Genetic Diversity and Utilization of Cultivated Eggplant Germplasm in Varietal Improvement
Abstract
1. Introduction
2. Economic Importance of Eggplant
2.1. Bioactive Compounds of Eggplant
2.1.1. Phenolic
2.1.2. Carotenoids
2.1.3. Glycoalkaloids
2.2. Antioxidant Capacity of Eggplant
3. Eggplant Origin and Domestication: First Insights
4. Global Germplasm Collection and Conservation
5. Strengthening Interdisciplinary Collaborations for Management and Utilization of Germplasm
6. Characterization of Eggplant Diversity
7. Classical Genetics and Traditional Breeding
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapman, M.A. Introduction: The importance of eggplant. In The Eggplant Genome; Springer: Cham, Switzerland, 2019; pp. 1–10. [Google Scholar]
- Mat-Sulaiman, N.N.; Rafii, M.Y.; Duangjit, J.; Ramlee, S.I.; Phumichai, C.; Oladosu, Y.; Datta, D.R.; Musa, I. Genetic variability of eggplant germplasm evaluated under open field and glasshouse cropping conditions. Agronomy 2020, 10, 436. [Google Scholar] [CrossRef]
- Weese, T.L.; Bohs, L. Eggplant origins: Out of Africa, into the Orient. Taxon 2010, 59, 49–56. [Google Scholar] [CrossRef]
- Meyer, R.S.; Karol, K.G.; Little, D.P.; Nee, M.H.; Litt, A. Phylogeographic relationships among Asian eggplants and new perspectives on eggplant domestication. Mol. Phylogenet. Evol. 2012, 63, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.S.; DuVal, A.E.; Jensen, H.R. Patterns and processes in crop domestication: An historical review and quantitative analysis of 203 global food crops. New Phytologist. 2012, 196, 29–48. [Google Scholar] [CrossRef]
- Doganlar, S.; Frary, A.; Daunay, M.C.; Lester, R.N.; Tanksley, S.D. Conservation of gene function in the solanaceae as revealed by comparative mapping of domestication traits in eggplant. Genetics 2002, 161, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Gramazio, P.; Prohens-Tomás, J.; Plazas-Ávila, M.D.L.O.; Mangino, G.; Herraiz-García, F.J.; Garcia-Fortea, E.; Vilanova-Navarro, S. Genomic tools for the enhancement of vegetable crops: A case in eggplant. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 1–13. [Google Scholar] [CrossRef]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and future use of wild relatives in crop breeding. Crop Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef]
- Collonier, C.; Fock, I.; Kashyap, V.; Rotino, G.L.; Daunay, M.C.; Lian, Y.; Mariska, I.K.; Rajam, M.V.; Servaes, A.; Ducreux, G.; et al. Applications of biotechnology in eggplant. Plant Cell Tiss. Org. Cult. 2001, 65, 91–107. [Google Scholar] [CrossRef]
- Toppino, L.; Valè, G.; Rotino, G.L. Inheritance of fusarium wilt resistance introgressed from Solanum aethiopicum gilo and aculeatum groups into cultivated eggplant (S. melongena) and development of associated PCR-based markers. Mol. Breed. 2008, 22, 237–250. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Z.; Zhou, X.; Feng, C.; Zhuang, Y. Improving the resistance of eggplant (Solanum melongena) to verticillium wilt using wild species Solanum linnaeanum. Euphytica 2015, 201, 463–469. [Google Scholar] [CrossRef]
- Kaushik, P.; Prohens, J.; Vilanova, S.; Gramazio, P.; Plazas, M. Phenotyping of eggplant wild relatives and interspecific hybrids with conventional and phenomics descriptors provides insight for their potential utilization in breeding. Front. Plant Sci. 2016, 7, 677. [Google Scholar] [CrossRef]
- FAOSTAT Database Collections. Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 6 May 2021).
- Raigón, M.D.; Prohens, J.; Muñoz-Falcón, J.E.; Nuez, F. Comparison of eggplant landraces and commercial varieties for fruit content of phenolics, minerals, dry matter and protein. J. Food Compos. Anal. 2008, 21, 370–376. [Google Scholar] [CrossRef]
- Helmja, K.; Vaher, M.; Gorbatšova, J.; Kaljurand, M. Characterization of bioactive compounds contained in vegetables of the Solanaceae family by capillary electrophoresis. Proc. Estonian Acad. Sci. 2007, 56, 172–186. [Google Scholar]
- Prohens, J.; Whitaker, B.D.; Plazas, M.; Vilanova, S.; Hurtado, M.; Blasco, M.; Stommel, J.R. Genetic diversity in morphological characters and phenolic acids content resulting from an interspecific cross between eggplant, Solanum melongena, and its wild ancestor (S. incanum). Ann. Appl. Biol. 2013, 162, 242–257. [Google Scholar] [CrossRef]
