,990.42 | 5.77 | E. reticulum |
| CcGH3.17d | Cc00_g04530 | 4103 | AT1G28130AtGH3.17 | 4 | 34,279,931 | 34,282,340 | 583 | 66,020.49 | 5.73 | Cytoplasmic |
| CcGH3.17e | Cc00_g04540 | 1774 | AT1G28130AtGH3.17 | 2 | 34,297,176 | 34,298,627 | 357 | 40,498.45 | 5.69 | Cytoplasmic |
| CcGH3.17f | Cc00_g04550 | 1284 | AT1G28130AtGH3.17 | 4 | 34,298,709 | 34,299,719 | 226 | 25,308.99 | 5.56 | Cytoplasmic |
| CcGH3.17g | Cc00_g28970 | 1939 | AT1G28130AtGH3.17 | 4 | 178,935,475 | 178,936,518 | 239 | 26,550.34 | 6.44 | Cytoplasmic |
| CcGH3.17h | Cc00_g28980 | 1961 | AT1G28130AtGH3.17 | 2 | 178,936,581 | 178,938,006 | 348 | 39,719.58 | 5.78 | Cytoplasmic |
| CcGH3.17i | Cc10_g16320 | 3565 | AT1G28130AtGH3.17 | 5 | 27,266,812 | 27,269,856 | 583 | 65,421.04 | 5.44 | Cytoplasmic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Hernández, H.A.; Quintana-Escobar, A.O.; Uc-Chuc, M.A.; Loyola-Vargas, V.M. Genome-Wide Analysis, Modeling, and Identification of Amino Acid Binding Motifs Suggest the Involvement of GH3 Genes during Somatic Embryogenesis of Coffea canephora. Plants 2021, 10, 2034. https://doi.org/10.3390/plants10102034
Méndez-Hernández HA, Quintana-Escobar AO, Uc-Chuc MA, Loyola-Vargas VM. Genome-Wide Analysis, Modeling, and Identification of Amino Acid Binding Motifs Suggest the Involvement of GH3 Genes during Somatic Embryogenesis of Coffea canephora. Plants. 2021; 10(10):2034. https://doi.org/10.3390/plants10102034
Chicago/Turabian StyleMéndez-Hernández, Hugo A., Ana O. Quintana-Escobar, Miguel A. Uc-Chuc, and Víctor M. Loyola-Vargas. 2021. "Genome-Wide Analysis, Modeling, and Identification of Amino Acid Binding Motifs Suggest the Involvement of GH3 Genes during Somatic Embryogenesis of Coffea canephora" Plants 10, no. 10: 2034. https://doi.org/10.3390/plants10102034
APA StyleMéndez-Hernández, H. A., Quintana-Escobar, A. O., Uc-Chuc, M. A., & Loyola-Vargas, V. M. (2021). Genome-Wide Analysis, Modeling, and Identification of Amino Acid Binding Motifs Suggest the Involvement of GH3 Genes during Somatic Embryogenesis of Coffea canephora. Plants, 10(10), 2034. https://doi.org/10.3390/plants10102034








