Morphological Variation in Scarlet Plume (Euphorbia fulgens Karw ex Klotzsch, Euphorbiaceae), an Underutilized Ornamental Resource of Mexico with Global Importance
Abstract
:1. Introduction
2. Results
2.1. Morphological Variation in Wild Populations
2.2. Morphological Variation in Cultivated Populations
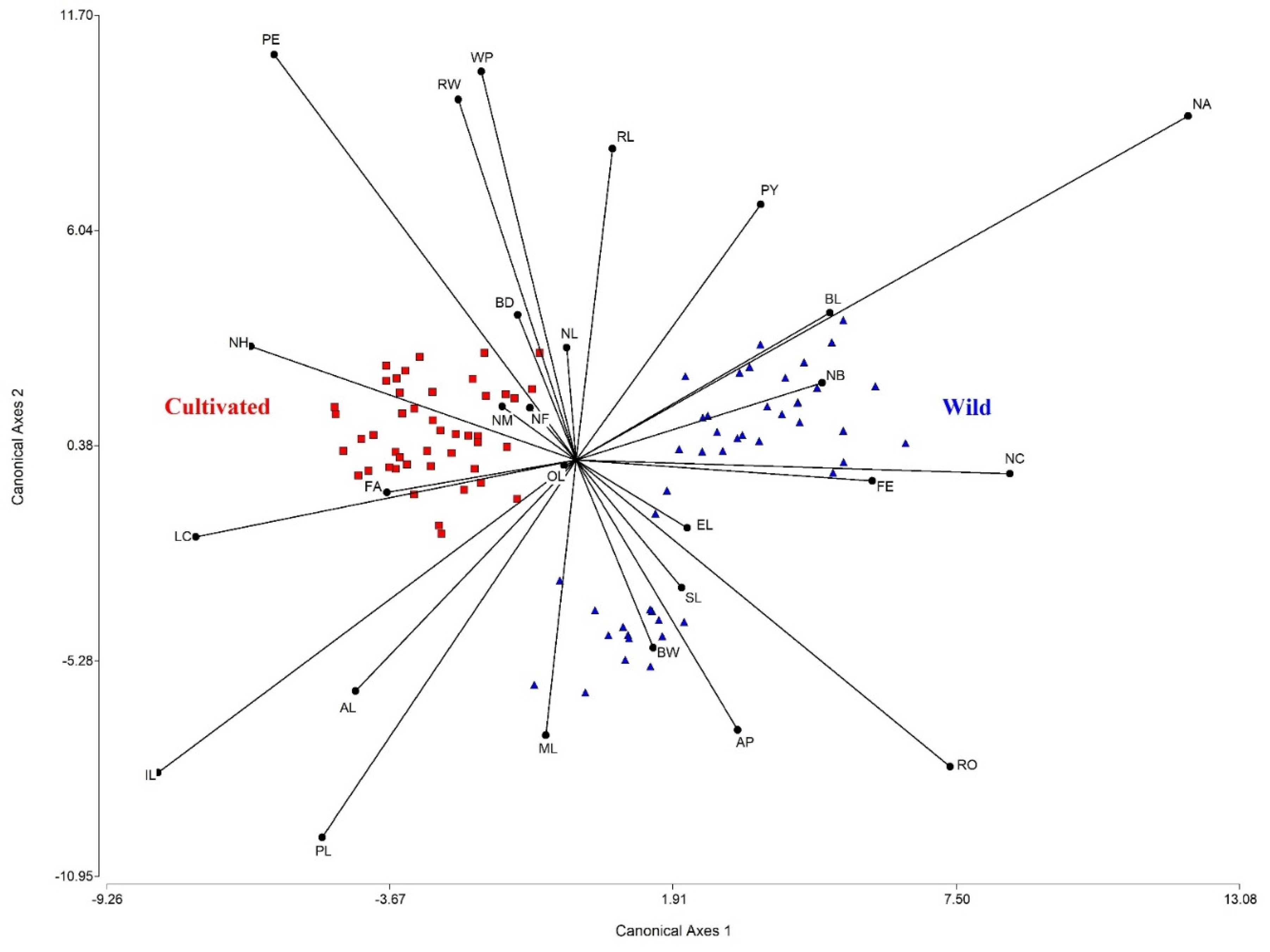
2.3. Morphometric Variation between Wild-Growing and Cultivated Populations of E. fulgens
3. Discussion
3.1. Morphological Variation in Wild Populations
3.2. Morphological Variation in Cultivated Populations
3.3. Morphological Variation between Wild-Growing and Cultivated Populations
4. Materials and Methods
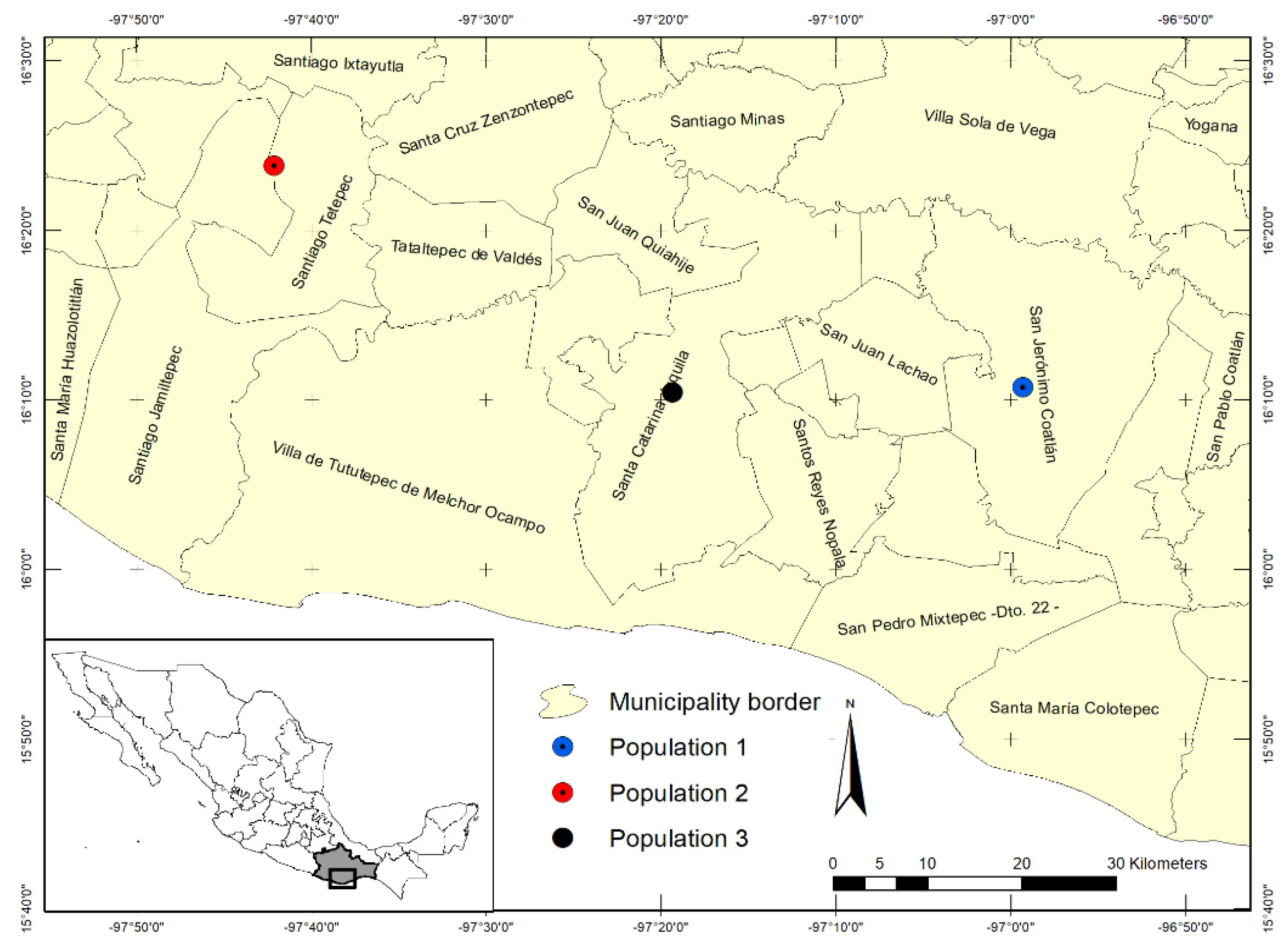
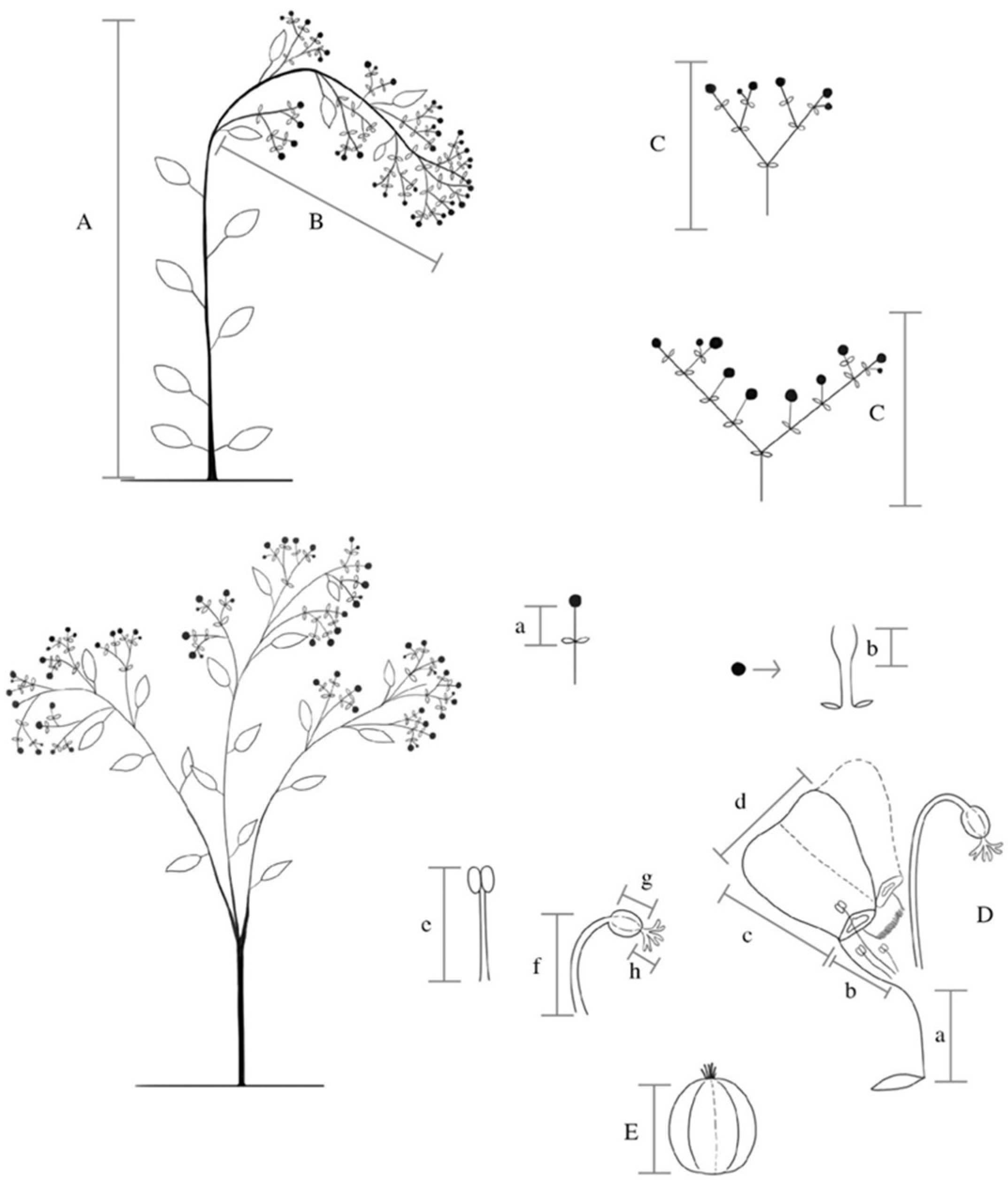
4.1. Morphological Variation in Wild-Growing Populations
4.2. Morphological Variation of Cultivated Populations of E. fulgens
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmid, B. Phenotypic variation in plants. Evol. Trends Plants 1992, 6, 45–60. [Google Scholar]
- Willmore, K.E.; Young, N.M.; Richtsmeier, J.T. Phenotypic variability: Its components, measurement and underlying developmental processes. Evol. Biol. 2007, 34, 99–120. [Google Scholar] [CrossRef]
- Casas, A.; Caballero, J.; Mapes, C.; Zárate, S. Manejo de la vegetación, domesticación de plantas y origen de la agricultura en Mesoamérica. Bol. Soc. Bot. Méx. 1997, 61, 31–47. [Google Scholar] [CrossRef]
- Casas, A.; Pickersgill, B.; Caballero, J.; Valiente-Banuet, A. Ethnobotany and domestication in xoconochtli, Stenocereus stellatus (Cactaceae), in the Tehuacán Valley and La Mixteca Baja, Mexico. Econ. Bot. 1997, 51, 279–292. [Google Scholar] [CrossRef]