- García-Salas, P.; Gómez-Caravaca, A.M.; Morales-Soto, A.; Segura-Carretero, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification and quantification of phenolic compounds in diverse cultivars of eggplant grown in different seasons by high-performance liquid chromatography coupled to diode array detector andelectrosprayquadrupole-time of flight-mass spectrometry. Food Res. Int. 2014, 57, 114–122. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautam, S.; Sharma, A. Browning of fresh-cut eggplant: Impact of cutting and storage. Postharvest Biol. Technol. 2012, 67, 44–51. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Kamdem, J.P.; Bolingon, A.A.; Athayde, M.L.; Lopes, S.R.; Waczuk, E.P.; Kade, I.J.; Adanlawo, I.G.; Rocha, J.B.T. African eggplant (Solanum anguivi Lam.) fruit with bioactive polyphenolic compounds exerts in vitro antioxidant properties and inhibits Ca2+ induced mitochondrial swelling. Asian Pac. J. Trop. Biomed. 2013, 3, 757–766. [Google Scholar] [CrossRef]
- Stommel, J.R.; Whitaker, B.D. Phenolic acid content and composition of eggplant fruit in a germplasmcore subset. J. Amer. Soc. Hort. Sci. 2003, 128, 704–710. [Google Scholar] [CrossRef]
- Gürbüz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef]
- Luthria, D.; Singh, A.P.; Wilson, T.; Vorsa, N.; Banuelos, G.S.; Vinyard, B.T. Influence of conventional and organic agricultural practices on the phenolic content in eggplant pulp: Plant-to-plant variation. Food Chem. 2010, 121, 406–411. [Google Scholar] [CrossRef]
- Sanchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Antifungal activity of secondary plant metabolites from potatoes (Solanum tuberosum L.): Glycoalkaloids and phenolic acids show synergistic effects. J. Appl. Microbiol. 2016, 120, 955–965. [Google Scholar] [PubMed]
- Zaro, M.J.; Keunchkarian, S.; Chaves, A.R.; Vicente, A.R.; Concellón, A. Changes in bioactive compounds and response to postharvest storage conditions in purple eggplants as affected by fruit developmental stage. Postharvest Biol. Technol. 2014, 96, 110–117. [Google Scholar] [CrossRef]
- Bagheri, M.; Bushehri, A.A.S.; Hassandokht, M.R.; Naghavi, M.R. Evaluation of solasonine content and expression patterns of SGT1 gene in different tissues of two Iranian eggplant (Solanum melongena L.) genotypes. Food Technol. Biotechnol. 2017, 55, 236–242. [Google Scholar] [CrossRef]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Nisha, P.; Nazar, P.A.; Jayamurthy, P. A comparative study on antioxidant activities of different varieties of Solanum melongena. Food Chem. Toxicol. 2009, 47, 2640–2644. [Google Scholar] [CrossRef]
- Taher, D.; Solberg, S.Ø.; Prohens, J.; Chou, Y.Y.; Rakha, M.; Wu, T.H. World vegetable center eggplant collection: Origin, composition, seed dissemination and utilization in breeding. Front. Plant Sci. 2017, 8, 1484. [Google Scholar] [CrossRef] [PubMed]
- Vorontsova, M.S.; Stern, S.; Bohs, L.; Knapp, S. African spiny Solanum (subgenus Leptostemonum, Solanaceae): A thorny phylogenetic tangle. Bot. J. Linn. Soc. 2013, 173, 176–193. [Google Scholar] [CrossRef]
- Knapp, S.; Vorontsova, M.S.; Prohens, J. Wild relatives of the eggplant (Solanum melongena L.: Solanaceae): New understanding of species names in a complex group. PLoS ONE 2013, 8, e57039. [Google Scholar] [CrossRef]
- Harlan, J.R.; de Wet, J.M.J. Toward a rational classification of cultivated plants. Taxon 1971, 20, 509–517. [Google Scholar] [CrossRef]
- Plazas, M.; Vilanova, S.; Gramazio, P.; Rodriguez-Burruezo, A.; Fita, A.; Herraiz, F.J.; Ranil, R.; Fonseka, R.; Niran, L.; Fonseka, H.; et al. Interspecific hybridization between eggplant and wild relatives from different genepools. J. Am. Soc. Hort. Sci. 2016, 141, 34–44. [Google Scholar] [CrossRef]
- Kouassi, B.; Prohens, J.; Gramazio, P.; Kouassi, A.B.; Vilanova, S.; Galan-Avila, A.; Herraiz, F.J.; Kouassi, A.; Segui-Simarro, J.M.; Plazas, M. Development of backcross generations and new interspecific hybrid combinations for introgression breeding in eggplant (Solanum melongena). Sci. Hortic. 2016, 213, 199–207. [Google Scholar] [CrossRef]
- Page, A.M.; Daunay, M.C.; Aubriot, X.; Chapman, M.A. Domestication of eggplants: A phenotypic and genomic insight. In The Eggplant Genome; Springer: Cham, Switzerland, 2019; pp. 193–212. [Google Scholar]
- Sękara, A.; Cebula, S.; Kunicki, E. Cultivated eggplants—Origin, breeding objectives and genetic resources, a review. Folia Hortic. 2007, 19, 97–114. [Google Scholar]
- GBIF (Global Biodiversity Information Facility). Free and Open Access to Biodiversity Data. 2021. Available online: http://www.gbif.org (accessed on 6 May 2021).
- GENESYS. The Global Gateway to Genetic Resources. 2021. Available online: https://www.genesys–pgr.org (accessed on 6 May 2021).
- AVGRIS. The AVRDC Vegetable Genetic Resources Information System. 2021. Available online: http://seed.worldveg.org/search/characterization/solanum_eggplant (accessed on 6 May 2021).
- Gangopadhyay, K.K.; Mahajan, R.K.; Kumar, G.; Yadav, S.K.; Meena, B.L.; Pandey, C.; Bisht, I.S.; Mishra, S.K.; Sivaraj, N.; Gambhir, R.; et al. Development of a core set in brinjal (Solanum melongena L.). Crop Sci. 2010, 50, 755–762. [Google Scholar] [CrossRef]
