Electrical Capacitance versus Minirhizotron Technique: A Study of Root Dynamics in Wheat–Pea Intercrops
Abstract
1. Introduction
2. Results
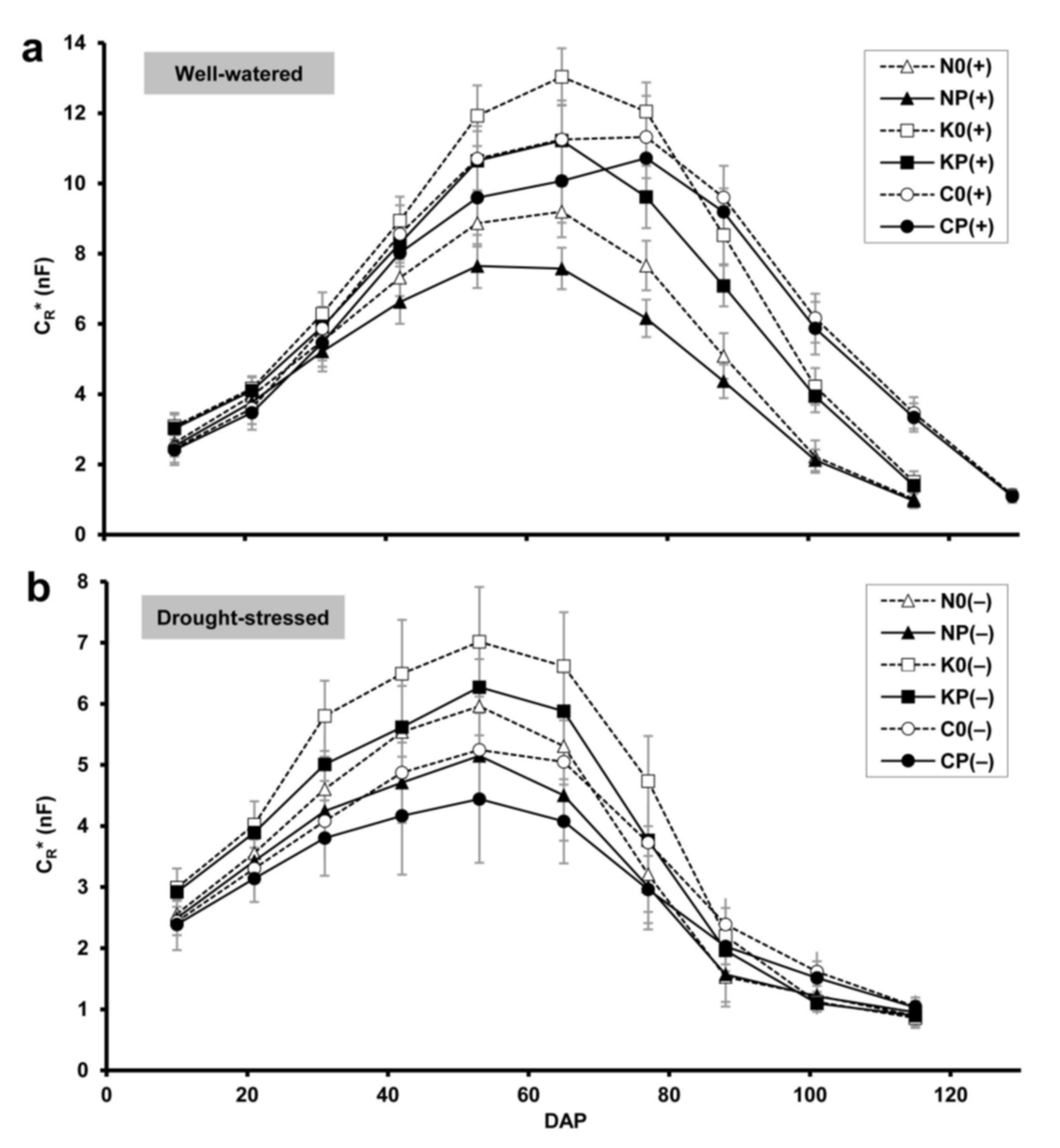
2.1. Root Electrical Capacitance
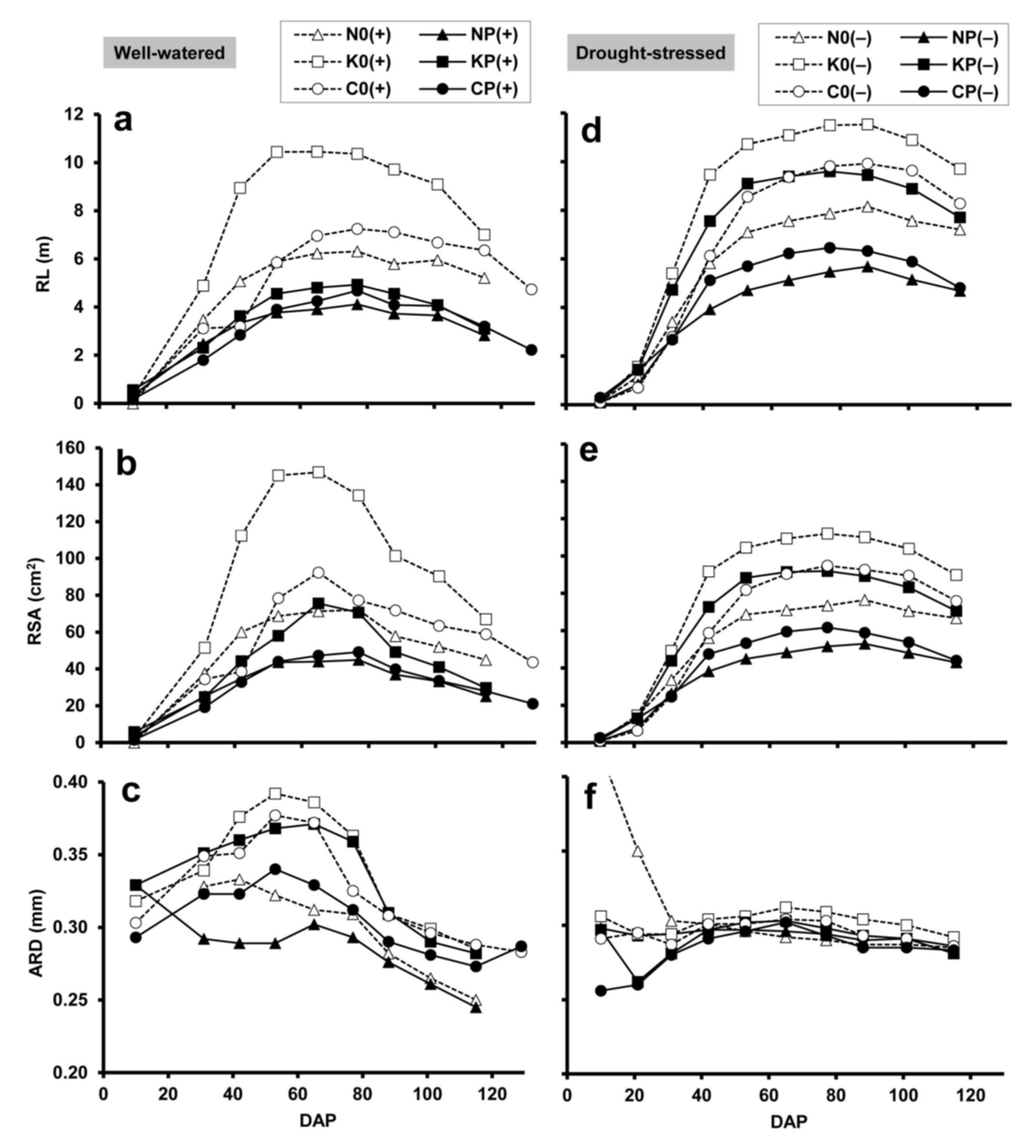
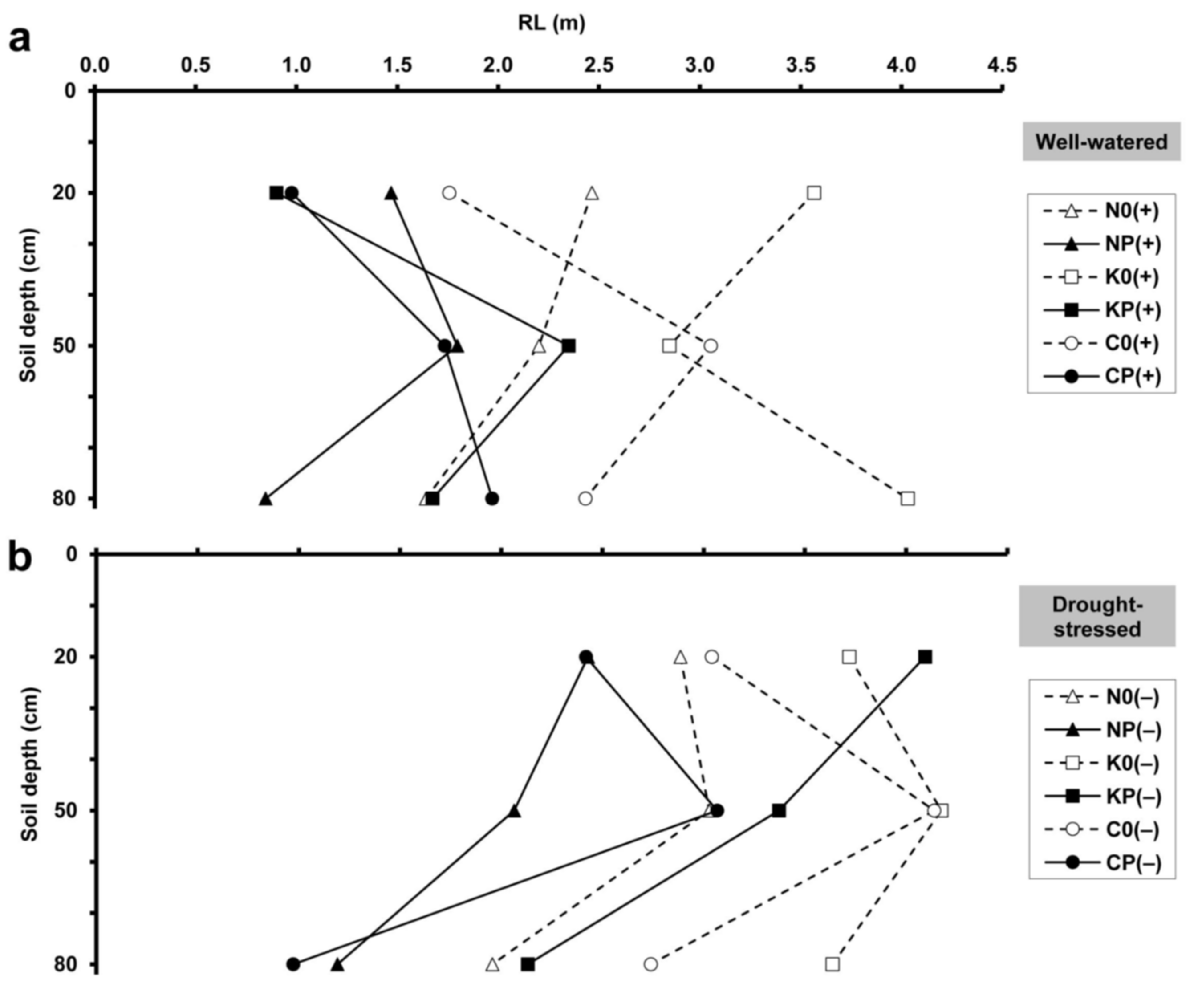
2.2. Minirhizotron Image Analysis
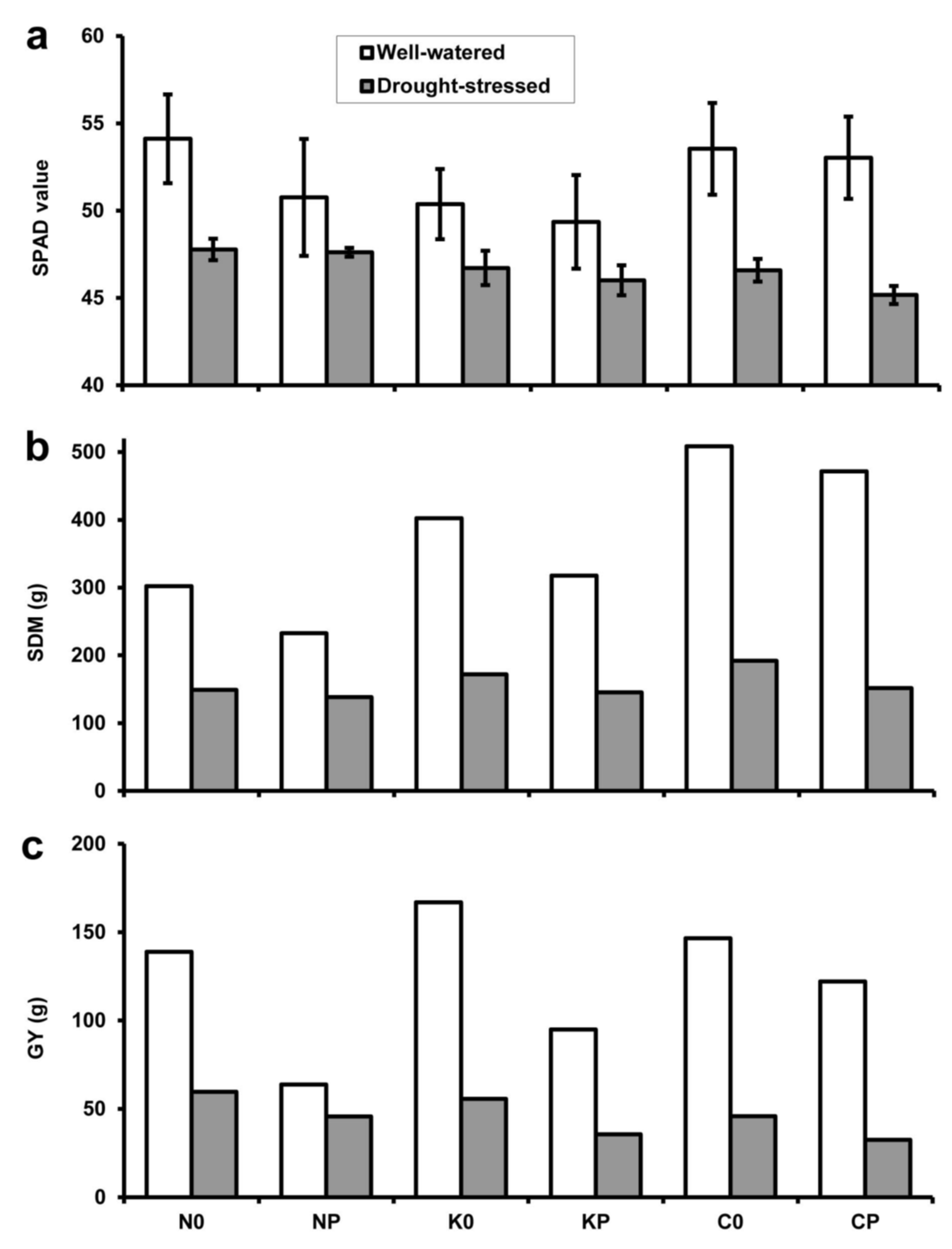
2.3. Chlorophyll Content and Yield Components
2.4. Relative Changes in Plant Parameters
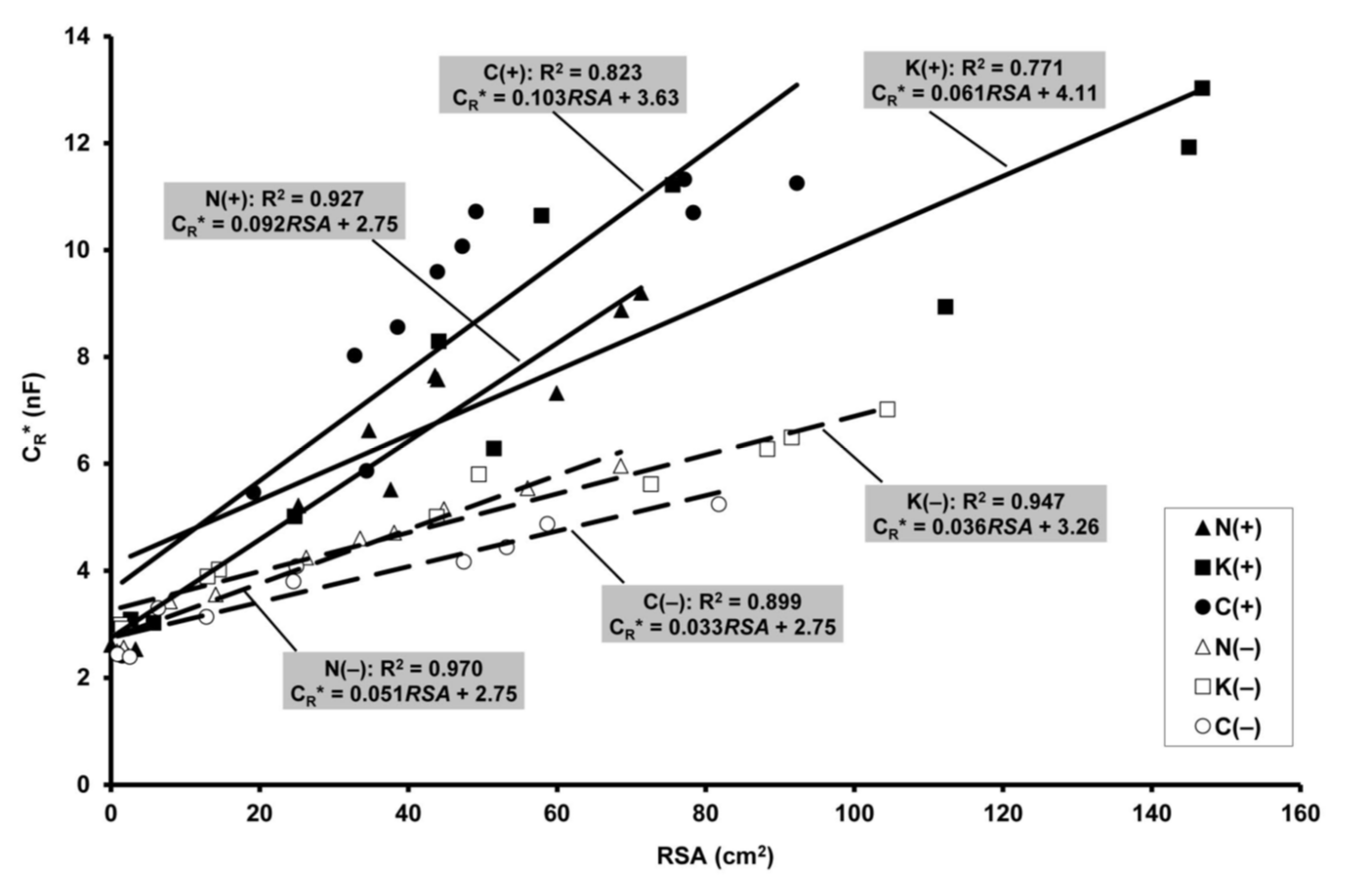
2.5. Relationship of Electrical Capacitance to Root Surface Area
3. Discussion
3.1. Root Dynamics
3.2. Effect of Drought and Intercropping
3.3. Benefits and Drawbacks of the Combined Approach of CR and MR
3.4. Conclusions
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Electrical Capacitance Measurements
4.3. Minirhizotron Technique
4.4. Leaf Chlorophyll Content and Post-Harvest Measurements
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Alternating current |
| ARD | Average root diameter |
| CR | Root electrical capacitance |
| CR* | Apparent (saturation) root electrical capacitance |
| DAP | Days after planting |
| GY | Grain yield |
| MR | Minirhizotron |
| RL | Root length |
| RSA | Root surface area |
| RSS | Root system size |
| SDM | Shoot dry mass |
| SWC | Soil water content |
References
- Faget, M.; Nagel, K.A.; Walter, A.; Herrera, J.M.; Jahnke, S.; Schurr, U.; Temperton, V.M. Root–root interactions: Extending our perspective to be more inclusive of the range of theories in ecology and agriculture using in-vivo analyses. Ann. Bot. 2013, 112, 253–266. [Google Scholar] [CrossRef]
