Metabolomics and Physiological Approach to Understand Allelopathic Effect of Horseradish Extract on Onion Root and Lettuce Seed as Model Organism
Abstract
:1. Introduction
2. Results and Discussion
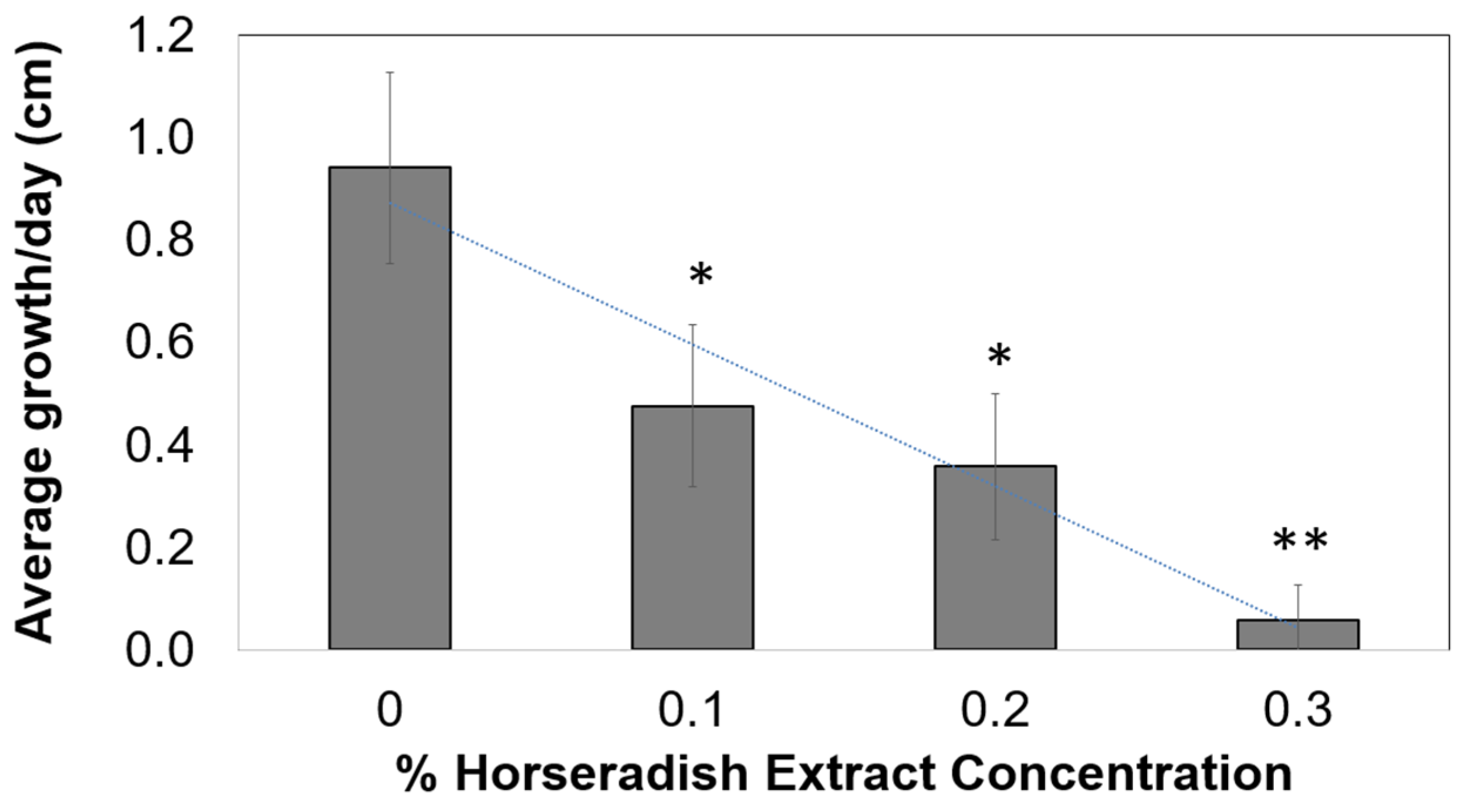
2.1. Onion Root Growth after Treatment with Various Concentrations of HRE
2.2. Onion Root Cell Mitotic Stage following Treatment with Various Concentrations of HRE
2.3. Onion Microscopic Characteristics during Cell Division following Treatment with Various Concentrations of HRE
2.4. Visualization of the Onion Root Tip following Treatment with Various Concentrations of HRE
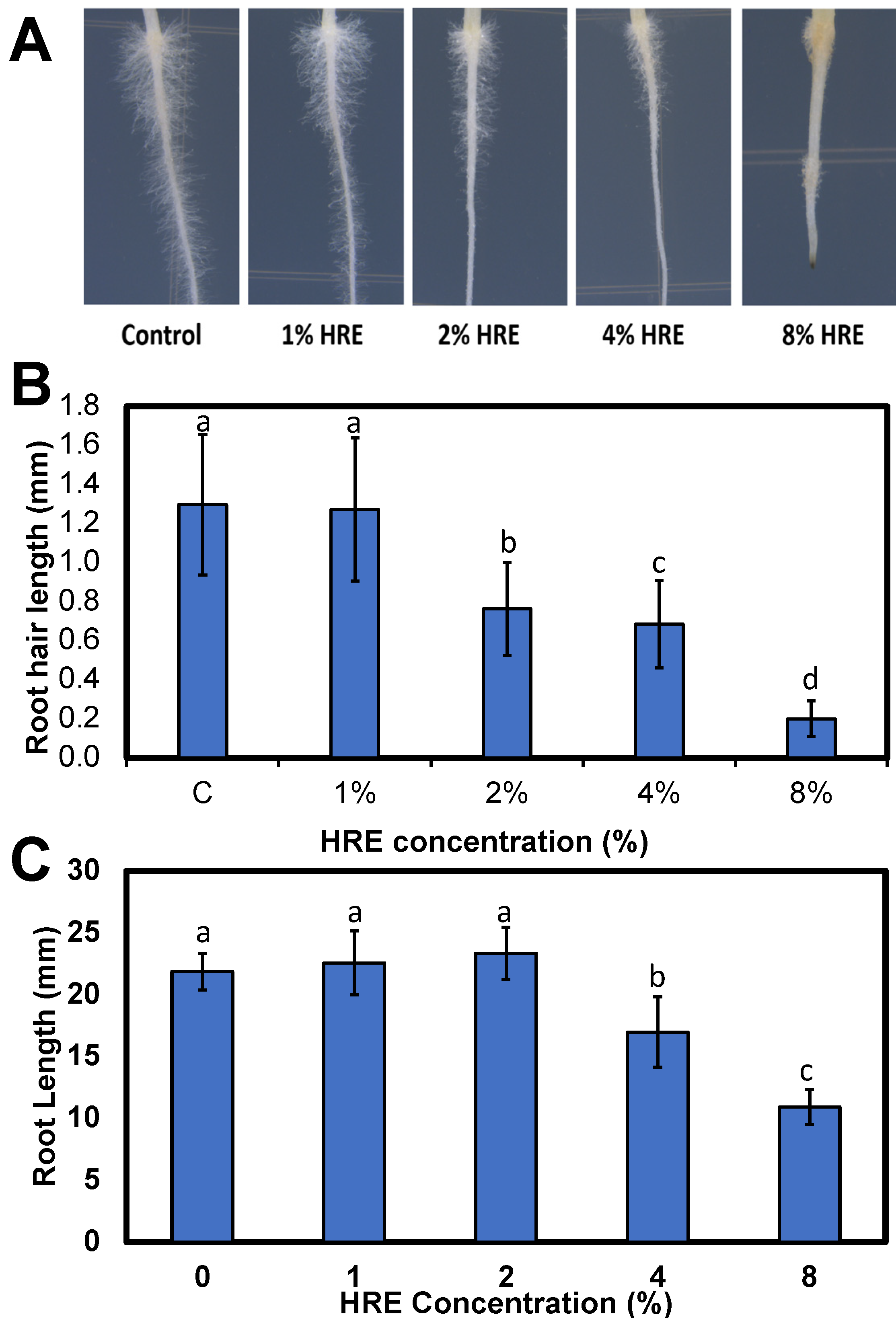
2.5. Lettuce Root and Root Hair Measurement
2.6. Metabolites Changes by HRE Treatment in Lettuce Seedlings
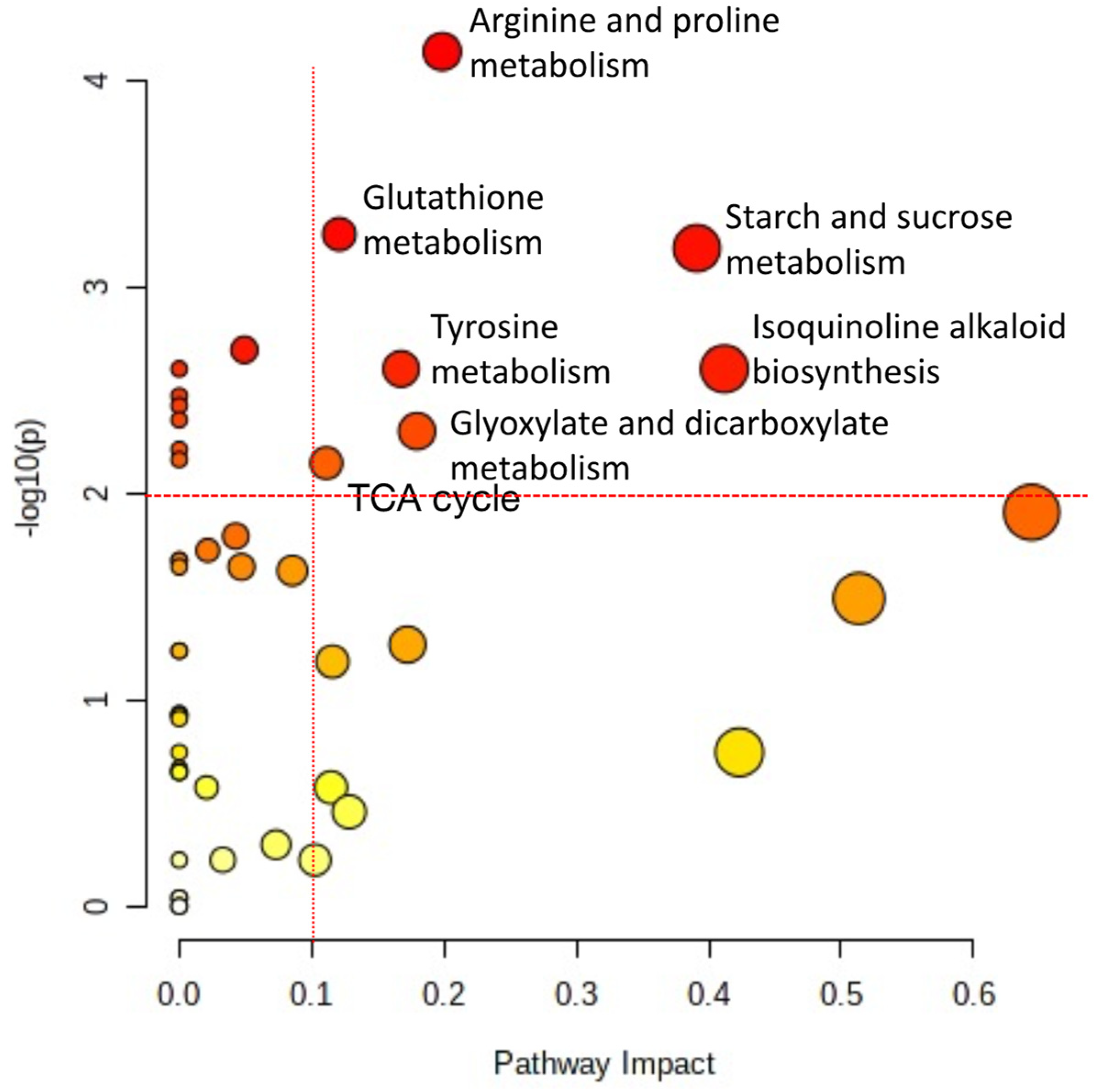
2.7. Metabolism Pathway Changes by HRE Treatment in Lettuce Seedlings
3. Materials and Methods
3.1. Horseradish Root Extract (HRE) Treatment and Onion Root Growth Rate Measurement
3.2. Cell Cycle Analysis
3.3. Accumulation of Hydrogen Peroxide and Dead Area Measurement
3.4. Horseradish Allelopatic Effect on Lettuce Growth
3.5. Primary Metabolite Extraction and Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and Stability of Glucosinolates and Their Breakdown Products in Foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.-M.; Jeffery, E.H.; Juvik, J.A.; Kushad, M.M. Correlation of Quinone Reductase Activity and Allyl Isothiocyanate Formation Among Different Genotypes and Grades of Horseradish Roots. J. Agric. Food Chem. 2015, 63, 2947–2955. [Google Scholar] [CrossRef]
- Shofran, B.G.; Purrington, S.T.; Breidt, F.; Fleming, H.P. Antimicrobial Properties of Sinigrin and Its Hydrolysis Products. J. Food Sci. 1998, 63, 621–624. [Google Scholar] [CrossRef]