- Blancas-Vásquez, J.; Casas, A.; Pérez-Salicrup, D.; Caballero, J. Ecological and socio-cultural factors influencing plant management in nahuatl communities of the Tehuacán Valley, Mexico. J. Ethnobiol. Ethnomed. 2013, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Cruise-Sanders, J.M.; Casas, A. Impactos evolutivos de las actividades humanas sobre las plantas: Manejo, domesticación y conservación in situ y ex situ. In Domesticación en el Continente Americano Investigación Para el Manejo Sustentable de Recursos Genéticos en el Nuevo Mundo; Casas, A., Torres-Guevara, J., Parra-Rondinel, F., Eds.; Editorial Morevalladolid: Morelia, Mexico, 2017; Volume 2, pp. 452–473. [Google Scholar]
- Casas, A.; Otero-Arnaiz, A.; Pérez-Negrón, E.; Valiente-Banuet, A. In situ management and domestication of plants in Mesoamerica. Ann. Bot. 2007, 100, 1101–1115. [Google Scholar] [CrossRef] [Green Version]
- De Freitas Lins Neto, E.M.; de Olivieira, I.F.; Britto, F.B.; de Albuquerque, J.P. Traditional knowledge, genetic and morphological diversity in populations of Spondias tuberosa Arruda (Analcardiaceae). Gen. Res. Crop. Evol. 2013, 60, 1389–1406. [Google Scholar] [CrossRef]
- Aguirre-Dugua, X.; Pérez-Negrón, E.; Casas, A. Phenotypic differentiation between wild and domesticated varieties of Crescentia cujete L. and culturally relevant uses of their fruits as bowls in the Yucatan Peninsula, Mexico. J. Ethnobiol. Ethnomed. 2013, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Betancourt-Olvera, M.; Nieto-Ángel, R.; Urbano, B.; González-Andrés, F. Analysis of the biodiversity of hawthorn (Crataegus spp.) from the morphological, molecular, and ethnobotanical approaches, and implications for genetic resource conservation in scenery of increasing cultivation: The case of Mexico. Gen. Res. Crop. Evol. 2018, 65, 897–916. [Google Scholar] [CrossRef]