- Maeght, J.-L.; Rewald, B.; Pierret, A. How to study deep roots–and why it matters. Front. Plant Sci. 2013, 4, 299. [Google Scholar] [CrossRef]
- Postic, F.; Doussan, C. Benchmarking electrical methods for rapid estimation of root biomass. Plant Methods 2016, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Ehosioke, S.; Nguyen, F.; Rao, S.; Kremer, T.; Placencia-Gomez, E.; Huisman, J.A.; Kemna, A.; Javaux, M.; Garré, S. Sensing the electrical properties of roots: A review. Vadose Zone J. 2020, 19, e20082. [Google Scholar] [CrossRef]
- Chloupek, O. The relationship between electric capacitance and some other parameters of plant roots. Biol. Plant. 1972, 14, 227–230. [Google Scholar] [CrossRef]
- Dalton, F.N. In-situ root extent measurements by electrical capacitance methods. Plant Soil 1995, 173, 157–165. [Google Scholar] [CrossRef]
- Cabal, C.; de Deurwaerder, H.P.T.; Matesanz, S. Field methods to study the spatial root density distribution of individual plants. Plant Soil 2021, 462, 25–43. [Google Scholar] [CrossRef]
- Dietrich, R.C.; Bengough, A.G.; Jones, H.G.; White, P.J. A new physical interpretation of plant root capacitance. J. Exp. Bot. 2012, 63, 6149–6159. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, R.C.; Bengough, A.G.; Jones, H.G.; White, P.J. Can root electrical capacitance be used to predict root mass in soil? Ann. Bot. 2013, 112, 457–464. [Google Scholar] [CrossRef]
- Peruzzo, L.; Chou, C.; Wu, Y.; Schmutz, M.; Mary, B.; Wagner, F.M.; Petrov, P.; Newman, G.; Blancaflor, E.B.; Liu, X.; et al. Imaging of plant current pathways for non-invasive root phenotyping using a newly developed electrical current source density approach. Plant Soil 2020, 450, 567–584. [Google Scholar] [CrossRef]