- Hecht, S.S. Chemoprevention by Isothiocyanates. In Cancer Chemoprevention; Humana Press: Totowa, NJ, USA, 2004; pp. 21–35. [Google Scholar]
- Petersen, J.; Belz, R.; Walker, F.; Hurle, K. Weed Suppression by Release of Isothiocyanates from Turnip-Rape Mulch. Agron. J. 2001, 93, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Elisabeth, G. Allelopathy of Aquatic Autotrophs. Crit. Rev. Plant Sci. 2003, 22, 313–339. [Google Scholar]
- Mallik, M.A.B.; Williams, R.D. Allelopathic Growth Stimulation of Plants and Microorganisms. Allelopath. J. 2005, 16, 175–198. [Google Scholar]
- Alves, M.; Filho, S.; Innecco, R.; Torres, S.B. Allelopathy of Plant Volatile Extracts on Seed Germination and Radicle Length of Lettuce. Pesqui. Agropecuária Bras. 2004, 39, 1083–1086. [Google Scholar] [CrossRef]
- Mohamed, F.I.; El-Ashry, Z.M. Cytogenetic Effect of Allelochemicals Brassica nigra L. Extracts on Pisum sativum L. World Appl. Sci. J. 2012, 20, 344–353. [Google Scholar]
- Turk, K.D.; al Tawaha, A.R. Inhibitory Effects of Aqueous Extracts of Black Mustard on Germination and Growth of Radish. Res. J. Agric. Biol. Sci. 2005, 1, 227–231. [Google Scholar]
- Åsberg, S.E.; Atle, M.B.; Anders, Ø. Allyl Isothiocyanate Affects the Cell Cycle of Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 364. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Wei, Z.; Bruce, A.S.; Sarah, M.A. Functional Proteomics of Arabidopsis Thaliana Guard Cells Uncovers New Stomatal Signaling Pathways. Plant Cell 2008, 20, 3210–3226. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.; Yukie, Y.; Kyoko, T.; Toru, K. Exogenously Applied Isothiocyanates Enhance Glutathione S-Transferase Expression in Arabidopsis but Act as Herbicides at Higher Concentrations. J. Plant Physiol. 