- Mao, W.; Jinxin, Y.; Sihachakr, D. Development of core subset for the collection of Chinese cultivated eggplants using morphological-based passport data. Plant Genet. Resour. 2008, 6, 33–40. [Google Scholar]
- Mueller, L.A.; Solow, T.H.; Taylor, N.; Skwarecki, B.; Buels, R.; Binns, J.; Lin, C.H.; Wright, M.; Ahrens, R.; Wang, Y.; et al. The SOL genomic network. A comparative resource for solanaceae biology and beyond. Plant Physiol. 2005, 138, 1310–1317. [Google Scholar] [CrossRef]
- Sadder, M.T.; Al-Shareef, R.M.; Hamdan, H. Assessment of genetic, morphological and agronomic diversity among the jordanian eggplant (S. melongena L.) landraces using random amplified polymorphic DNA (RAPD). Acta Hortic. 2007, 745, 303–311. [Google Scholar] [CrossRef]
- Sakata, Y.; Monma, S.; Narikawa, T.; Komochi, S. Evaluation of resistance to bacterial wilt and verticillium wilt in eggplants (Solanum melongena L.) collected in Malaysia. J. Jpn. Soc. Hortic. 1996, 65, 81–88. [Google Scholar] [CrossRef]
- Lester, R.N.; Jaeger, P.M.L.; Bleijendaal, S.; Bleijendaal, H.P.O.; Holloway, H.L.O. African eggplants: A review of collecting in West Africa. Plant Genet. Resour. Newsl. 1990, 81–82, 17–26. [Google Scholar]
- Gousset, C.; Collonier, C.; Mulya, K.; Mariska, I.; Rotino, L.G.; Besse, P.; Servaes, A.; Sihachakr, D. Solanum torvum, as a useful source of resistance against bacterial and fungal disease for improvement of eggplant (S. melongena L.). Plant Sci. 2004, 168, 319–327. [Google Scholar] [CrossRef]
- Castañeda-Álvarez, N.P.; Khoury, C.K.; Achicanoy, H.A.; Bernau, V.; Dempewolf, H.; Eastwood, R.J. Global conservation priorities for crop wild relatives. Nat. Plants 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Daunay, M.C.; Aubriot, X.; Hennart, J.W.; Haanstra, J.; Moquet, F.; Majde, M. Eggplant (Solanum melongena L.) and its relatives: Overview of 50 years of research and breeding. In Innovations in Genetics and Breeding of Capsicum and Eggplant, Proceedings of the 17th EUCARPIA Meeting on Genetics and Breeding of Capsicum and Eggplant, Avigno, France, 11–13 September 2019; Lefebvre, V., Daunay, M.C., Eds.; Institut National de la Recherche Agronomique (INRA) Centre de recherche Provence-Alpes-Côte d’Azur 228 route de l’aérodrome CS 40 509-Domaine Saint Paul, Site Agroparc: Avignon, France, 2019; pp. 23–25. [Google Scholar]
- Aubriot, X.; Daunay, M.C. Eggplants and relatives: From exploring their diversity and phylogenetic relationships to conservation challenges. In The Eggplant Genome; Springer: Cham, Switzerland, 2019; pp. 91–134. [Google Scholar]
- Musa, I.; Rafii, M.Y.; Ahmad, K.; Ramlee, S.I.; Md-Hatta, M.A.; Oladosu, Y.; Halidu, J. Effects of grafting on morphophysiological and yield characteristic of eggplant (Solanum melongena L.) grafted onto wild relative rootstocks. Plants 2020, 9, 1583. [Google Scholar] [CrossRef]
- IBPGR. Descriptors for Eggplant. International Board for Plant Genetic Resources (IBPGR): Rome, Italy, 1990. Available online: https://www.bioversityinternational.org/fileadmin/user_upload/online_library/publications/pdfs/401.pdf (accessed on 6 May 2021).
- Kumar, G.; Meena, B.L.; Kar, R.; Tiwari, S.K.; Gangopadhyay, K.K.; Bisht, I.S.; Mahajan, R.K. Morphological diversity in brinjal (Solanum melongena L.) germplasm accessions. Plant Genet. Res. 2008, 6, 232–236. [Google Scholar] [CrossRef]
- Polignano, G.; Uggenti, P.; Bisignano, V.; Della-Gatta, C. Genetic divergence analysis in eggplant (Solanum melongena L.) and allied species. Genet. Resour. Crop Evol. 2010, 57, 171–181. [Google Scholar] [CrossRef]
- Karihaloo, J.L.; Gottlieb, L.D. Allozyme variation in the eggplant, Solanum melongena L. (Solanaceae). Theor. Appl. Genet. 1995, 90, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Lester, R.N. Chloroplast DNA diversity in eggplant (Solanum melongena L.) and related species. Euphytica 1997, 97, 295–301. [Google Scholar] [CrossRef]
- Furini, A.; Wunder, J. Analysis of eggplant (Solanum melongena) related germplasm: Morphological and AFLP data contribute to phylogenetic interpretation and germplasm utilization. Theor. Appl. Genet. 2004, 108, 197–208. [Google Scholar] [CrossRef]
- Datta, D.R.; Rafii, M.Y.; Misran, A.; Jusoh, M.; Oladosu, Y.; Arolu, F.; Haque, A.; Sulaiman, N.M. Genetic diversity in eggplant (Solanum melongena L.) germplasm from three secondary geographical origins of diversity using SSR markers. Biocell 2021. [Google Scholar] [CrossRef]
- Tümbilen, Y.; Frary, A.; Daunay, M.C.; Doganlar, S. Application of EST SSRs to examine genetic diversity in eggplant and its close relatives. Turk. J. Biol. 2011, 35, 125–136. [Google Scholar]
- Singh, A.K.; Singh, M.; Singh, A.K.; Singh, R.; Kumar, S.; Kalloo, G. Genetic diversitywithin the genus Solanum (Solanaceae) as revealed by RAPD markers. Curr. Sci. 2006, 90, 711–716. [Google Scholar]