- Ozier-Lafontaine, H.; Bajazet, T. Analysis of root growth by impedance spectroscopy (EIS). Plant Soil 2005, 277, 299–313. [Google Scholar] [CrossRef]
- Čermák, J.; Ulrich, R.; Staněk, Z.; Koller, J.; Aubrecht, L. Electrical measurement of tree root absorbing surfaces by the earth impedance method: 2. Verification based on allometric relationships and root severing experiments. Tree Physiol. 2006, 26, 1113–1121. [Google Scholar] [CrossRef][Green Version]
- Ellis, T.; Murray, W.; Paul, K.; Kavalieris, L.; Brophy, J.; Williams, C.; Maass, M. Electrical capacitance as a rapid non-invasive indicator of root length. Tree Physiol. 2013, 33, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Cseresnyés, I.; Rajkai, K.; Takács, T. Indirect monitoring of root activity in soybean cultivars under contrasting moisture regimes by measuring electrical capacitance. Acta Physiol. Plant. 2016, 38, 121. [Google Scholar] [CrossRef]
- Cseresnyés, I.; Szitár, K.; Rajkai, K.; Füzy, A.; Mikó, P.; Kovács, R.; Takács, T. Application of electrical capacitance method for prediction of plant root mass and activity in field-grown crops. Front. Plant Sci. 2018, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Chloupek, O.; Dostál, V.; Středa, T.; Psota, V.; Dvořáčková, O. Drought tolerance of barley varieties in relation to their root system size. Plant Breed. 2010, 129, 630–636. [Google Scholar] [CrossRef]
- Aulen, M.; Shipley, B. Non-destructive estimation of root mass using electrical capacitance on ten herbaceous species. Plant Soil 2012, 355, 41–49. [Google Scholar] [CrossRef]