2010, 167, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Nishino, H.; Iwashima, A. Isothiocyanates Inhibit Cell Cycle Progression of Hela Cells at G2/M Phase. Anticancer Drugs 1993, 4, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Cancer-Preventive Isothiocyanates: Measurement of Human Exposure and Mechanism of Action. Mutat Res. 2004, 555, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kawakami, M.; Yoshihiro, A.; Miyoshi, N.; Ohigashi, H.; Kawai, K.; Osawa, T.; Uchida, K. Involvement of the Mitochondrial Death Pathway in Chemopreventive Benzyl Isothiocyanate-Induced Apoptosis. J. Biol. Chem. 2002, 277, 8492–8499. [Google Scholar] [CrossRef] [Green Version]
- Firbas, P.; Tomaž, A. Chromosome Damage Studies in the Onion Plant Allium cepa L. Caryologia 2014, 67, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Dane, F.; Dalgiç, Ö. The Effects of Fungicide Benomyl (Benlate) on Growth and Mitosis in Onion (Allium Cepa L.) Root Apical Meristem. Acta Biol. Hung. 2005, 56, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.; Chiu, Y.-C.; Richards-Babb, M.; Jessica, M.B.; Ku, K.-M. Demonstration of Allelopathy of Horseradish Root Extract on Lettuce Seed. Biochem. Mol. Biol. Educ. 2019, 47, 333–340. [Google Scholar] [CrossRef]
- Sodaiezadeh, H.; Rafieiolhossaini, M.; Havlik, J.; van Damme, P. Allelopathic Activity of Different Plant Parts of Peganum harmala L. and Identification of Their Growth Inhibitors Substances. Plant Growth Regul. 2009, 59, 227–236. [Google Scholar] [CrossRef]
- Jafariehyazdi, E.; Javidfar, F. Comparison of Allelopathic Effects of Some Brassica Species in Two Growth Stages on Germination and Growth of Sunflower. PlantSoil Environ. 2011, 57, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Øverby, A.; Mette, S.; Bævre, O.; Thangstad, P.; Atle, M.B. Disintegration of Microtubules in Arabidopsis Thaliana and Bladder Cancer Cells by Isothiocyanates. Front. Plant Sci. 2015, 6, 6. [Google Scholar]