- Aros, D.; Meneses, C.; Infante, R. Genetic diversity of wild species and cultivated varieties of alstroemeria estimated through morphological descriptors and RAPD marker. Sci. Hortic. 2006, 108, 86–90. [Google Scholar] [CrossRef]
- Trejo, L.; Rosell, J.A.; Olson, M.E. Nearly 200 years of sustained selection have not overcome the leaf area-stem size relationship in the poinsettia. Evol. Appl. 2018, 11, 1401–1411. [Google Scholar] [CrossRef]
- Ferreira de Melo, C.A.; Magalhães-Souza, M.; Viana, A.P.; Azevedo-Santosm, E.; de Oliveira-Souza, V.; Corrêa, R.X. Morphological characterization and genetic parameter estimation in backcrossed progenies of Passiflora, L. for ornamental use. Sci. Hortic. 2016, 212, 91–103. [Google Scholar] [CrossRef]
- Mladenović, E.; Cvejić, S.; Jocić, S.; Ĉukanović, J.; Jocković, M.; Malizdža, G. Variability of morphological characters among ornamental sunflower collection. Genetika 2017, 49, 573–582. [Google Scholar] [CrossRef]
- Aros, D.; Suazo, M.; Rivas, C.; Zapata, P.; Úbeda, C.; Bridgen, M. Molecular and mophological characterization of new interspecific hybrids of alstroemeria originated from A. caryophylleae scented lines. Euphytica 2019, 215, 93. [Google Scholar] [CrossRef] [Green Version]
- Dalda-Şekerci, A.; Karaman, K.; Yetişir, H. Characterization of ornamental pumpkin (Cucurbita pepo L. var. ovifera (L.) Alef.) genotypes: Molecular, morphological and nutritional properties. Gen. Res. Crop. Evol. 2020, 67, 533–547. [Google Scholar] [CrossRef]