- Carlson, C.H.; Smart, L.B. Electrical capacitance as a predictor of root dry weight in shrub willow (Salix; Salicaceae) parents and progeny. Appl. Plant Sci. 2016, 4, 1600031. [Google Scholar] [CrossRef]
- Wu, W.; Duncan, R.W.; Ma, B.-L. Quantification of canola root morphological traits under heat and drought stresses with electrical measurements. Plant Soil 2017, 415, 229–244. [Google Scholar] [CrossRef]
- Svačina, P.; Středa, T.; Chloupek, O. Uncommon selection by root system size increases barley yield. Agron. Sustain. Dev. 2014, 34, 545–551. [Google Scholar] [CrossRef]
- Vamerali, T.; Bandiera, M.; Mosca, G. Minirhizotrons in modern root studies. In Measuring Roots; Mancuso, S., Ed.; Springer: Berlin, Germany, 2012; pp. 341–361. [Google Scholar] [CrossRef]
- Muñoz-Romero, V.; Benítez-Vega, J.; López-Bellido, L.; López-Bellido, R.J. Monitoring wheat root development in a rainfed vertisol: Tillage effect. Eur. J. Agron. 2010, 33, 182–187. [Google Scholar] [CrossRef]
- Milchunas, D.G. Estimating root production: Comparison of 11 methods in shortgrass steppe and review of biases. Ecosystems 2009, 12, 1381–1402. [Google Scholar] [CrossRef]
- Postic, F.; Beauchêne, K.; Gouache, D.; Doussan, C. Scanner-based minirhizotrons help to highlight relations between deep roots and yield in various wheat cultivars under combined water and nitrogen deficit conditions. Agronomy 2019, 9, 297. [Google Scholar] [CrossRef]
- Bedoussac, L.; Justes, E. The efficiency of a durum wheat–winter pea intercrop to improve yield and wheat grain protein concentration depends on N availability during early growth. Plant Soil 2010, 330, 19–35. [Google Scholar] [CrossRef]
- Pankou, C.; Lithourgidis, A.; Dordas, C. Effect of irrigation on intercropping systems of wheat (Triticum aestivum L.) with pea (Pisum sativum L.). Agronomy 2021, 11, 283. [Google Scholar] [CrossRef]