- Yin, P.; Tomoya, K.; Meilan, H.; Donkena, K.V.; Charles, Y.F.Y. Phenethyl Isothiocyanate Induces Cell Cycle Arrest and Reduction of Alpha- and Beta-Tubulin Isotypes in Human Prostate Cancer Cells. Cell Biol. Int. 2009, 33, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Mi, L.; Xiao, Z.; Hood, B.L.; Dakshanamurthy, S.; Wang, X.; Govind, S.; Conrads, T.P.; Veenstra, T.D.; Chung, F.L. Covalent Binding to Tubulin by Isothiocyanates. A Mechanism of Cell Growth Arrest and Apoptosis. J. Biol. Chem. 2008, 283, 22136–22146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musk, S.R.R.; Smith, T.K.; Johnson, I.T. On the Cytotoxicity and Genotoxicity of Allyl and Phenethyl Isothiocyanates and Their Parent Glucosinolates Sinigrin and Gluconasturtiin. Mutat. Res. Lett. 1995, 348, 19–23. [Google Scholar] [CrossRef]
- Liszkay, A.; van der Zalm, E.; Schopfer, P. Production of Reactive Oxygen Intermediates (O2 ˙−, H2O2, and ˙OH) by Maize Roots and Their Role in Wall Loosening and Elongation Growth. Plant Physiol. 2004, 136, 3114–3123. [Google Scholar] [CrossRef] [Green Version]
- Gutteridge, B.; Halliwell, J.M.C. Free Radicals in Biology and Medicine; Clarendon Press: Oxford, UK, 1989. [Google Scholar]
- Leymarie, J.; Giedré, V.; Hai, H.H.; Emmanuel, G.; Virginie, C.; Patrice, M.; Françoise, C.; Hayat, E.-M.B.; Christophe, B. Role of Reactive Oxygen Species in the Regulation of Arabidopsis Seed Dormancy. Plant Cell Physiol. 2011, 53, 96–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, K.L.; Chaudhuri, A.; Kar, R.K. Superoxide and Its Metabolism During Germination and Axis Growth of Vigna radiata (L.) Wilczek Seeds. Plant Signal. Behav. 2014, 9, e29278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, K.L.; Mukherjee, A.; Kar, R.K. Early axis growth during seed germination is gravitropic and mediated by ROS and calcium. J. Plant Physiol. 2017, 216, 181–187. [Google Scholar] [CrossRef]