- Bruschi, P.; Vendramin, G.G.; Bussotti, F.; Grossoni, P. Morphological and molecular diversity among Italian populations of Quercus petraea (Fagaceae). Ann. Bot. 2003, 91, 707–716. [Google Scholar] [CrossRef]
- Ouinsavi, C.; Sokpon, N. Morphological variation and ecological structure of Iroko (Milicia excelsa Welw. C. C. Berg) populations across different biogeographical Zonas in Benin. Int. J. For. Res. 2010, 2010, 658396. [Google Scholar] [CrossRef]
- López-Gómez, V.; Zedillo-Avelleyra, P.; Anaya-Hong, S.; González-Lozada, E.; Cano-Santana, Z. Efecto de la orientación de la ladera sobre la estructura poblacional y ecomorfología de Neobuxbaumia tetetzo (Cactaceae). Bot. Sci. 2012, 90, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Ouédrago, L.; Fuchs, D.; Schaefer, H.; Kiendrebeogo, M. Morphological and molecular characterization of Zanthoxylum zanthoxyloides (Rutaceae) from Burkina Faso. Plants 2019, 8, 353. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Vázquez, B.N.; Gómez-Cárdenas, M.; Gutiérrez-Vázquez, M.H.; Mallén-Rivera, C. Variación fenotípica de poblaciones naturales de Pinus oocarpa Schiede ex Schltdl. en Chiapas. Rev. Mex. Cien. 2013, 4, 46–60. [Google Scholar]
- Arteaga, M.C.; Bello-Bedoy, R.; León de la Luz, J.L.; Delgadillo, J.; Domínguez, R. Phenotypic variation of flowering and vegetative morphological traits along the distribution for the endemic species Yucca capensis (Agavaceae). Bot. Sci. 2015, 93, 765–770. [Google Scholar] [CrossRef]
- Matesanz, S.; Rubio-Teso, M.L.; García-Fernández, A.; Escudero, A. Habitat fragmentation differentially affects genetic variation, phenotypic plasticity and survival in populations of a Gypsum endemic. Front. Plant. Sci. 2017, 8, 843. [Google Scholar] [CrossRef]