- Li, H.; Chen, H.; Zhuang, T.; Chen, J. Analysis of genetic variation in eggplant and related Solanum species using sequence-related amplified polymorphism markers. Sci. Hort. 2010, 125, 19–24. [Google Scholar] [CrossRef]
- Behera, T.K.; Sharma, P.; Singh, B.K.; Kumar, G.; Kumar, R.; Mohapatra, T.; Singh, N.K. Assessment of genetic diversity and species relationships in eggplant (Solanum melongena L.) using STMS markers. Sci. Hort. 2006, 107, 352–357. [Google Scholar] [CrossRef]
- Muñoz-Falcón, J.E.; Prohen, J.; Vilanova, S.; Nuez, F. Characterization, diversity, and relationships of the Spanish striped (Listada) eggplants: A model for the enhancement and protection of local heirlooms. Euphytica 2008, 164, 405–419. [Google Scholar] [CrossRef]
- Frary, A.; Doganlar, S.; Daunay, M.C. Eggplant. In Genome Mapping and Molecular Breeding in Plants; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 5, pp. 231–257. [Google Scholar]
- Daunay, M.C. Eggplant. In Handbook of Crop Breeding, Vegetables II: Fabaceae, Liliaceae, Umbelliferae, and Solanaceae; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; pp. 163–220. [Google Scholar]
- Sharma, S.; Sharma, A.; Katoch, V. Biotechnological interventions in eggplant (Solanum melongena L.). Hortic. Sci. Biotechnol. 2020, 95, 273–285. [Google Scholar] [CrossRef]
- Bletsos, F.A.; Roupakias, D.G.; Thanassoulopoulos, C.C. Gene transfer from wild Solanum species to eggplant cultivars: Prospects and limitations. Acta Hort. 2000, 522, 71–78. [Google Scholar] [CrossRef]
- Bletsos, F.A.; Stavropoulos, N.I.; Papdopoulou, P.D. Evaluation of eggplant (Solanum melongena L.) germplasmfor resistance to Verticillium wilt. Adv. Hort. Sci. 2004, 18, 33–37. [Google Scholar]
- Iwamoto, Y.; Hirai, M.; Ohmido, N.; Fukui, K.; Ezura, H. Fertile somatic hybrids between S. integrifolium and S. sanitwongsei (syn. S. kurzii) as candidates for bacterial wilt resistant rootstock of eggplant. Plant Biotechnol. 2007, 24, 179–184. [Google Scholar] [CrossRef][Green Version]
- Rizza, F.; Mennella, G.; Collonnier, C.; Sihachakr, D.; Kashyap, V.; Rajam, M.; Rotino, G. Androgenic dihaploids from somatic hybrids between Solanum melongena and S. aethiopicum group gilo as a source of resistance to Fusarium oxysporum f. sp. melongenae. Plant Cell Rep. 2002, 20, 1022–1032. [Google Scholar] [CrossRef]
- Rotino, G.L.; Sihachakr, D.; Rizza, F.; Valè, G.; Tacconi, M.G.; Alberti, P.; Mennella, G.; Sabatini, E.; Toppino, L.; D’Alessandro, A.; et al. Current status in production and utilization of dihaploids from somatic hybrids between eggplant (Solanum melongena L.) and its wild relatives. Acta Physiol. Plant 2005, 27, 723–733. [Google Scholar] [CrossRef]
- Yu, Y.; Ye, W.; He, L.; Cai, X.; Liu, T.; Liu, J. Introgression of bacterial wilt resistance from eggplant to potato via protoplast fusion and genome components of the hybrids. Plant Cell Rep. 2013, 32, 1687–1701. [Google Scholar] [CrossRef] [PubMed]
- Rotino, G.L.; Dorr, I.; Pedrazzini, E.; Perri, E. Fusione di protoplasti tra melanzana (Solanum melongena L.) e Solanum selvatici. In Proceedings of the XXXIX Convegno Annuale S.I.G.A., Vasto Marina, Italy, 23–25 October 1995; p. 157. [Google Scholar]
- Asao, H.; Arai, S.; Sato, T.; Hirai, M. Characteristics of a somatic hybrid between Solanum melongena L. and Solanum sanitwongsei Craib. Breed Sci. 1994, 44, 301–305. [Google Scholar] [CrossRef]
- Daunay, M.C.; Chaput, M.H.; Sihachakr, D.; Allot, M.; Vedel, F.; Ducreux, G. Production and characterization of fertile somatic hybrids of eggplant (Solanum melongena L.) with Solanum aethiopicum L. Theor. Appl. Genet. 1993, 85, 841–850. [Google Scholar] [CrossRef]
- Kameya, T.; Miyazawa, N.; Toki, S. Production of somatic hybrids between Solanum melongena L. and S. integrifolium Poir. Jpn. J. Breed. 1990, 40, 429–434. [Google Scholar] [CrossRef]
- Sihachakr, D.; Ducreux, G.; Vedel, F.; Allot, M.; San, L.H.; Servaes, A. Somatic hybridization of eggplant (Solanum melongena L.) with Solanum nigrum L. by protoplast electrofusion. In Proceedings of the International Conference on the Impact of Biotechnology on Agriculture, Amiens, France, 10–12 July 1989. [Google Scholar]
- Sihachakr, D.; Haicour, R.; Chaput, M.H.; Barrientos, E.; Ducreux, G.; Rossignol, L. Somatic hybrid plants produced by electrofusion between Solanum melongena L. and Solanum torvum Sw. Theor. Appl. Genet. 1989, 77, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guri, A.; Sink, K.C. Organelle composition in somatic hybrids between an atrazine resistant biotype of Solanum nigrum and Solanum melongena. Plant Sci. 1988, 58, 51–58. [Google Scholar] [CrossRef]
- Guri, A.; Sink, K.C. Interspecific somatic hybrid plants between eggplant Solanum melongena L. and Solanum torvum. Theor. Appl. Genet. 1988, 76, 490–496. [Google Scholar] [CrossRef]