- Knörzer, H.; Graeff-Hönninger, S.; Guo, B.; Wang, P.; Claupein, W. The rediscovery of intercropping in China: A traditional cropping system for future Chinese agriculture. In Climate Change, Intercropping, Pest Control and Beneficial Microorganisms. Sustainable Agriculture Reviews 2; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 13–44. [Google Scholar] [CrossRef]
- Aziz, M.; Mahmood, A.; Asif, M.; Ali, A. Wheat-based intercropping: A review. J. Anim. Plant Sci. 2015, 25, 896–907. [Google Scholar]
- Klimek-Kopyra, A.; Zając, T.; Oleksy, A.; Kulig, B. Biological and production responses of intercropped plants of pea, spring wheat, and linseed. Acta Agrobot. 2018, 71, 1737. [Google Scholar] [CrossRef]
- Ghaley, B.B.; Hauggaard-Nielsen, H.; Høgh-Jensen, H.; Jensen, E.S. Intercropping of wheat and pea as influenced by nitrogen fertilization. Nutr. Cycl. Agroecosyst. 2005, 73, 201–212. [Google Scholar] [CrossRef]
- Amossé, C.; Jeuffroy, M.-H.; David, C. Relay intercropping of legume cover crops in organic winter wheat: Effects on performance and resource availability. Field Crop. Res. 2013, 145, 78–87. [Google Scholar] [CrossRef]
- Naudin, C.; Corre-Hellou, G.; Pineau, S.; Crozat, Y.; Jeuffroy, M.-H. The effect of various dynamics of N availability on winter pea–wheat intercrops: Crop growth, N partitioning and symbiotic N2 fixation. Field Crop. Res. 2010, 119, 2–11. [Google Scholar] [CrossRef]