- Winkler, D.E.; Yu-Chan Lin, M.; Delgadillo, J.; Chapin, K.J.; Huxman, T.E. Early life history responses and phenotypic shifts in a rare endemic plant responding to climate change. Conserv. Physiol. 2019, 7, coz076. [Google Scholar] [CrossRef]
- Rünger, W.; Albert, G. Influence of temperature, soil moisture and CCC on the flowering of Euphorbia fulgens. Sci. Hortic. 1975, 3, 393–403. [Google Scholar] [CrossRef]
- Van Leeuwen, P.J. Post-harvest treatment of Euphorbia fulgens. Acta Hortic. 1986, 181, 467–471. [Google Scholar] [CrossRef]
- Canul-Ku, J.; García-Pérez, F.; Ramírez-Rojas, S.G.; Osuna-Canizalez, F.J. Estrategias para el mejoramiento genético de Nochebuena (Euphorbia pulcherrima Willd. ex Klotzsch). Investigación Agropecuaria 2010, 7, 44–54. [Google Scholar]
- Gwali, S.; Nakabonge, G.; Lamoris-Okullo, J.G.; Eilu, G.; Nyeko, P.; Vuzi, P. Morphological variation among sea tree (Vitellaria paradoxa subsp. nilotica) ‘ethnovarieties’ in Uganda. Gen. Res. Crop. Evol. 2012, 59, 1883–1898. [Google Scholar] [CrossRef]
- Araiza-Lizarde, N.; Alcaraz-Meléndez, L.; Angulo-Escalante, M.A.; Reynoso-Granados, T.; Cruz-Hernández, P.; Ortega-Nieblas, M.; Valdez-Zamudio, D. Caracterización y distribución de germoplasma silvestre de Jatropha curcas L. (Euphorbiaceae) en el Noroeste de México. Polibotánica 2016, 42, 137–152. [Google Scholar] [CrossRef]
- Beltrán-Rodríguez, L.; Romero-Manzanares, A.; Luna-Cavazos, M.; García-Moya, E. Variación arquitectónica y morfológica de Hintonia latiflora (Rubiaceae) en relación a la cosecha de corteza y factores ambientales. Rev. Biol. Trop. 2017, 65, 900–916. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Arias, A.; Palomino-Hasbach, G.; Terrazas, T.; García-Velasquez, A.; Pimienta-Barrios, E. Variación anatómica y morfológica en especies y entre poblaciones de Opuntia en la porción sur del desierto Chihuahuense. Bol. Soc. Bot. Mex. 2008, 83, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Parker, I.M.; López, I.; Petersen, J.J.; Anaya, N.; Cubilla-Rios, L.; Potter, D. Domestication syndrome in Caimito (Chysophyllum cainito L.): Fruit and seed characteristics. Econ. Bot. 2010, 64, 161–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre-Dugua, X.; Eguiarte, L.E.; González-Rodríguez, A.; Casas, A. Round and large: Morphological and genetic consequences of artificial selection on the gourd tree Crescentia cujete by the Maya of the Yucatan Peninsula, Mexico. Ann. Bot. 2012, 109, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rinna, R.; Morawetz, J.J.; Haevermans, T.; Aubriot, X.; Berry, P.E. Molecular phylogenetics and classification of Euphorbia subgenus Chamaesyce (Euphorbiaceae). Taxon 2012, 61, 764–789. [Google Scholar] [CrossRef] [Green Version]
- UPOV. Guidelines for the Conduct of Tests for Distinctness, Homogeneity and Stability. Euphorbia fulgens. International Union for the Protection of New Varieties of Plants. 1988. Available online: https://www.upov.int/genie/es/details.xhtml?cropId=2262 (accessed on 20 February 2017).
- The Royal Horticultural Society. Colour Chart; The Royal Horticutural Society: London, UK, 2001. [Google Scholar]
- Izenman, A.J. Linear Discriminant Analysis. In Modern Multivariate Statistical Techniques; Springer Texts in Statistics: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available online: http://www.infostat.com.ar (accessed on 12 January 2020).