- Sihachakr, D.; Haicour, R.; Serraf, I.; Barrientos, E.; Herbreteau, C.; Ducreux, G.; Souvannavong, V. Electrofusion for the production of somatic hybrid plants of Solanum melongena L. and Solanum khasianum C.B. Clark. Plant Sci. 1988, 57, 215–223. [Google Scholar] [CrossRef]
- Gleddie, S.; Keller, W.; Setterfield, G. Production and characterization of somatic hybrids between Solanum melongena L. and S. sisymbriifolium Lam. Theor. Appl. Genet. 1986, 71, 613–621. [Google Scholar] [CrossRef]
- Collonnier, C.; Fock, I.; Mariska, I.; Servaes, A.; Vedel, F.; Siljak-Yakovlev, S.; Sihachakr, D. GISH confirmation of somatic hybrids between Solanum melongena and S. torvum: Assessment of resistance to both fungal and bacterial wilts. Plant Physiol. Biochem. 2003, 41, 459–470. [Google Scholar] [CrossRef]
- Donzella, G.; Spena, A.; Rotino, G.L. Transgenic parthenocarpic eggplants: Superior germplasmfor increased winter production. Mol. Breed. 2000, 6, 79–86. [Google Scholar] [CrossRef]
- Kikuchi, K.; Honda, I.; Matsuo, S.; Fukuda, M.; Saito, T. Stability of fruit set in newly selected parthenocarpic eggplant lines. Sci. Hort. 2008, 115, 111–116. [Google Scholar] [CrossRef]
- Saito, T.; Yoshida, T.; Monma, S.; Matsunaga, H.; Sato, T.; Saito, A.; Yamada, T. Development of the parthenocarpic eggplant cultivar ‘Anominori’. Jpn. Agric. Res. Quart. 2009, 43, 123–127. [Google Scholar] [CrossRef]
- Mennella, G.; Rotino, G.L.; Fibiani, M.; D’Alessandro, A.; Francese, G.; Toppino, L.; Cavallanti, F.; Acciarri, N.; Lo-Scalzo, R. Characterization of health—Related compounds in eggplant (Solanum melongena L.) lines derived from introgression of allied species. J. Agric. Food Chem. 2010, 58, 7597–7603. [Google Scholar] [CrossRef]
- Hanson, P.M.; Yang, R.; Tsou, S.C.; Ledesma, D.; Engle, L.; Leeb, T. Diversity in eggplant (Solanum melongena) for superoxide scavenging activity, total phenolics, and ascorbic acid. J. Food Comp. Anal. 2006, 19, 594–600. [Google Scholar] [CrossRef]
- Prohens, J.; Rodríguez-Burruezo, A.; Raigón, M.D.; Nuez, F. Total phenolic concentration and browning susceptibility in a collection of different varietal types and hybrids of eggplant: Implications for breeding for higher nutritional quality and reduced browning. J. Am. Soc. Hortic. Sci. 2007, 132, 638–646. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Yokoyama, W.E.; Hong, Y.J.; Prohens, J. α-solasonine and α-solamargine contents of gboma (Solanum macrocarpon L.) and scarlet (Solanum aethiopicum L.) eggplants. J. Agric. Food Chem. 2010, 58, 5502–5508. [Google Scholar] [CrossRef] [PubMed]
- Shiu, L.Y.; Chang, L.C.; Liang, C.H.; Huang, Y.S.; Sheu, H.M.; Kuo, K.W. Solamargine induces apoptosis and sensitizes breast cancer cells to cisplatin. Food Chem. Toxicol. 2007, 45, 2155–2164. [Google Scholar] [CrossRef]
- Hall, C.A.; Hobby, T.; Cipollini, M. Efficacy and mechanisms of solasonine- and solamargineinduced cytolysis on two strains of Trypanosoma cruzii. J. Chem. Ecol. 2006, 32, 2405–2416. [Google Scholar] [CrossRef]
- Bubici, G.; Cirulli, M. Screening and selection of eggplant and wild related species for resistance to Leveillula taurica. Euphytica 2008, 164, 339–345. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pandey, P.K.; Kalloo, G.; Chaurasia, S.N.S. Phomopsis blight in brinjal and sources of resistance. Indian Phytopathol. 2002, 55, 507–509. [Google Scholar]
- Yamakawa, K. Use of rootstocks in Solanaceous fruit-vegetable production in Japan. Jpn. Agric. Res. Q. 1982, 15, 175–179. [Google Scholar]
- Schaff, D.A.; Jelenkovic, G.; Boyer, C.D.; Pollack, B.L. Hybridization and fertility of hybrid derivatives of Solatium melongena L. and Solanum macrocarpon L. Theor. Appl. Genet. 1982, 62, 149–153. [Google Scholar] [CrossRef]
- Ramasamy, S. Insect and Mite Pests on Eggplant: A Field Guide for Indentification and Management; AVRDC—The World Vegetable Center: Shanhua, Taiwan, 2009. [Google Scholar]
- Lal, O.P.; Sharma, R.K.; Verma, T.S.; Bhagchandani, P.M. Resistance in brinjal to shoot and fruit borer. Veg. Sci. 1976, 3, 111–116. [Google Scholar]
- Rao, G.R. Cytogenic relationship and barrier to gene exchange between Solanum melongena L. and Solanum hispidum Pers. Caryologia 1980, 33, 429–433. [Google Scholar] [CrossRef]
- Sharma, D.R.; Chawdhury, J.B.; Ahuja, U.; Dhankhar, B.S. Interspecific hybridization in the genus Solanum. A cross between S. melongena and S. khasianum throughout embryo culture. Z. Pflanzenzücht. 1980, 248–253. [Google Scholar]
- Boiteux, L.S.; Charchar, J.M. Genetic resistance to root-knot nematode (Meloidogyne javanica) in eggplant (Solanum melongena). Plant Breed. 1996, 115, 198–200. [Google Scholar] [CrossRef]
- AVRDC. AVRDC 1998 Report; Asian Vegetable Research and Development Center: Tainan, Taiwan, 1999; pp. 32–36. [Google Scholar]