- Fageria, N.K. The Role of Plant Roots in Crop Production; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Hodgkinson, L.; Dodd, I.C.; Binley, A.; Ashton, R.W.; White, R.P.; Watts, C.W.; Whalley, W.R. Root growth in field-grown winter wheat: Some effects of soil conditions, season and genotype. Eur. J. Agron. 2017, 91, 74–83. [Google Scholar] [CrossRef]
- Jha, S.K.; Gao, Y.; Liu, H.; Huang, Z.; Wang, G.; Liang, Y.; Duan, A. Root development and water uptake in winter wheat under different irrigation methods and scheduling for North China. Agr. Water Manage. 2017, 182, 139–150. [Google Scholar] [CrossRef]
- Yang, B.; Wang, P.; You, D.; Liu, W. Coupling evapotranspiration partitioning with root water uptake to identify the water consumption characteristics of winter wheat: A case study in the North China Plain. Agr. For. Meteorol. 2018, 259, 296–304. [Google Scholar] [CrossRef]
- Herrera, J.M.; Stamp, P.; Liedgens, M. Interannual variability in root growth of spring wheat (Triticum aestivum L.) at low and high nitrogen supply. Eur. J. Agron. 2007, 26, 317–326. [Google Scholar] [CrossRef]
- Döring, T.; Wolfe, M.; Jones, H.; Pearce, H.; Zhan, J. Breeding for resilience in wheat–Nature’s choice. In Eucarpia 2nd Conference of the Organic and Low-Input Agriculture Section; Goldringer, I., Dawson, J.C., Rey, F., Vettoretti, A., Chable, V., Lammerts van Bueren, E., Finckh, M., Barot, S., Eds.; INRA: Paris, France, 2010; pp. 47–50. [Google Scholar]
- De Micco, V.; Aronne, G. Morpho-anatomical traits for plant adaptation to drought. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Heidelberg, Germany, 2012; pp. 37–62. [Google Scholar] [CrossRef]
- Hooker, J.E.; Hendrick, R.; Atkinson, D. The measurement and analysis of fine root longevity. In Root Methods: A Handbook; Smit, A.L., Bengough, A.G., Engels, C., van Noordwijk, M., Pellerin, S., van de Geijn, S.C., Eds.; Springer: Berlin, Germany, 2000; pp. 273–304. [Google Scholar] [CrossRef]