| Variable | p-Value | Population 1 | Population 2 | Population 3 |
|---|---|---|---|---|
| Plant height | 0.0696 | 145.53 a | 158.63 a | 180.63 a |
| Basal diameter | 0.0240 | 0.78 ab | 0.64 b | 1.02 a |
| Petiole length | 0.0007 | 3.46 a | 2.94 b | 2.95 b |
| Blade length | 0.0001 | 7.99 c | 9.03 b | 9.73 a |
| Blade width | 0.0204 | 2.75 a | 2.36 b | 2.45 ab |
| Blade width/Blade length | 0.0001 | 0.35 a | 0.26 b | 0.25 b |
| Petiole length/Blade length | 0.0001 | 0.43 a | 0.33 b | 0.30 b |
| Leaf area | 0.8267 | 14.43 a | 13.77 a | 14.08 a |
| Leaf perimeter | 0.4161 | 27.88 a | 27.46 a | 28.73 a |
| Leaf Feret diameter | 0.0504 | 11.12 b | 11.58 ab | 12.01 a |
| Leaf roundness | <0.0001 | 0.34 a | 0.26 b | 0.25 b |
| Number of branches with inflorescences | 0.0037 | 4.20 a | 1.80 b | 4.07 a |
| Inflorescence length | 0.0080 | 12.07 b | 22.92 a | 18.72 ab |
| Number of cyathia per axillary cyme | 0.1527 | 3.03 a | 3.37 a | 3.07 a |
| Longest cyme length | 0.0068 | 2.72 ab | 2.87 a | 2.41 b |
| Number of axillary cymes | 0.9085 | 14.27 a | 13.40 a | 13.73 a |
| Number of hermaphrodite cyathia | 0.0001 | 1.53 b | 9.30 a | 0.73 b |
| Number of male cyathia | 0.0011 | 14.33 a | 7.13 b | 8.17 b |
| Number of female cyathia | 0.5249 | 1.03 a | 0.60 a | 0.67 a |
| Peduncle length | 0.0001 | 1.53 a | 1.05 b | 1.51 a |
| Involucre length | 0.0007 | 0.31 b | 0.30 b | 0.33 a |
| Petal appendage length | 0.6668 | 0.54 a | 0.54 a | 0.53 a |
| Petal appendage width | 0.0044 | 0.50 a | 0.50 a | 0.44 b |
| Pedicel length | 0.0033 | 0.98 a | 0.85 b | 1.02 a |
| Ovary length | 0.0136 | 0.32 a | 0.29 b | 0.31 ab |
| Style length | 0.0117 | 0.15 a | 0.12 ab | 0.11 b |
| Stamen length | <0.0001 | 0.46 c | 0.67 a | 0.53 b |
| Petal appendages colour | <0.0001 | 1.57 b | 3.20 a | 1.30 b |
| Leaf blade colour in the superior third of inflorescence | 0.3721 | 1.00 a | 1.00 a | 1.03 a |
| Intensity of leaf blade colour in the superior third of inflorescence | 0.3721 | 1.00 a | 1.00 a | 1.13 a |
| Variable | p-Value | Population 1 | Population 2 | Population 3 |
|---|---|---|---|---|
| Plant height | 0.0007 | 88.50 b | 83.69 b | 110.87 a |
| Basal diameter | 0.0102 | 0.44 b | 0.44 b | 0.49 a |
| Petiole length | 0.0024 | 3.25 a | 2.73 b | 2.60 b |
| Blade length | 0.0032 | 7.74 b | 8.51 ab | 8.88 a |
| Blade width | <0.0001 | 2.41 a | 1.38 c | 1.92 b |
| Blade width/Blade length | <0.0001 | 0.32 a | 0.15 c | 0.21 b |
| Petiole length/Blade length | <0.0001 | 0.43 a | 0.33 b | 0.29 b |
| Internode length | 0.0712 | 1.77 a | 1.95 a | 2.08 a |
| Leaf area | 0.0002 | 13.58 a | 9.38 b | 12.59 a |
| Leaf perimeter | 0.7648 | 29.44 a | 29.50 a | 30.11 a |
| Leaf Feret diameter | 0.0422 | 10.61 b | 11.20 ab | 11.55 a |
| Leaf roundness | <0.0001 | 0.31 a | 0.17 b | 0.21 b |
| Number of branches with inflorescences | 0.0877 | 31.13 a | 25.87 a | 28.47 a |
| Inflorescence length | 0.1890 | 20.13 a | 16.97 a | 21.92 a |