- Parker, B.L.; Talekar, N.S.; Skinner, M. Field Guide: Insect Pests of Selected Vegetables in Tropical and Subtropical Asia; Asian Vegetable Research and Development Center: Tainan, Taiwan, 1995. [Google Scholar]
- Sidhu, A.S.; Dhatt, A.S. Current status of brinjal research in India. Acta Hortic. 2007, 752, 243–248. [Google Scholar] [CrossRef]
- Wang, J.F.; Chen, N.C.; Li, H.M. Resistance sources to bacterial wilt in eggplant (Solanum melongena). In Bacterial Wilt Disease; Springer: Berlin/Heidelberg, Germany, 1998; pp. 284–289. [Google Scholar]
- Tani, E.; Kizis, D.; Markellou, E.; Papadakis, I.; Tsamadia, D.; Leventis, G.; Karapanos, I. Cultivar-dependent responses of eggplant (Solanum melongena L.) to simultaneous Verticillium dahliae infection and drought. Front. Plant Sci. 2018, 9, 1181. [Google Scholar] [CrossRef]
- Naegele, R.P.; Boyle, S.; Quesada-Ocampo, L.M.; Hausbeck, M.K. Genetic diversity, population structure, and resistance to Phytophthora capsici of a worldwide collection of eggplant germplasm. PLoS ONE 2014, 9, e95930. [Google Scholar]
- Chen, N.C. Evaluation of Elite Eggplant Cultivars. AVRDC 1998 Report; World Vegetable Center: Tainan, Taiwan, 1998; pp. 30–32. [Google Scholar]
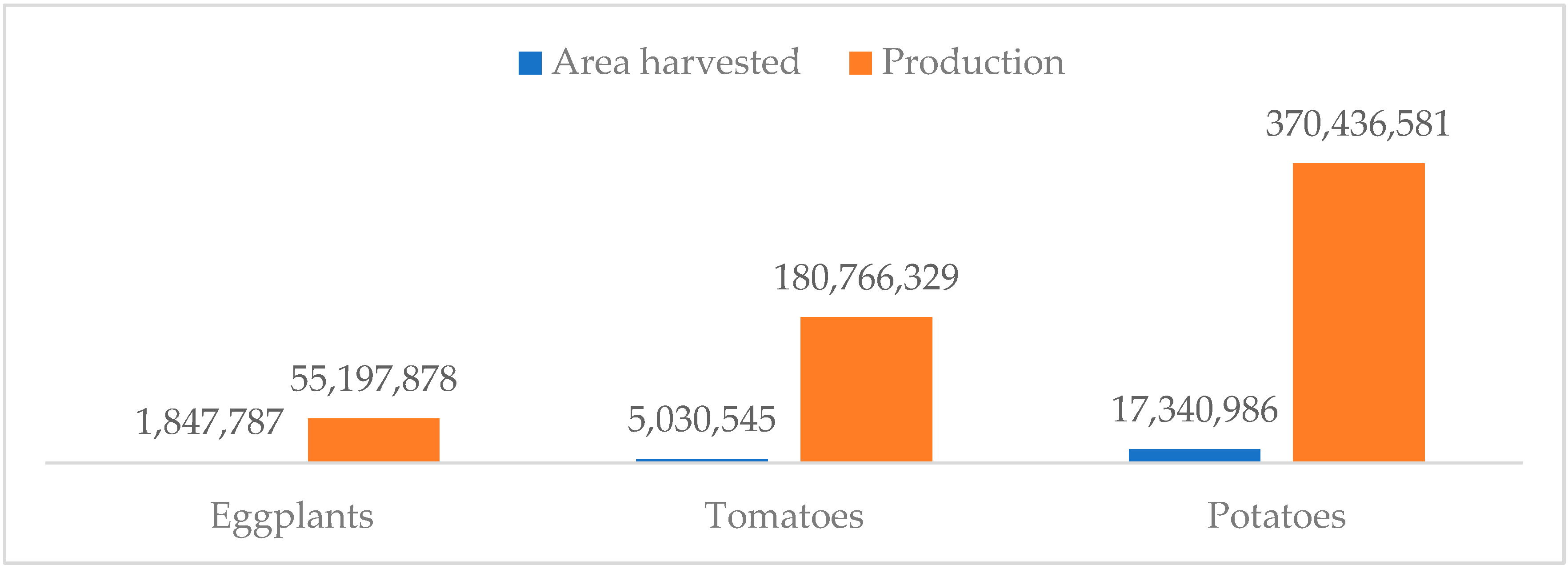
| Area | Area Harvested | Production | Area | Area Harvested | Production |
|---|---|---|---|---|---|
| China | 782,998 | 35,590,700 | Spain | 3470 | 245,150 |
| India | 727,000 | 12,680,000 | Mexico | 2333 | 185,234 |
| Egypt | 43,818 | 1,180,240 | Algeria | 6047 | 184,145 |
| Turkey | 23,337 | 822,659 | Syrian Arab Republic | 8342 | 154,807 |
| Iran | 21,350 | 670,158 | Iraq | 8660 | 136,749 |
| Indonesia | 43,954 | 575,392 | Sri Lanka | 9877 | 134,863 |
| Japan | 8650 | 301,700 | Kazakhstan | 4812 | 108,065 |
| Italy | 9550 | 300,620 | United States of America | 2614 | 105,302 |
| Philippines | 21,819 | 249,890 | Rest of the world | 119,173 | 1,572,468 |
| Total | 1,847,804 | 55,198,142 |
| Nutrient (Unit) | Amount | Nutrient (Unit) | Amount |
|---|---|---|---|
| Proximates | Vitamins | ||
| Sugars, total (g) | 3.53 | Vitamin K (Phylloquinone) (μg) | 3.5 |
| Fibre, total dietary (g) | 3 | Vitamin E (α-tocopherol) (mg) | 0.3 |
| Carbohydrate, (g) | 5.88 | Vitamin A, IU (IU) | 23 |
| Total lipid (fat) (g) | 0.18 | Vitamin A, RAE (μg) | 1 |
| Protein (g) | 0.98 | Folate, DFE (μg) | 22 |
| Energy (kcal) | 25 | Vitamin B6 (mg) | 0.084 |
| Water (g) | 92.3 | Niacin (mg) | 0.649 |
| Minerals | Riboflavin (mg) | 0.037 | |
| Zinc, Zn (mg) | 0.16 | Thiamin (mg) | 0.039 |
| Sodium, Na (mg) | 2 | Vitamin C (mg) | 2.2 |
| Potassium, K (mg) | 229 | Lipids | |
| Phosphorus, P (mg) | 24 | Cholesterol (mg) | 0 |
| Magnesium, Mg (mg) | 14 | Fatty acids, total polysaturated (g) | 0.076 |
| Iron, Fe (mg) | 0.23 | Fatty acids, total monosaturated (g) | 0.016 |
| Calcium, Ca (mg) | 9 | Fatty acids, total saturated (g) | 0.034 |
| Scientific Name | GBIF [34] | GENESYS [35] | AVGRIS [36] |
|---|---|---|---|
| Solanum nigrum | 211,385 | 44 | 20 |
| Solanum americanum | 27,624 | 43 | 189 |
| Solanum melongena | 21,852 | 4056 | 2256 |
| Solanum torvum | 12,775 | 115 | 39 |
| Solanum villosum | 11,590 | 48 | 17 |
| Solanum sisymbriifolium | 7054 | 4 | 10 |
| Solanum nigrescens | 4794 | 1 | 2 |
| Solanum aethiopicum | 4230 | 590 | 60 |
| Solanum anguivi | 4098 | 6 | 39 |
| Solanum anguivi | 4098 | 23 | 4 |
| Solanum seaforthianum | 3713 | 3 | 5 |
| Solanum linnaeanum | 3327 | 4 | 3 |
| Solanum linnaeanum | 3327 | 3 | 3 |
| Solanum capsicoides | 2638 | 1 | 1 |
| Solanum viarum | 2237 | 3 | 17 |
| Solanum incanum | 2008 | 28 | 3 |
| Solanum aculeatissimum | 1873 | 46 | 19 |
| Solanum violaceum | 1606 | 1 | 59 |
| Solanum scabrum | 1400 | 148 | 55 |
| Solanum macrocarpon | 1365 | 95 | 42 |
| Solanum lasiocarpum | 1076 | 31 | 34 |
| Solanum virginianum | 1032 | 1 | 3 |
| Solanum virginianum | 1032 | 3 | 3 |
| Solanum trilobatum | 207 | 10 | 7 |
| Solanum ferox | 150 | 11 | 8 |
| Solanum insanum | 110 | 11 | 16 |
| Grand total | 1,518,222 | 5204 | 2907 |
| Seedling Traits | Unit | Scale |
|---|---|---|
| Germination period | no. | Number of days from sowing to first seed germination |
| Cotyledonous leaf color | - | 7 = Violet; 5 = Light violet; 3 = Green |
| Cotyledonous leaf length | mm | N = 10 |
| Cotyledonous leaf width | mm | N = 10 |
| Cotyledon length to width ratio | - | 9 = Very high (>5.0); 7 = High (~3.5); 5 = Intermediate (~2.5); 3 = Low (~2.2); 1 = Very low (<2.0) |
| Vegetative Traits | ||
| Plant breadth at flowering stage | cm | 9 = Very strong (>130); 7 = Broad (~90); 5 = Intermediate; 3 = Narrow (~40); 1 = Very narrow (<30) |
| Plant height at flowering stage | cm | 9 = Very tall (>150); 7 = Tall (~100); 5 = Intermediate (~60); 3 = Short (~30); 1 = Very short (<20) |
| Plant growth habit | - | 7 = Prostrate; 5 = Intermediate; 3 = Upright; 1 = Very upright |