- Monti, M.; Pellicanò, A.; Santonoceto, C.; Preiti, G.; Pristeri, A. Yield components and nitrogen use in cereal–pea intercrops in Mediterranean environment. Field Crop. Res. 2016, 196, 379–388. [Google Scholar] [CrossRef]
- Mariotti, M.; Masoni, A.; Ercoli, L.; Arduini, I. Above- and below-ground competition between barley, wheat, lupin and vetch in a cereal and legume intercropping system. Grass Forage Sci. 2009, 64, 401–412. [Google Scholar] [CrossRef]
- Pelzer, E.; Bazot, M.; Makowski, D.; Corre-Hellou, G.; Naudin, C.; Al Rifaï, M.; Baranger, E.; Bedoussac, L.; Biarnès, V.; Boucheny, P.; et al. Pea–wheat intercrops in low-input conditions combine high economic performances and low environmental impacts. Eur. J. Agron. 2012, 40, 39–53. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef]
- Monostori, I.; Árendás, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vágújfalvi, A. Relationship between SPAD value and grain yield can be affected by cultivar, environment and soil nitrogen content in wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef]
- Asseng, S.; Ritchie, J.T.; Smucker, A.J.M.; Robertson, M.J. Root growth and water uptake during water deficit and recovering in wheat. Plant Soil 1998, 201, 265–273. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Li, Q.; Ma, D.; Lu, H.; Feng, W.; Xie, Y.; Zhu, Y.; Gou, T. Effects of different irrigation and nitrogen regimes on root growth and its correlation with above-ground plant parts in high-yielding wheat under field conditions. Field Crop. Res. 2014, 165, 138–149. [Google Scholar] [CrossRef]