- Bournonville, C.F.; Díaz-Ricci, J.C. Quantitative Determination of Superoxide in Plant Leaves Using a Modified Nbt Staining Method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef]
- Bartosz, G. Oxidative Stress in Plants. Acta Physiol. Plant. 1997, 19, 47–64. [Google Scholar] [CrossRef]
- Gapper, C.; Dolan, L. Control of Plant Development by Reactive Oxygen Species. Plant Physiol. 2006, 141, 341–345. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.; et al. Reactive Oxygen Species Produced by Nadph Oxidase Regulate Plant Cell Growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Ciniglia, C.; Mastrobuoni, F.; Scortichini, M.; Petriccione, M. Oxidative Damage and Cell-Programmed Death Induced in Zea mays L. By Allelochemical Stress. Ecotoxicology 2015, 24, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-J.; Ku, K.-M. Optimization of Allyl Isothiocyanate Sanitizing Concentration for Inactivation of Salmonella Typhimurium on Lettuce Based on Its Phenotypic and Metabolome Changes. Food Chem. 2021, 364, 130438. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Chi, W.-C.; Trinh, N.N.; Cheng, K.-T.; Chen, Y.-A.; Lin, T.-C.; Lin, Y.-C.; Huang, L.-Y.; Huang, H.-J.; Chiang, T.-Y. Alleviation of Allelochemical Juglone-Induced Phytotoxicity in Tobacco Plants by Proline. J. Plant Interact. 2015, 10, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Verslues, P.E.; Sharma, S. Proline Metabolism and Its Implications for Plant-Environment Interaction. In The Arabidopsis Book; The American Society of Plant Biologists: Rockville, MD, USA, 2010; Volume 8. [Google Scholar]