| Stem ridging | - | 7 = Prominent; 5 = Intermediate; 3 = Shallow; 0 = Absent |
| Degree of stem pubescence | - | 4 = Very many; 3 = Many; 2 = Intermediate; 1 = Few; 0 = Absent |
| Spines on stem | - | 7 = Long; 5 = Intermediate; 3 = Short; 0 = Absent; |
| Number of primary branches per plant | no. | 9 = Very strong (>30); 7 = Strong (~20); 5 = Intermediate (~10); 3 = Weak (~5); 1 = Very weak (~2) |
| Petiole length | mm | 9 = Very long (>100); 7 = Long (~50); 5 = Intermediate (~30); 3 = Short (~10); 1 = Very short (<5); 0 = None |
| Petiole color | - | 9 = Dark brown; 7 = Dark violet; 3 = Violet; 2 = Greenish violet; 1 = Green |
| Leaf blade length | cm | 7 = Long (~30); 5 = Intermediate (~20); 3 = Short (~10) |
| Leaf blade width (maximum width) | cm | 7 = Wide (~15); 5 = Intermediate (~10); 3 = Narrow (~5) |
| Leaf blade tip angle | - | 9 = Very obtuse (>160°); 7 = Obtuse (~110°); 5 = Intermediate (~75°); 3 = Acute (~45°); 1 = Very acute (<15°) |
| Leaf blade lobes | - | 9 = Very strong; 7 = Strong; 5 = Intermediate; 3 = Weak; 1 = Very weak |
| Leaf blade color (upper surface) | - | 9 = Violet; 7 = Greenish violet; 5 = Dark green; 3 = Green; 1 = Light green |
| Leaf hairs (no. of hair per mm2 on lower surface of the leaf) | no. | 9 = Very many (>200); 7 = Many (100–200); 5 = Intermediate (50–100); 3 = Few (20–50); 1 = Very few (<20) |
| Leaf prickles (no. of leaf prickles on upper surface of the leaf) | no. | 9 = Very many (>20); 7 = Many (11–20); 5 = Intermediate (6–10); 3 = Few (3–5); 1 = Very few (1–2); 0 = None |
| Inflorescence Traits | ||
| Flowering time | no. | Number of days from sowing until first flower opening |
| Sepal length | cm | N = 5 |
| Petal length | cm | N = 5 |
| Stamen length | cm | N = 5 |
| Style Exsertion | - | 7 = Exerted; 5 = Intermediate; 3 = Inserted |
| Pollen production | - | 7 = High; 5 = Medium; 3 = Low; 0 = None |
| Relative style length | mm | 7 = Long (~5); 5 = Intermediate (~3); 3 = Short (~1); |
| Corolla color | - | 9 = Bluish violet; 7 = Light violet; 5 = Pale violet; 3 = White; 1 = Greenish white; 0 = Yellow |
| Seed Traits | ||
| 100 seeds weight | gm | - |
| Seed size (diameter) | mm | 7 = Large (~4); 5 = Intermediate (~3); 3 = Small (~2) |
| Seed density | - | 7 = Dense; 5 = Intermediate; 3 = Scarce |
| Number of seeds per fruit | - | 9 = Very many (>500); 7 = Many (~300); 5 = Intermediate (~100); 3 = Few (~50); 1 = Very few (<10); 0 = None |
| Seed color | - | 9 = Black; 6 = Brown black; 5 = Brown; 4 = Brownish yellow; 3 = Grey yellow; 2 = Light yellow; 1 = White |
| Fruit Traits | ||
| Fruiting date | no. | Days to 50% mature fruits per plant |
| Fruit breadth (diameter at broadest part) | cm | 9 = Very large (>10); 7 = Large (~5); 5 = Intermediate (~3); 3 = Small (~2); 1 = Very small (<1) |
| Fruit length (from base of calyx to tip of fruit) | cm | 9 = Very long (>20); 7 = Long (~10); 5 = Intermediate (~5); 3 = Short (~2); 1 = Very short (<1) |
| Fruit length/breadth ratio | - | 9 = Several times as long as broad; 8 = Three times as long as broad; 7 = Twice as long as broad; 5 = Slightly longer than broad; 3 = As long as broad; 1 = Broader than long |
| Fruit calyx prickles (N = 10) | no. | 9 = Very many (>30); 7 = Many (~20); 5 = Intermediate (~10); 3 = Few (~5); 1 = Very few (<3); 0 = None |
| Fruit cross-section | - | 9 = Very irregular; 7 = Many grooves (~8); 5 = Few grooves (~4); 3 = Elliptic, no grooves; 1 = Circular, no grooves |
| Fruit pedicel prickles | no. | 9 = Very many (>30); 7 = Many (~20); 5 = Intermediate (~10); 3 = Few (~5); 1 = Very few (<3); 0 = None |
| Fruit pedicel thickness | mm | 9 = Very thick (>10); 7 = Thick (~5); 5 = Intermediate (~3); 3 = Thin (~2); 1 = Very thin (<1) |
| Fruit pedicel length | mm | 9 = Very long (~75); 7 = Long (~50); 5 = Intermediate (~25); 3 = Short (~10); 1 = Very short (<5) |
| Fruit color at commercial ripeness | - | 9 = Black; 8 = Purple black; 7 = Purple; 6 = Lilac gray; 5 = Scarlet red; 4 = Fire red; 3 = Deep yellow; 2 = Milk white; 1 = Green |
| Fruit curvature | - | 9 = U shaped; 8 = Sickle shaped; 7 = Snake shaped; 5 = Curved; 3 = Slightly curved; 1 = None |
| Fruit yield per plant | gm | 9 = Very high (>5000); 7 = High (~2500); 5 = Intermediate (~1000); 3 = Low (~500); 1 = Very low (<250) |
| Fruit flesh density | - | 9 = Very dense; 7 = Dense; 5 = Average density; 3 = Loose (crumbly); 1 = Very loose (spongy) |
| Fruit color at physiological ripeness | - | 9 = Black; 8 = Light brown; 7 = Scarlet red; 6 = Poppy red; 5 = Fired red; 4 = Deep orange; 3 = Yellow orange; 2 = Deep yellow; 1 = Green |
| Fruit position | - | 9 = Pendant; 7 = Semi-pendant; 5 = Horizontal; 3 = Semi-erect; 1 = Erect |
| Fruit apex shape | - | 7 = Depressed; 5 = Rounded; 3 = Protruded |
| Varietal mixture condition | - | 7 = Serious mixture; 5 = Medium mixture; 3 = Slight mixture; 0 = Pure |