- IUSS Working Group. World Reference Base (WRB) for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Meier, U. (Ed.) Growth Stages of Mono- and Dicotyledonous Plants. BBCH Monograph, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry: Berlin, Germany, 2001. [Google Scholar]
- Liu, L.; Gan, Y.; Bueckert, R.; van Rees, K. Rooting systems of oilseed and pulse crops I: Temporal growth patterns across the plant developmental periods. Field Crop. Res. 2011, 122, 256–263. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cseresnyés, I.; Kelemen, B.; Takács, T.; Füzy, A.; Kovács, R.; Megyeri, M.; Parádi, I.; Mikó, P. Electrical Capacitance versus Minirhizotron Technique: A Study of Root Dynamics in Wheat–Pea Intercrops. Plants 2021, 10, 1991. https://doi.org/10.3390/plants10101991
Cseresnyés I, Kelemen B, Takács T, Füzy A, Kovács R, Megyeri M, Parádi I, Mikó P. Electrical Capacitance versus Minirhizotron Technique: A Study of Root Dynamics in Wheat–Pea Intercrops. Plants. 2021; 10(10):1991. https://doi.org/10.3390/plants10101991
Chicago/Turabian StyleCseresnyés, Imre, Bettina Kelemen, Tünde Takács, Anna Füzy, Ramóna Kovács, Mária Megyeri, István Parádi, and Péter Mikó. 2021. "Electrical Capacitance versus Minirhizotron Technique: A Study of Root Dynamics in Wheat–Pea Intercrops" Plants 10, no. 10: 1991. https://doi.org/10.3390/plants10101991
APA StyleCseresnyés, I., Kelemen, B., Takács, T., Füzy, A., Kovács, R., Megyeri, M., Parádi, I., & Mikó, P. (2021). Electrical Capacitance versus Minirhizotron Technique: A Study of Root Dynamics in Wheat–Pea Intercrops. Plants, 10(10), 1991. https://doi.org/10.3390/plants10101991