| Number of cyathia per axillary cyme | 0.0180 | 3.47 ab | 2.93 b | 3.93 a |
| Longest cyme length | 0.0471 | 4.23 ab | 4.74 a | 3.77 b |
| Number of axillary cymes | 0.1277 | 14.33 a | 10.67 a | 12.40 a |
| Number of hermaphrodite cyathia | 0.0633 | 33.87 a | 20.60 a | 29.80 a |
| Number of male cyathia | 0.5016 | 14.47 a | 11.13 a | 10.40 a |
| Number of female cyathia | 0.0298 | 5.87 a | 2.13 b | 4.87 ab |
| Internode length in inflorescence | 0.2370 | 1.05 a | 1.12 a | 1.87 a |
| Peduncle length | 0.1880 | 1.13 a | 1.39 a | 1.19 a |
| Involucre length | 0.8454 | 0.32 a | 0.32 a | 0.33 a |
| Cyathium diameter | 0.2550 | 1.40 a | 1.41 a | 1.27 a |
| Petal appendage length | 0.0352 | 0.55 a | 0.54 a | 0.45 a |
| Petal appendage width | 0.7939 | 0.49 a | 0.51 a | 0.48 a |
| Pedicel length | 0.0636 | 0.83 a | 0.89 a | 0.73 a |
| Ovary length | 0.2002 | 0.30 a | 0.30 a | 0.27 a |
| Style length | 0.3040 | 0.10 a | 0.12 a | 0.11 a |
| Stamen length | 0.0156 | 0.70 a | 0.68 ab | 0.59 b |
| Petal appendages colour | 0.5167 | 3.40 a | 3.53 a | 3.07 a |
| Leaf blade colour in the superior third of inflorescence | 0.0018 | 1.60 a | 1.20 b | 1.00 b |
| Intensity of leaf blade colour in the superior third of inflorescence | 0.0011 | 2.87 a | 1.53 b | 1.07 b |
| Fruit length | 0.1489 | 0.69 a | 0.67 a | 0.60 a |
| Fruit diameter | 0.5954 | 0.60 a | 0.58 a | 0.56 a |
| Fruit number | 0.6511 | 5.67 a | 4.40 a | 4.93 a |
| Seed number | 0.9118 | 11.00 a | 10.40 a | 9.80 a |
| Number of vegetative sprouts | 0.2209 | 6.07 a | 4.47 a | 4.40 a |
| Population | Altitude (msnm) | Vegetation Type | Sand | Silt | Clay | Organic Matter |
|---|---|---|---|---|---|---|
| 1 | 1421 | OPF | 53.3 | 29.3 | 17.2 | 4.48 |
| 2 | 1130 | OPF | 55.5 | 31.3 | 13.2 | 7.07 |
| 3 | 1160 | MMF, SVMMF | 65.9 | 16.0 | 18.1 | 7.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Nicolás, M.; Colinas-León, T.; Alia-Tejacal, I.; Peña-Ortega, G.; González-Andrés, F.; Beltrán-Rodríguez, L. Morphological Variation in Scarlet Plume (Euphorbia fulgens Karw ex Klotzsch, Euphorbiaceae), an Underutilized Ornamental Resource of Mexico with Global Importance. Plants 2021, 10, 2020. https://doi.org/10.3390/plants10102020
Pérez-Nicolás M, Colinas-León T, Alia-Tejacal I, Peña-Ortega G, González-Andrés F, Beltrán-Rodríguez L. Morphological Variation in Scarlet Plume (Euphorbia fulgens Karw ex Klotzsch, Euphorbiaceae), an Underutilized Ornamental Resource of Mexico with Global Importance. Plants. 2021; 10(10):2020. https://doi.org/10.3390/plants10102020
Chicago/Turabian StylePérez-Nicolás, Mónica, Teresa Colinas-León, Iran Alia-Tejacal, Gisela Peña-Ortega, Fernando González-Andrés, and Leonardo Beltrán-Rodríguez. 2021. "Morphological Variation in Scarlet Plume (Euphorbia fulgens Karw ex Klotzsch, Euphorbiaceae), an Underutilized Ornamental Resource of Mexico with Global Importance" Plants 10, no. 10: 2020. https://doi.org/10.3390/plants10102020