- Barickman, T.C.; Ku, K.-M.; Sams, C.E. Differing Precision Irrigation Thresholds for Kale (Brassica oleracea L. var. acephala) Induces Changes in Physiological Performance, Metabolites, and Yield. Environ. Exp. Bot. 2020, 180, 104253. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, S.J.; Lee, J.S.; Hong, Y.P.; Chae, S.H.; Ku, K.M. Carbon Dioxide Pretreatment and Cold Storage Synergistically Delay Tomato Ripening through Transcriptional Change in Ethylene-Related Genes and Respiration-Related Metabolism. Foods 2021, 10, 744. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-C. Chemical Weapons for Competition between Plants, Allelopathy. Mag. Korea Concr. Inst. 2016, 28, 64–66. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simpson, T.; Ku, K.-M. Metabolomics and Physiological Approach to Understand Allelopathic Effect of Horseradish Extract on Onion Root and Lettuce Seed as Model Organism. Plants 2021, 10, 1992. https://doi.org/10.3390/plants10101992
Simpson T, Ku K-M. Metabolomics and Physiological Approach to Understand Allelopathic Effect of Horseradish Extract on Onion Root and Lettuce Seed as Model Organism. Plants. 2021; 10(10):1992. https://doi.org/10.3390/plants10101992
Chicago/Turabian StyleSimpson, Tyler, and Kang-Mo Ku. 2021. "Metabolomics and Physiological Approach to Understand Allelopathic Effect of Horseradish Extract on Onion Root and Lettuce Seed as Model Organism" Plants 10, no. 10: 1992. https://doi.org/10.3390/plants10101992
APA StyleSimpson, T., & Ku, K.-M. (2021). Metabolomics and Physiological Approach to Understand Allelopathic Effect of Horseradish Extract on Onion Root and Lettuce Seed as Model Organism. Plants, 10(10), 1992. https://doi.org/10.3390/plants10101992