| Fruit color distribution at commercial ripeness | - | 7 = Striped; 5 = Netted; 3 = Mottled; 1 = Uniform |
| Fruit shape | - | 7 = About 3/4 way from base to tip; 5 = About 1/2 way from base to tip; 3 = About 1/4 way from base to tip |
| Fruit flavor | - | 7 = Sweet; 5 = Intermediate; 3 = Bitter |
| Relative fruit calyx length | mm | N = 10 |
| Number of locules per fruit | no. | N = 10 |
| Number of fruit per plant | no. | Total number of fruit per plant |
| Parents | Fusion | Hybrid Characteristics | Source |
|---|---|---|---|
| S. melongena ×S. tuberosum | Electrical | Introgression of bacterial wilt resistance to Solanum tuberosum from Solanum melongena | [70] |
| S. integrifolium ×S. sanitwongsei | UV | Ralstonia solanacearum Resistance | [67] |
| S. melongena ×S. aethiopicum gr. Aculeatum | Electrical | Fertile and fusarium wilt resistant | [71] |
| S. melongena ×S. sanitwongsei | polyethylene glycol | Fertile and bacterial wilt resistant | [72] |
| S. melongena ×S. aethiopicum gr. Aculeatum | Electrical | Ralstonia solanacearum Resistant and high-yielding | [73] |
| S. melongena ×S. integrifolium | - | Fertile and bacterial wilt resistant | [74] |
| S. melongena ×S. nigrum | Electrical | Sterile and atrazine resistant | [75] |
| S. melongena ×S. torvum | Electrical | Sterile hybrid resistance to nematodes and Verticillium dahlia | [76] |
| S. melongena ×S. nigrum | polyethylene glycol | Sterile and atrazine resistant | [77] |
| S. melongena ×S. torvum | polyethylene glycol | Sterile hybrid partial resistance to mites and resistance to Verticillium wilt | [78] |
| S. melongena × S.khasianum | Electrical | Sterile and Leucinodes orbonalis resistant | [79] |
| Solanum melongena ×S. sisymbrifolium | polyethylene glycol | Sterile hybrid resistant to mites and nematodes | [80] |
| S. melongena ×S. torvum | Electrical | Ralstonia solanacearum and Verticillium dahlia resistant | [81] |
| Trait | Source | Reference |
|---|---|---|
| Powdery mildew (Leveillula taurica) | S. pseudocapsicum, S. aviculare, S. aculeatissimum, S. linnaeanum, | [91] |
| Phomopsis fruit rot (Phomopsis vexans) | Solanum xanthocarpum, S nigrum, S gila, S indicum, S. khasianum, S sisymbrifolium | [92] |
| Fusarium wilt (Fusarium oxysporum f. sp. melongenae) | S integrifolium | [93] |
| Spider mite (Tetranychus urticae) | S. sisymbrifolium,S. pseudocapsium, S. mamosum, S. integrifolium, S. macrocarpon, | [94] |
| Eggplant fruit and shoot borer (Leucinodes orbonalis Guenee) | S. melongena: VI047451; S incanum; S integrifolium; S hispidum; S. khasianum, | [95,96,97,98] |
| Root Knot Nematode (Meloidogyne javanica) | S. melongena: A-264-A; S. torvum ‘CNPH 610′ | [99] |
| Leafhopper (Amrasca devastans Distant) | S. melongena: VI035835, VI035822, VI034971 | [100] |
| Spotted beetles (Epilachna vigintioctopunctata Fabricius) | Shankar Vijay, Hissar Selection 14, Arka Shirish | [101] |
| Little leaf disease | Solanum hispidum; S. melongena: Nurki, Hisar Shyamal, H-10 | [97,102] |
| Bacterial wilt (BW), (Ralstonia solanacearum) | BG 219; EG 203; BG 192; TS 3; S. melongena: TS90, TS87, TS69, TS47, VI034885 and TS3 | [100,103] |
| Verticillium wilt (Verticillium spp.) | Skoutari, EMI, S. linnaeanum | [11,104] |
| Phytophthora blight (Phytophthora capsici) | PI413784 | [105] |
| leaf mosaic virus | S. hispidum | [97] |
| aphids | S. hispidum | [97] |
| High antioxidant activity | S. aethiopicum: S00197, S. melongena: S00022, S00062 | [86] |
| Early maturity | S. melongena: VI046110 | [100] |
| High yielding | S. melongena: EG235, EG233, VI44067, VI047332, VI046097, VI037736, VI046110, VI047333, VI045551 | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Salisu, M.A.; Olaniyan, B.A.; Fagbohun, I.K.; Muftaudeen, T.K. Genetic Diversity and Utilization of Cultivated Eggplant Germplasm in Varietal Improvement. Plants 2021, 10, 1714. https://doi.org/10.3390/plants10081714
Oladosu Y, Rafii MY, Arolu F, Chukwu SC, Salisu MA, Olaniyan BA, Fagbohun IK, Muftaudeen TK. Genetic Diversity and Utilization of Cultivated Eggplant Germplasm in Varietal Improvement. Plants. 2021; 10(8):1714. https://doi.org/10.3390/plants10081714
Chicago/Turabian StyleOladosu, Yusuff, Mohd Y. Rafii, Fatai Arolu, Samuel Chibuike Chukwu, Monsuru Adekunle Salisu, Bolanle Amudalat Olaniyan, Ifeoluwa Kayode Fagbohun, and Taoheed Kolawole Muftaudeen. 2021. "Genetic Diversity and Utilization of Cultivated Eggplant Germplasm in Varietal Improvement" Plants 10, no. 8: 1714. https://doi.org/10.3390/plants10081714
APA StyleOladosu, Y., Rafii, M. Y., Arolu, F., Chukwu, S. C., Salisu, M. A., Olaniyan, B. A., Fagbohun, I. K., & Muftaudeen, T. K. (2021). Genetic Diversity and Utilization of Cultivated Eggplant Germplasm in Varietal Improvement. Plants, 10(8), 1714. https://doi.org/10.3390/plants10081714






